

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198694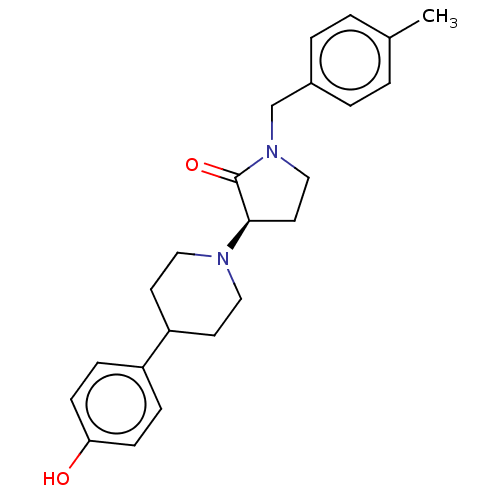 (US9221796, 23b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198665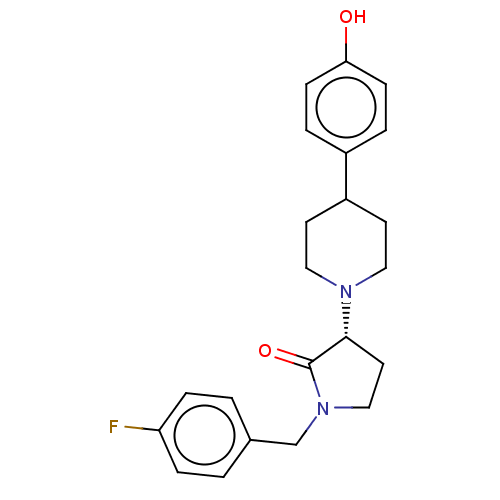 (US9221796, 2b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50169541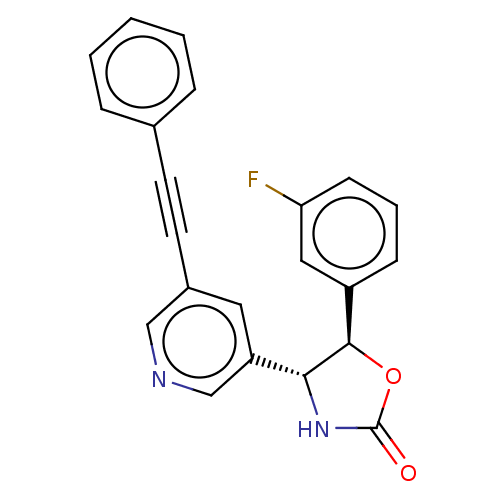 (CHEMBL3805542) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198728 (US9221796, 46, P-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198726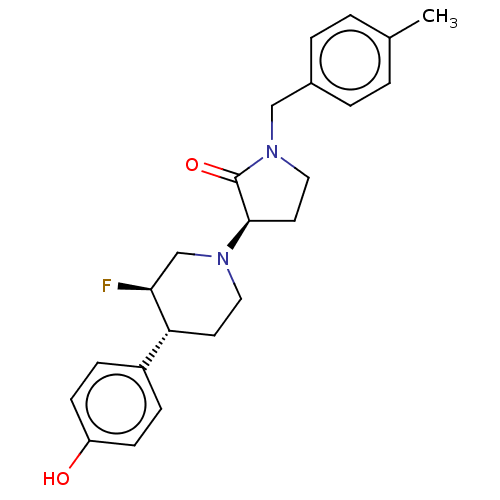 (US9221796, 46, P-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330324 (CHEMBL4170867) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM198728 (US9221796, 46, P-4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to GluN2B receptor in human cortex | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330409 (CHEMBL4168402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50330410 (CHEMBL4161899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Reversible inhibition of rat liver S- adenosyl-L-homocysteine hydrolase using SAH as substrate by spectrophotometric method | J Med Chem 63: 6315-6386 (2020) Article DOI: 10.1021/acs.jmedchem.9b01877 BindingDB Entry DOI: 10.7270/Q2NG4V6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM198735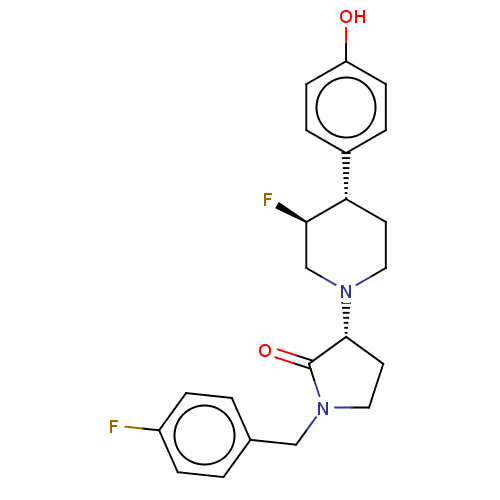 (US9221796, 48, P-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... | ACS Med Chem Lett 9: 472-477 (2018) Article DOI: 10.1021/acsmedchemlett.8b00080 BindingDB Entry DOI: 10.7270/Q2PR7ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174392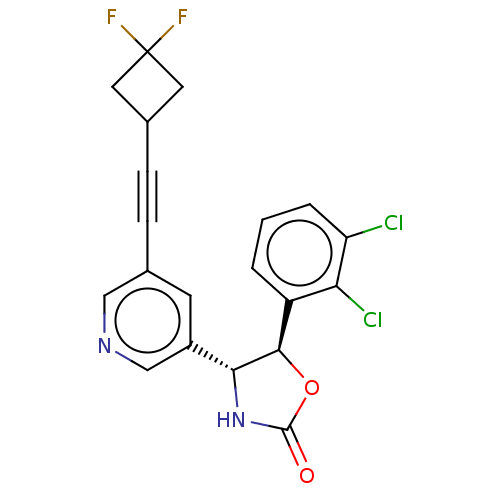 (US9688669, Example 124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174258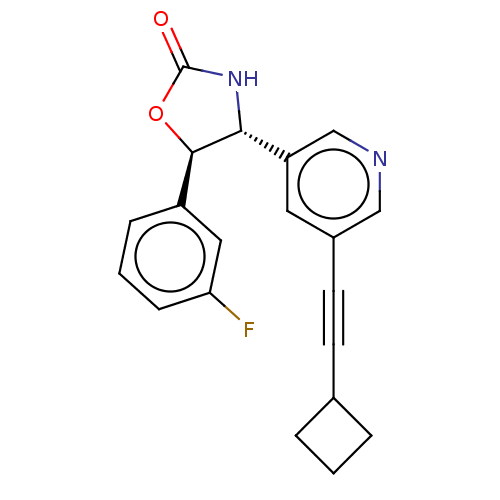 (US9688669, Example 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174255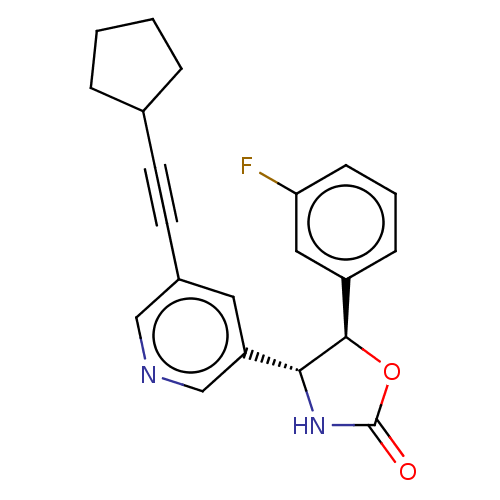 (US9688669, Example 86) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174275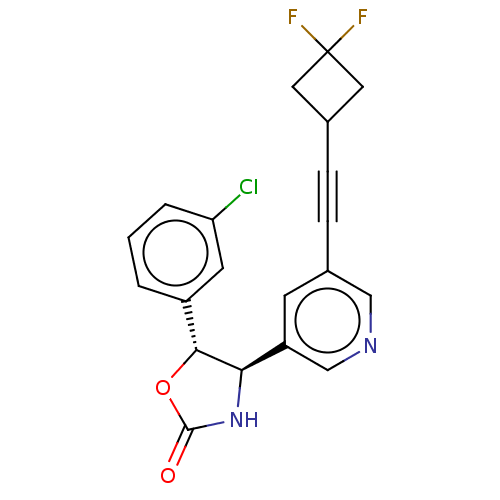 (US9688669, Example 108) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174267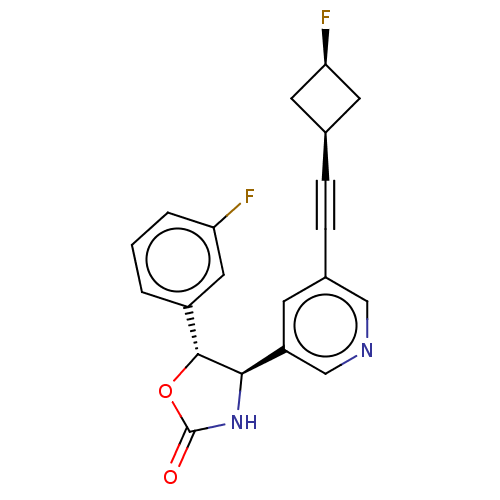 (US9688669, Example 98) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174391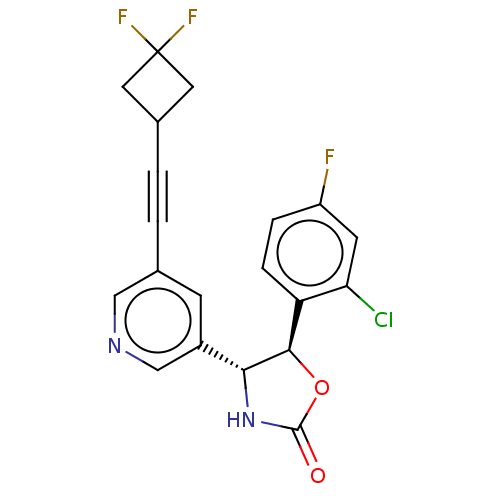 (US9688669, Example 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM168300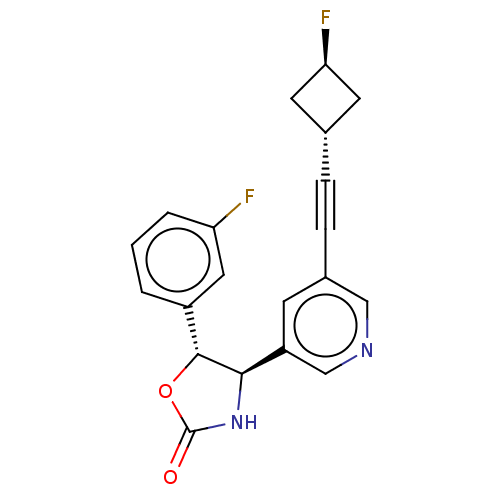 (US9688669, Example 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174252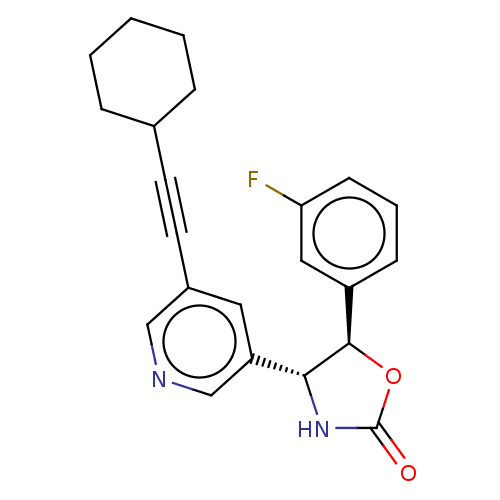 (US9688669, Example 83) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174277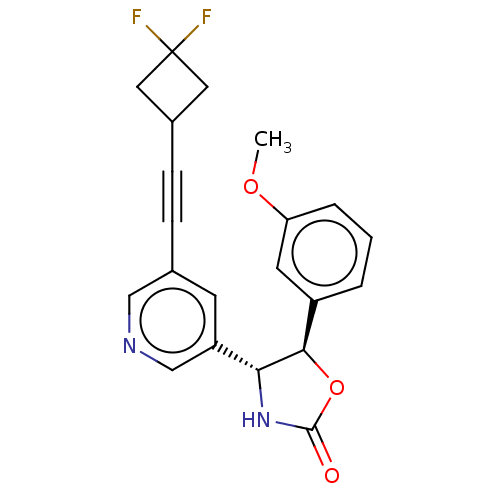 (US9688669, Example 110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174306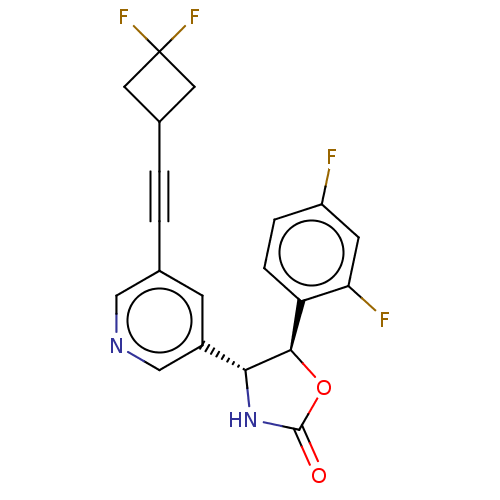 (US9688669, Example 120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174266 (US9688669, Example 97) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174276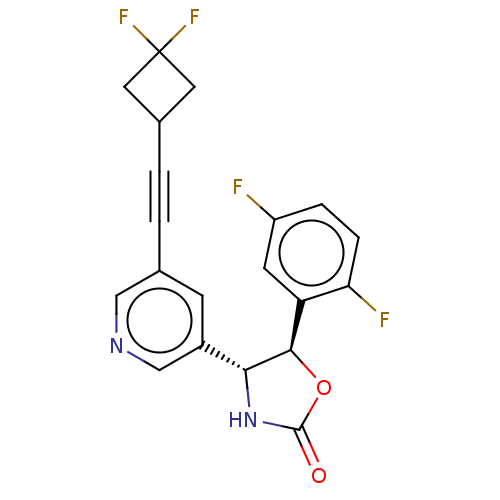 (US9688669, Example 109) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174282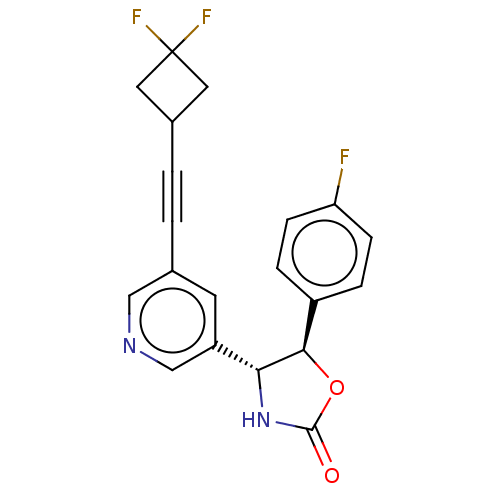 (US9688669, Example 115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174257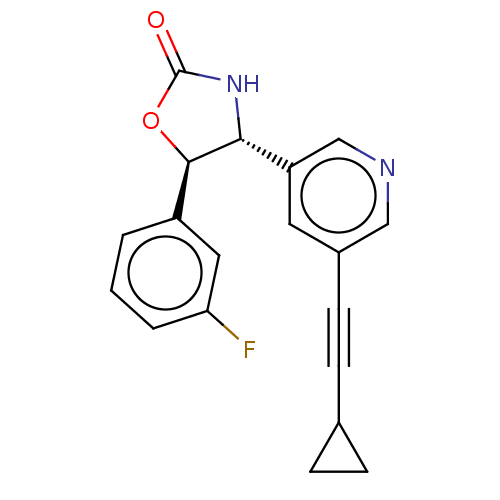 (US9688669, Example 88) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174284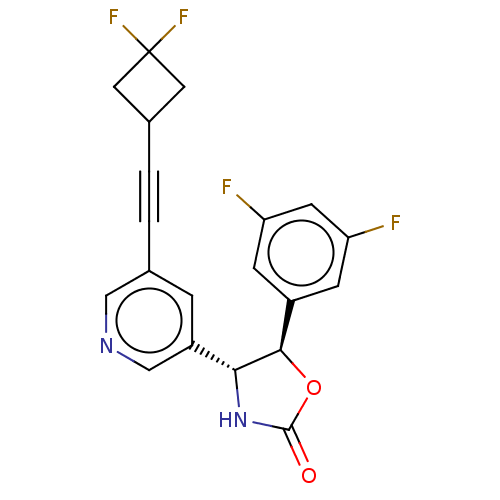 (US9688669, Example 117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174331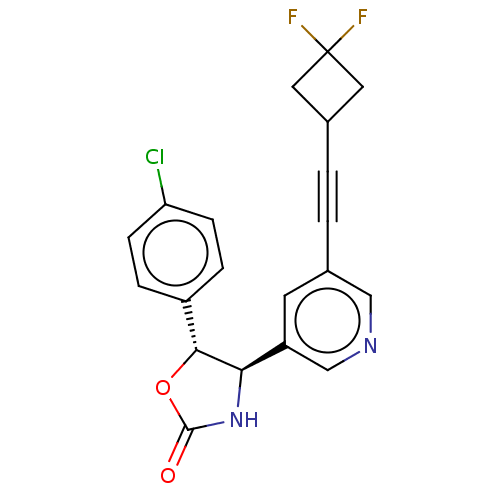 (US9688669, Example 121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174263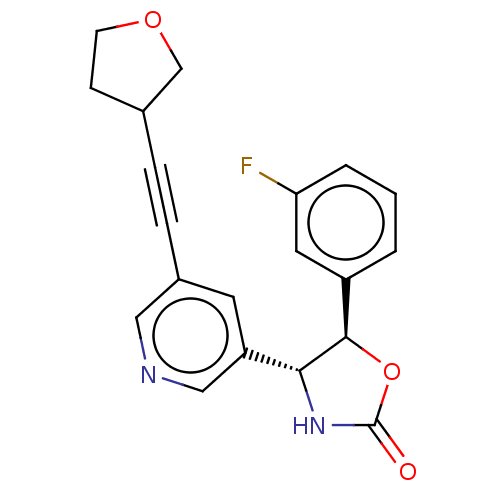 (US9688669, Example 94) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174264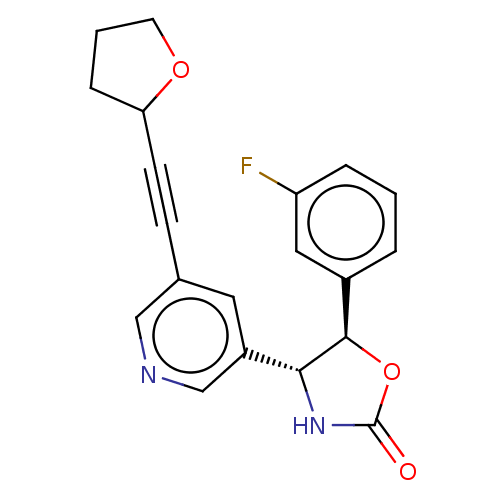 (US9688669, Example 95) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174262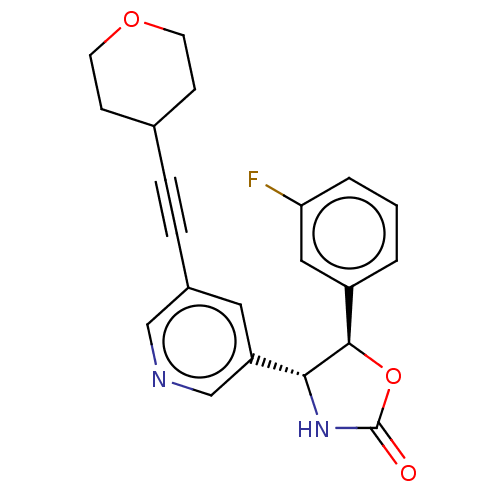 (US9688669, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174273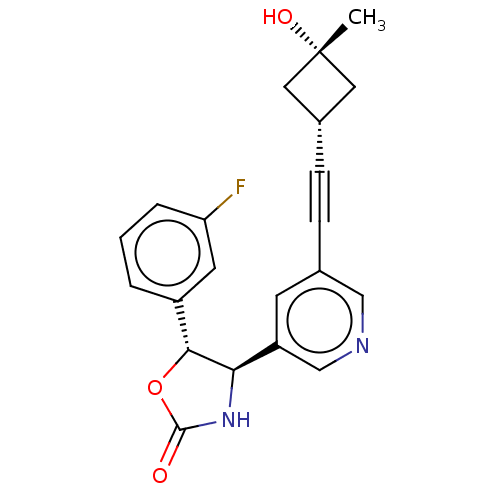 (US9688669, Example 105) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174270 (US9688669, Example 102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174271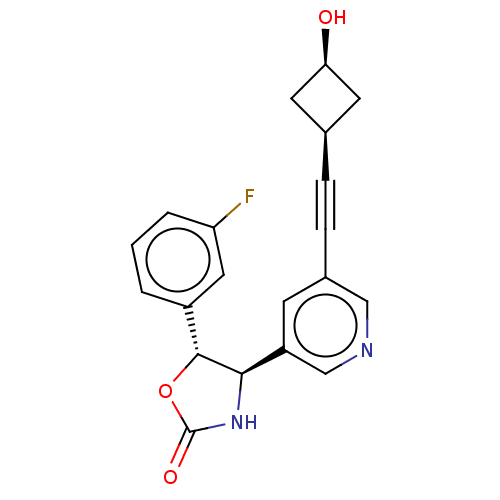 (US9688669, Example 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174272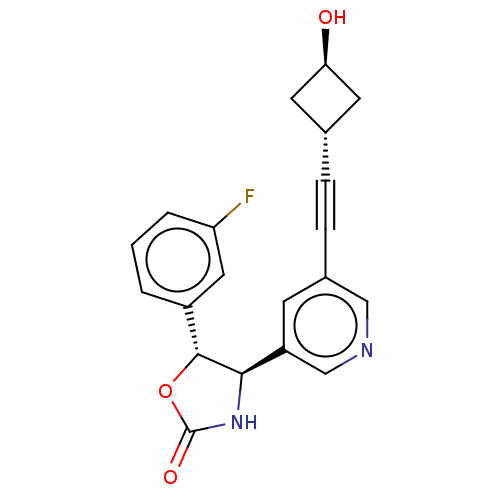 (US9688669, Example 104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50088500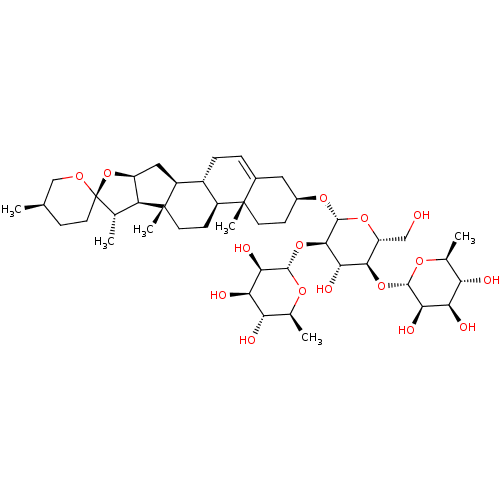 (CHEBI:74023 | Dioscin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University Curated by ChEMBL | Assay Description Cellular uptake in HEK293 cells expressing OATP1B3 (unknown origin) assessed as inhibition of telmisartan-mediated drug transport | Drug Metab Dispos 41: 994-1003 (2013) Article DOI: 10.1124/dmd.112.049452 BindingDB Entry DOI: 10.7270/Q2D220B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50088500 (CHEBI:74023 | Dioscin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University Curated by ChEMBL | Assay Description Cellular uptake in HEK293 cells expressing OATP1B3 (unknown origin) assessed as inhibition of cyclosporin A-mediated drug transport | Drug Metab Dispos 41: 994-1003 (2013) Article DOI: 10.1124/dmd.112.049452 BindingDB Entry DOI: 10.7270/Q2D220B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174265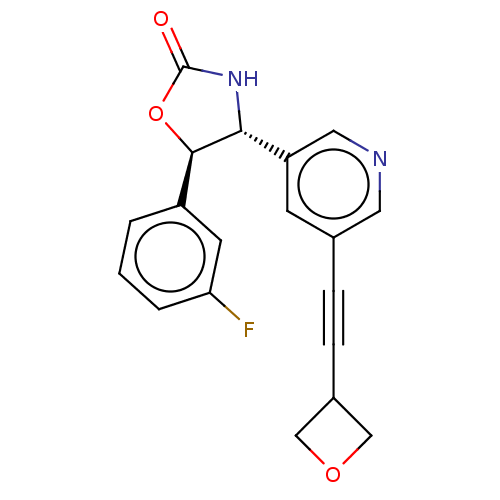 (US9688669, Example 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130903 (CHEMBL3632950) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Fixed inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by Dixon and Lineweaver-Burk plot analysis | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM179971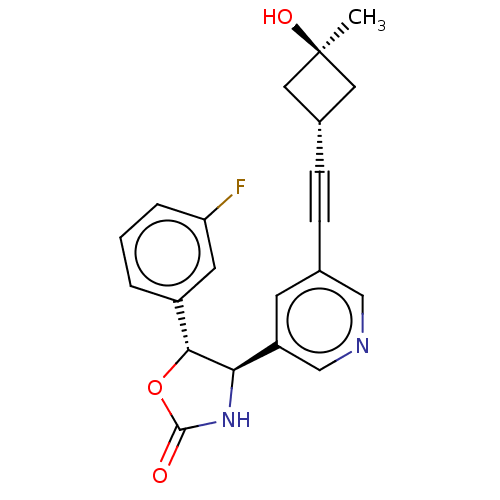 (US9688669, Example 106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50088500 (CHEBI:74023 | Dioscin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University Curated by ChEMBL | Assay Description Cellular uptake in HEK293 cells expressing OATP1B3 (unknown origin) assessed as inhibition of rifampicin-mediated drug transport | Drug Metab Dispos 41: 994-1003 (2013) Article DOI: 10.1124/dmd.112.049452 BindingDB Entry DOI: 10.7270/Q2D220B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM174269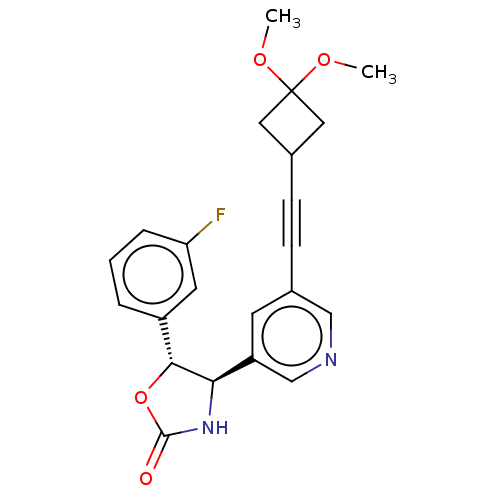 (US9688669, Example 101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGluR5 expressed in cell membranes after 60 mins by liquid scintillation counting method | Bioorg Med Chem Lett 26: 5871-5876 (2016) Article DOI: 10.1016/j.bmcl.2016.11.014 BindingDB Entry DOI: 10.7270/Q2SB47RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier organic anion transporter family member 1B3 (Homo sapiens (Human)) | BDBM50088500 (CHEBI:74023 | Dioscin) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian Medical University Curated by ChEMBL | Assay Description Cellular uptake in HEK293 cells expressing OATP1B3 (unknown origin) assessed as inhibition of (-)-epigallocatechin gallate-mediated drug transport | Drug Metab Dispos 41: 994-1003 (2013) Article DOI: 10.1124/dmd.112.049452 BindingDB Entry DOI: 10.7270/Q2D220B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50458163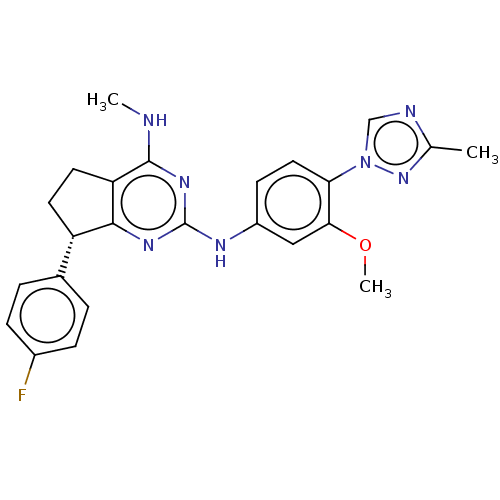 (CHEMBL4209316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time-dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant by Kitz-Wilson plot analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127530 BindingDB Entry DOI: 10.7270/Q24X5CFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50553647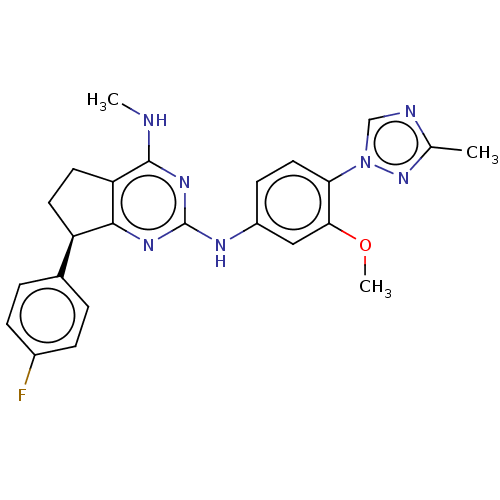 (CHEMBL4793756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time-dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant by Kitz-Wilson plot analysis | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127530 BindingDB Entry DOI: 10.7270/Q24X5CFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50458163 (CHEMBL4209316) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of CYP3A4 in human liver microsomes assessed as inhibition constant | Citation and Details Article DOI: 10.1021/acsmedchemlett.8b00541 BindingDB Entry DOI: 10.7270/Q24171P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine decarboxylase (Hafnia alvei) | BDBM50540321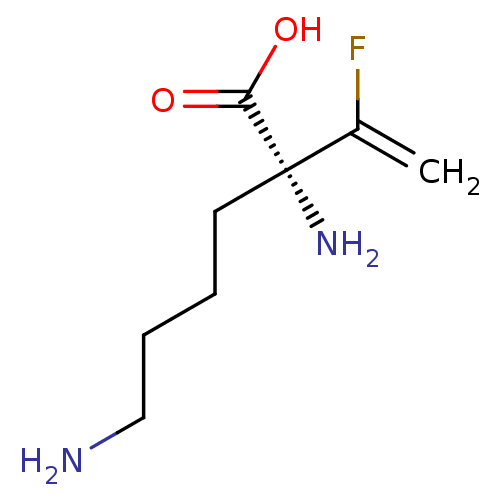 (CHEMBL4646127) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Time dependent inhibition of Hafnia alvei lysine decarboxylase using L-lysine by Kitz-Wilson analysis | J Med Chem 63: 6315-6386 (2020) Article DOI: 10.1021/acs.jmedchem.9b01877 BindingDB Entry DOI: 10.7270/Q2NG4V6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine decarboxylase (Hafnia alvei) | BDBM50540320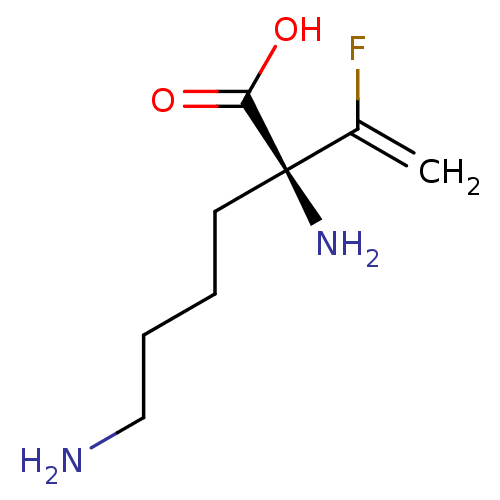 (CHEMBL4640604) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Time dependent inhibition of Hafnia alvei lysine decarboxylase using L-lysine by Kitz-Wilson analysis | J Med Chem 63: 6315-6386 (2020) Article DOI: 10.1021/acs.jmedchem.9b01877 BindingDB Entry DOI: 10.7270/Q2NG4V6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM207586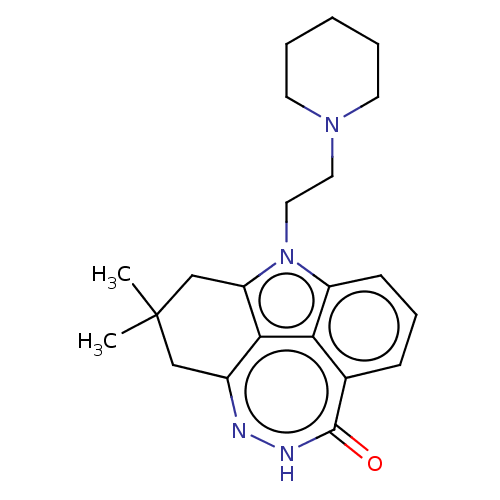 (US10501467, Example 31 | US9260440, 31 | US9617273...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PARP-2 (unknown origin) pre-incubated for 30 mins before addition of activated DNA and NAD by chemiluminescent assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01346 BindingDB Entry DOI: 10.7270/Q2474FGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300149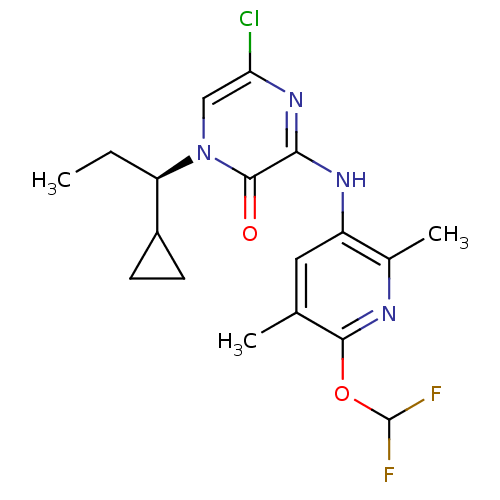 ((R)-5-Chloro-1-(1-cyclopropylpropyl)-3-(6-(difluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM50300145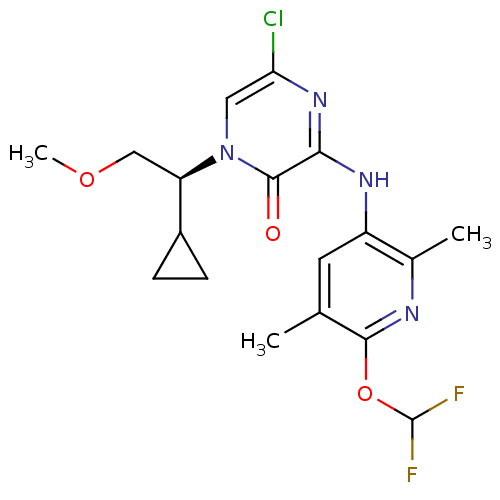 ((S)-5-Chloro-1-(cyclopropyl-2-methoxyethyl)-3-[6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor in rat frontal cortex homogenate by binding titration assay | J Med Chem 52: 7653-68 (2009) Article DOI: 10.1021/jm900716v BindingDB Entry DOI: 10.7270/Q2CN740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 896 total ) | Next | Last >> |