

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035500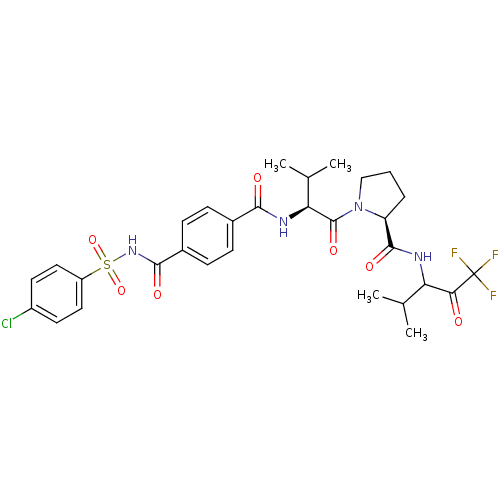 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035489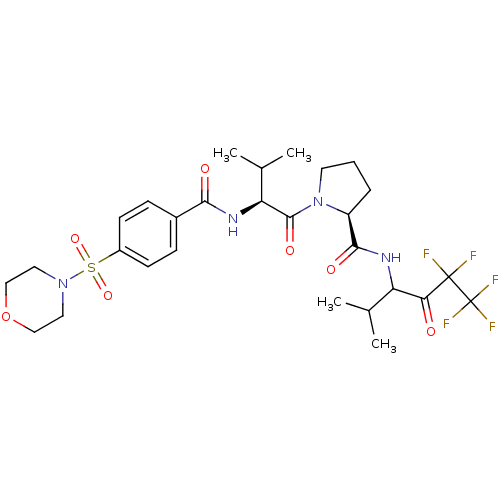 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-sulfonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035500 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035528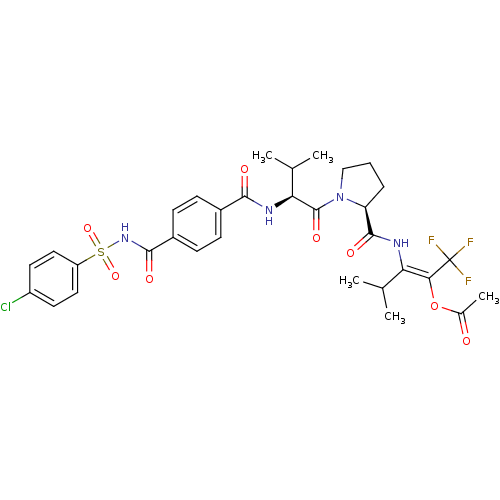 (Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037811 (CHEMBL123016 | {(S)-1-[(S)-3-Benzylcarbamoyl-1-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the ability to inhibit HIV-protease. | J Med Chem 37: 3684-92 (1994) BindingDB Entry DOI: 10.7270/Q2ZW1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035488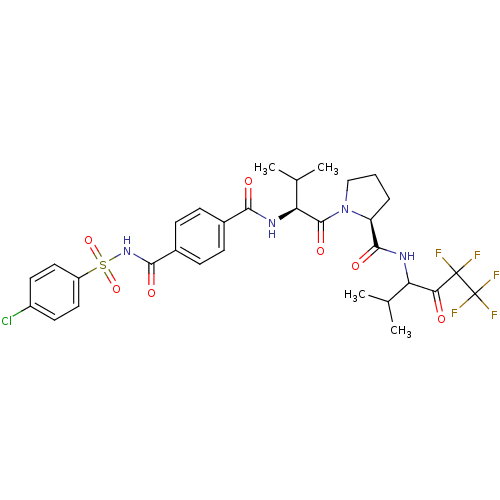 ((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 100 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50279944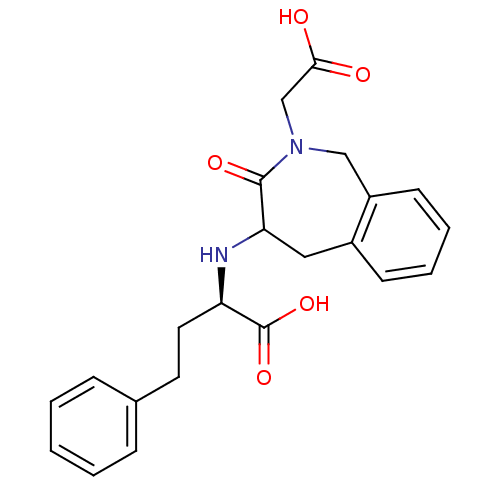 ((R)-2-(2-Carboxymethyl-3-oxo-2,3,4,5-tetrahydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant of the compound was tested angiotensin converting enzyme | Bioorg Med Chem Lett 1: 309-312 (1991) Article DOI: 10.1016/S0960-894X(01)80814-3 BindingDB Entry DOI: 10.7270/Q208657V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035498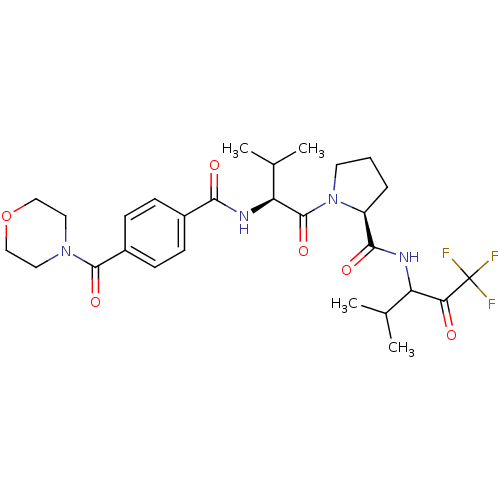 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035498 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035535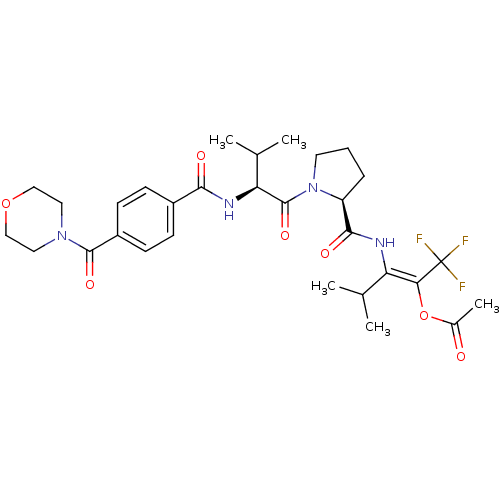 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035492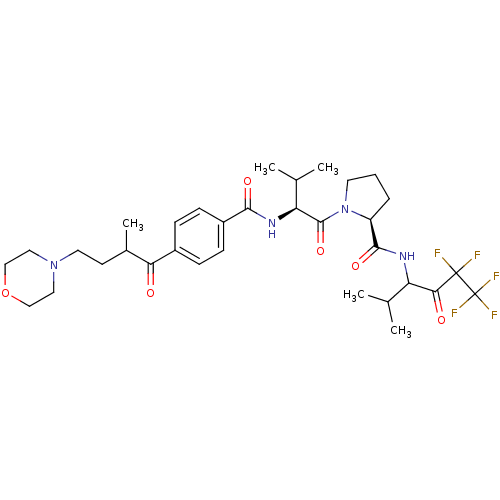 ((S)-1-{(S)-3-Methyl-2-[4-(2-methyl-4-morpholin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50037812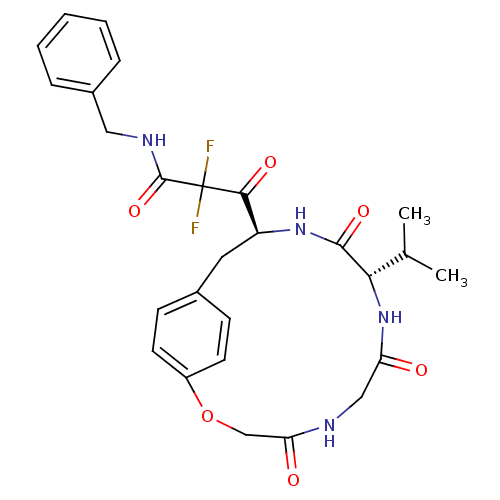 (CHEMBL331994 | MDL-104168 | N-Benzyl-2,2-difluoro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the ability to inhibit HIV-protease. | J Med Chem 37: 3684-92 (1994) BindingDB Entry DOI: 10.7270/Q2ZW1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035494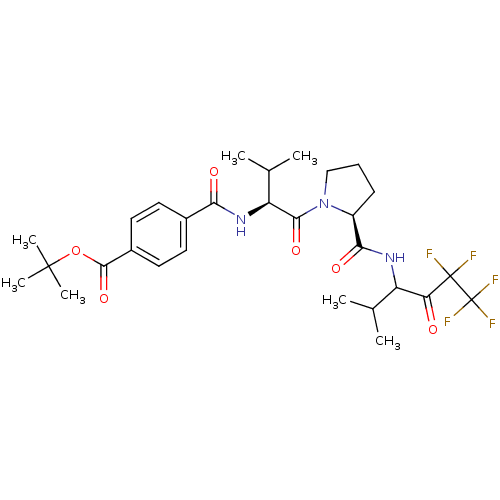 (CHEMBL429359 | N-{(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035485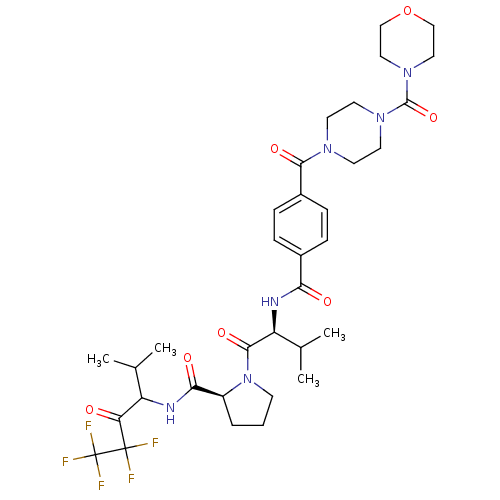 ((S)-1-((S)-3-Methyl-2-{4-[4-(morpholine-4-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495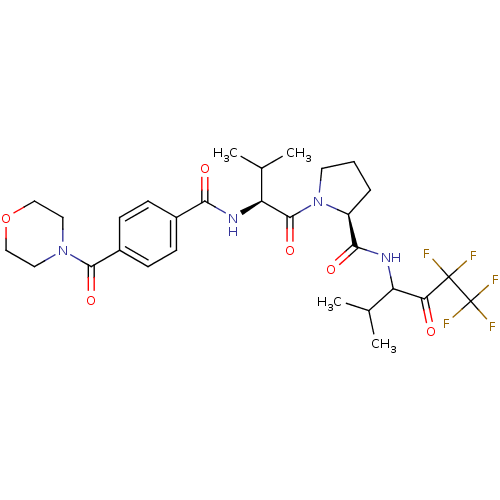 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035532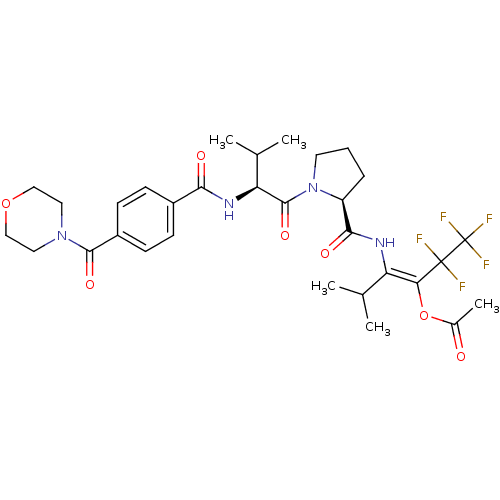 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035531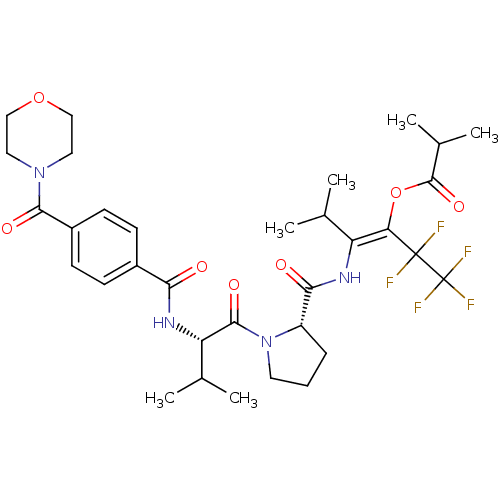 (CHEMBL74332 | Isobutyric acid (E)-3-methyl-2-[((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035530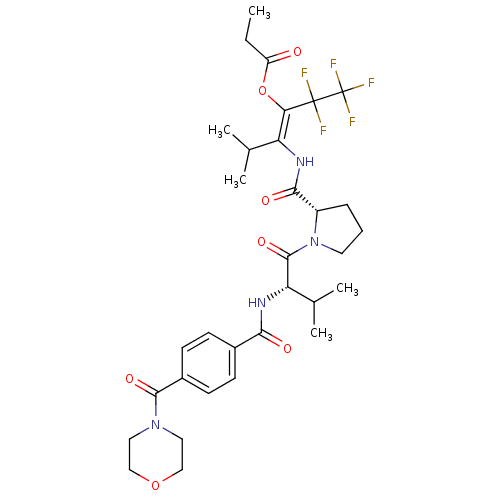 (CHEMBL74651 | Propionic acid (E)-3-methyl-2-[((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in the presence of pig liver esterase, in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035487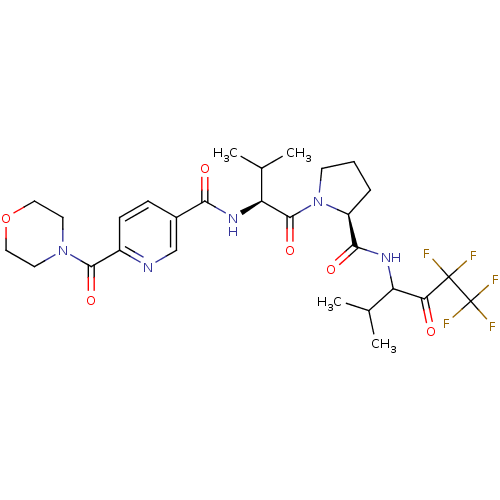 (CHEMBL140639 | N-{(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035502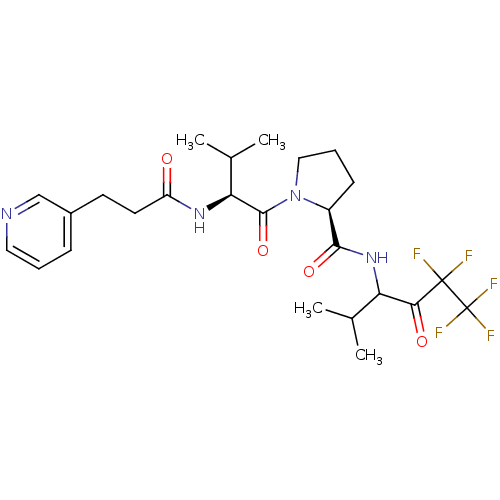 ((S)-1-[(S)-3-Methyl-2-(3-pyridin-3-yl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035505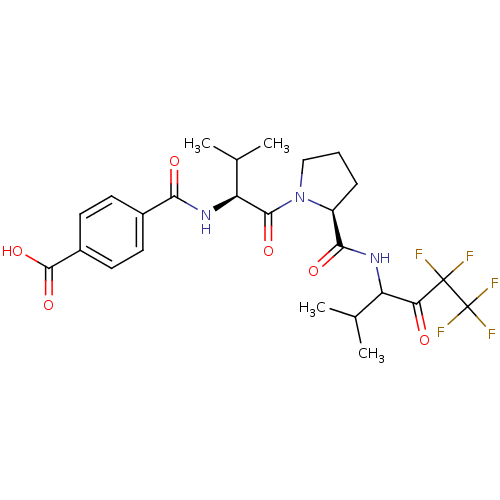 (CHEMBL140763 | N-{(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035497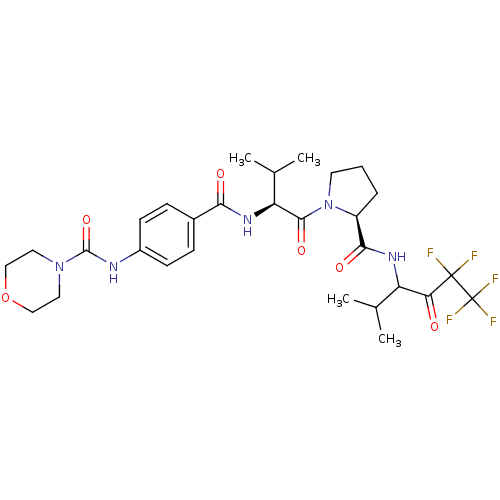 (CHEMBL141423 | Morpholine-4-carboxylic acid (4-{(S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035486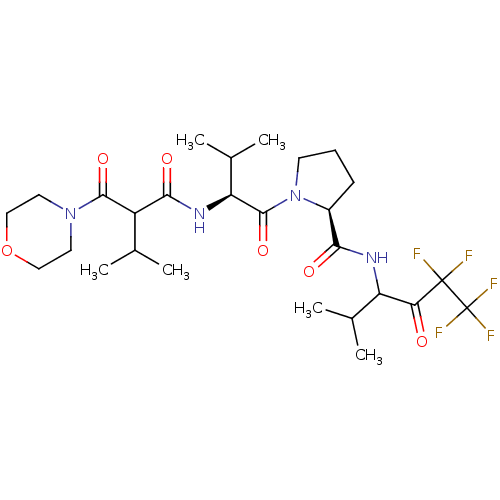 ((S)-1-{(S)-3-Methyl-2-[3-methyl-2-(morpholine-4-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035501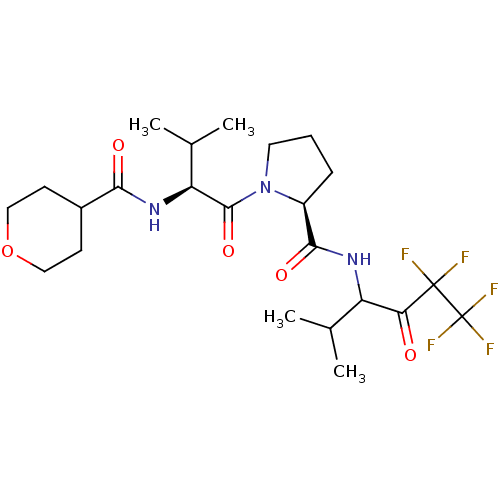 ((S)-1-{(S)-3-Methyl-2-[(tetrahydro-pyran-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035499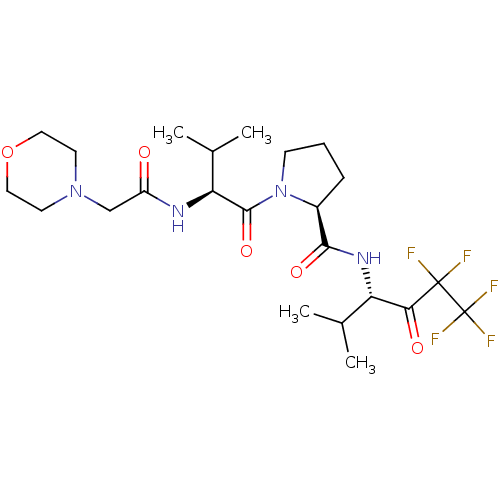 ((S)-1-[(S)-3-Methyl-2-(2-morpholin-4-yl-acetylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035504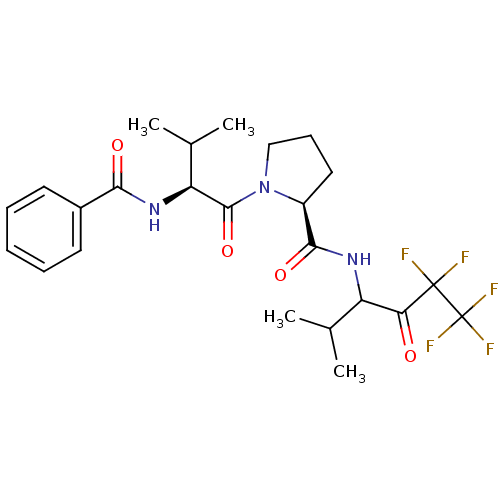 ((S)-1-((S)-2-Benzoylamino-3-methyl-butyryl)-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035493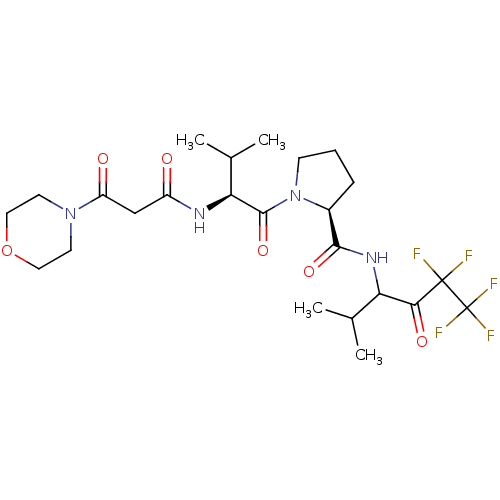 ((S)-1-[(S)-3-Methyl-2-(3-morpholin-4-yl-3-oxo-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 25 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282635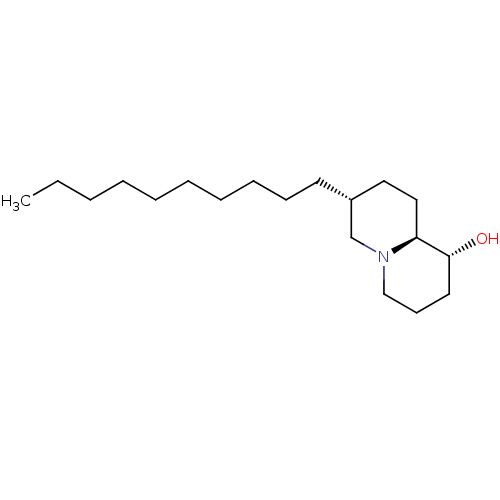 ((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035490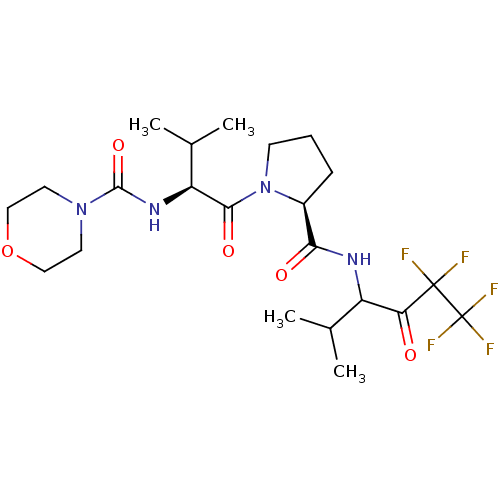 (CHEMBL141517 | Morpholine-4-carboxylic acid {(S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 50 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035496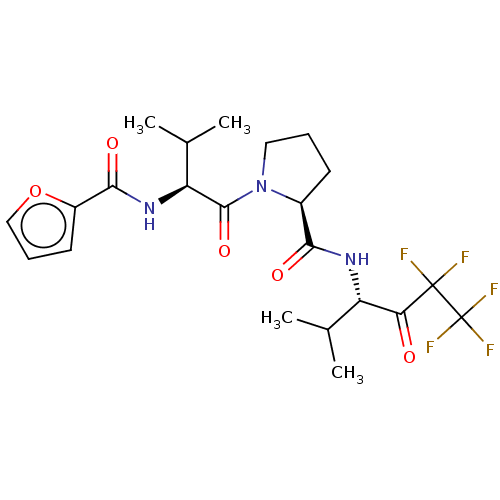 ((S)-1-{(S)-2-[(Furan-2-carbonyl)-amino]-3-methyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase was determined at a dose of 45 mg/kg | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035491 (CHEMBL59091 | Morpholine-4-carboxylic acid {(S)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035491 (CHEMBL59091 | Morpholine-4-carboxylic acid {(S)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035506 ((S)-1-[(S)-2-(3,3-Dimethyl-ureido)-3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035528 (Acetic acid (E)-2-[((S)-1-{(S)-2-[4-(4-chloro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282634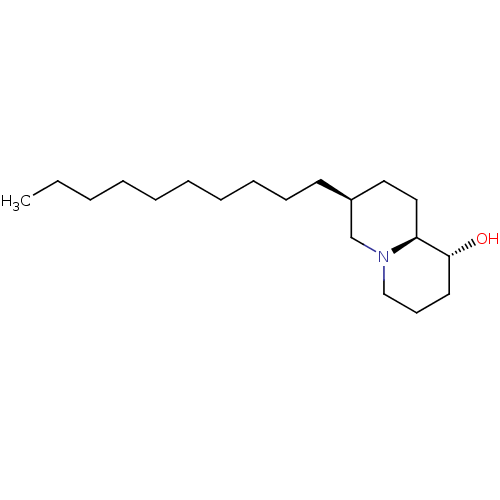 ((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035503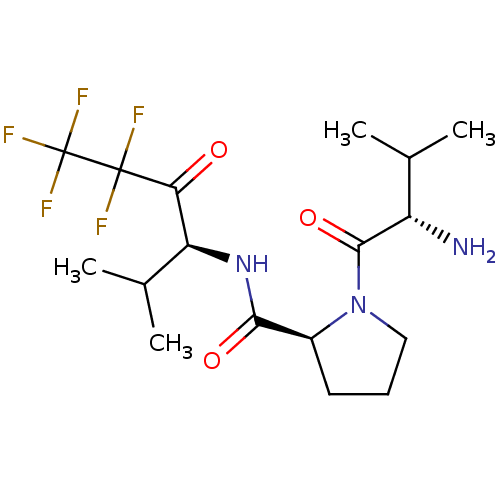 ((S)-1-((S)-2-Amino-3-methyl-butyryl)-pyrrolidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil elastase | J Med Chem 37: 4538-53 (1995) BindingDB Entry DOI: 10.7270/Q28P615X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035532 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035533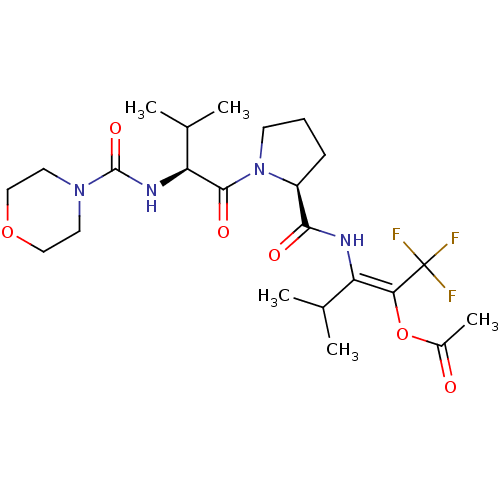 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035533 (Acetic acid (E)-3-methyl-2-[((S)-1-{(S)-3-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035536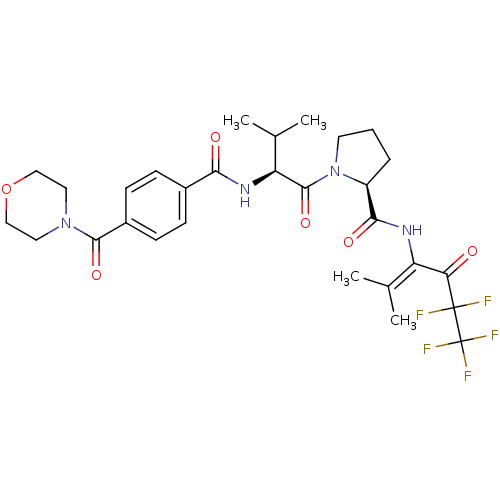 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50281462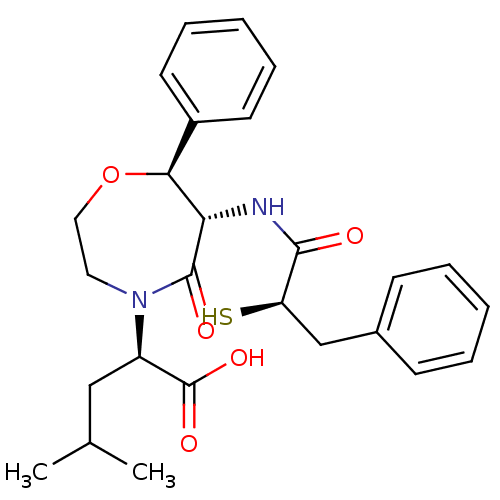 ((R)-2-[(6S,7S)-6-((R)-2-Mercapto-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against Angiotensin I converting enzyme | Bioorg Med Chem Lett 3: 231-234 (1993) Article DOI: 10.1016/S0960-894X(01)80882-9 BindingDB Entry DOI: 10.7270/Q21G0M5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035534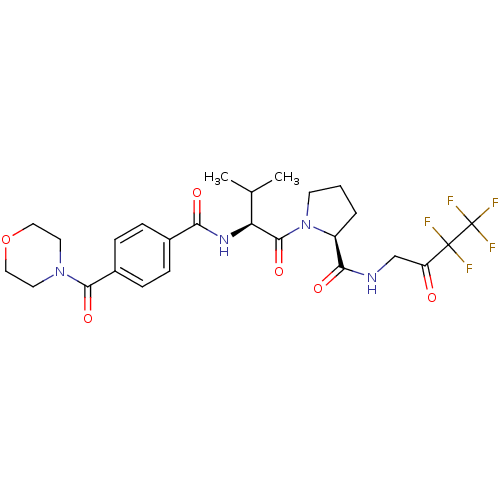 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50281462 ((R)-2-[(6S,7S)-6-((R)-2-Mercapto-3-phenyl-propiony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound against neutral endopeptidase | Bioorg Med Chem Lett 3: 231-234 (1993) Article DOI: 10.1016/S0960-894X(01)80882-9 BindingDB Entry DOI: 10.7270/Q21G0M5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035529 (CHEMBL74545 | {(S)-2-Methyl-1-[(S)-2-(3,3,4,4,4-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound for purified Human neutrophil elastase was determined in vitro. | J Med Chem 38: 223-33 (1995) BindingDB Entry DOI: 10.7270/Q2CC0ZPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282635 ((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase (OSC) | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50282634 ((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase | Bioorg Med Chem Lett 4: 1317-1318 (1994) Article DOI: 10.1016/S0960-894X(01)80352-8 BindingDB Entry DOI: 10.7270/Q23T9H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||