

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110268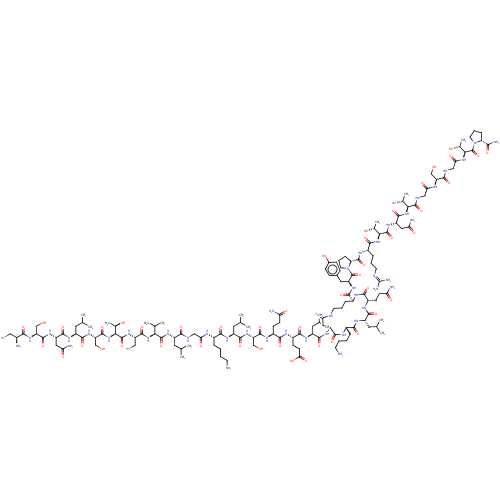 (CHEMBL2369895 | CSNLSTCVLGKLSQELc[DKLQK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50024170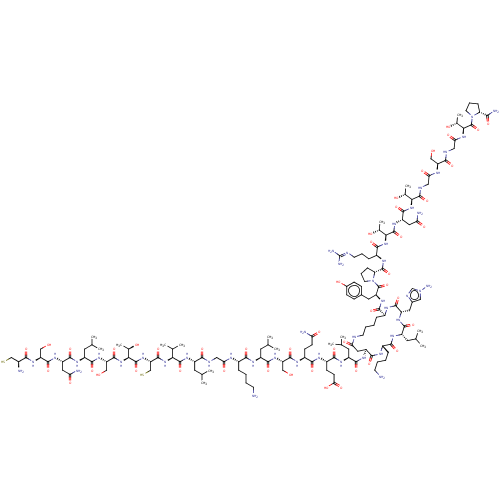 (CHEMBL2369912) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110272 (CHEMBL2369907 | CSNLSTCVLGKLSQELc[DKLHK]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527524 (CHEMBL4566742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110265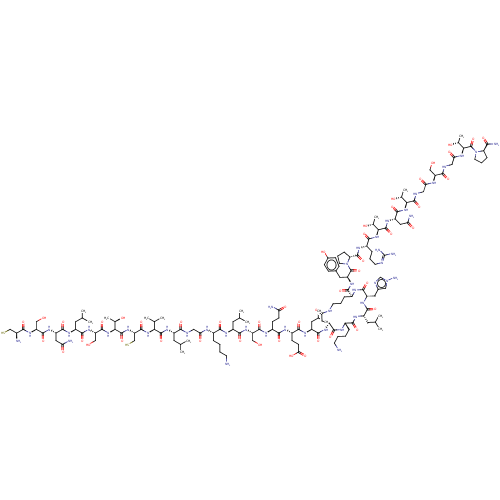 (CHEMBL2369886 | CSNLSTCVLGKLSQELc[DKLHO]YPRTNTGSGT...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110275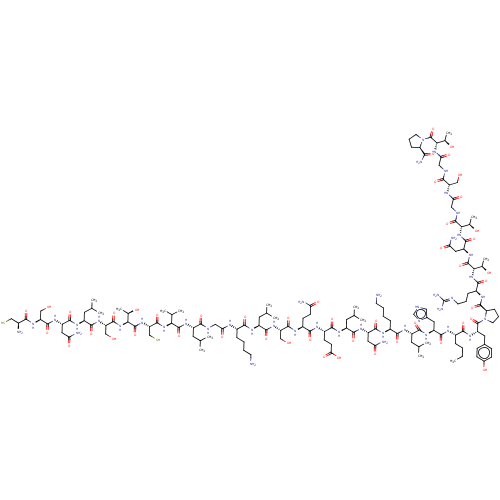 (CGNLSTCBLGTYTQDF[DKFHO]YPQTAIGVGAP-amide | CHEMBL2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527552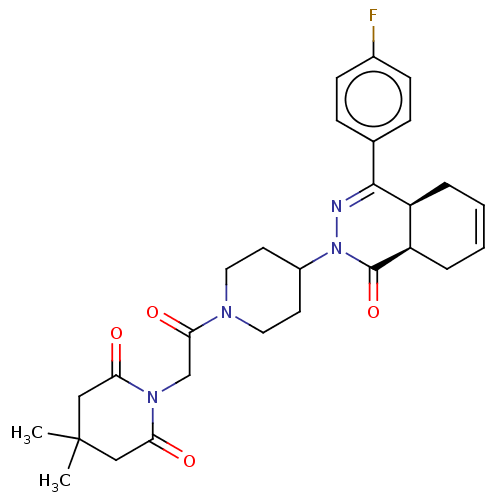 (CHEMBL4435111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527532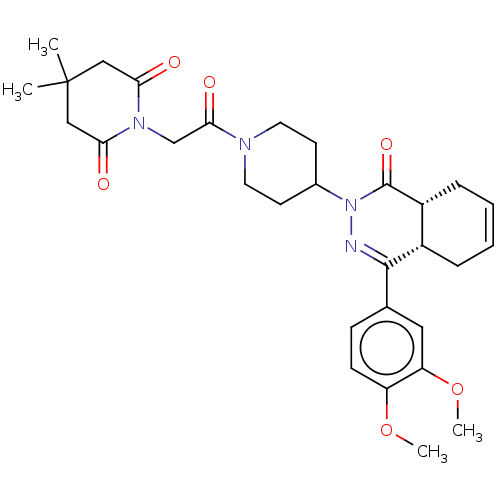 (CHEMBL4441748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527537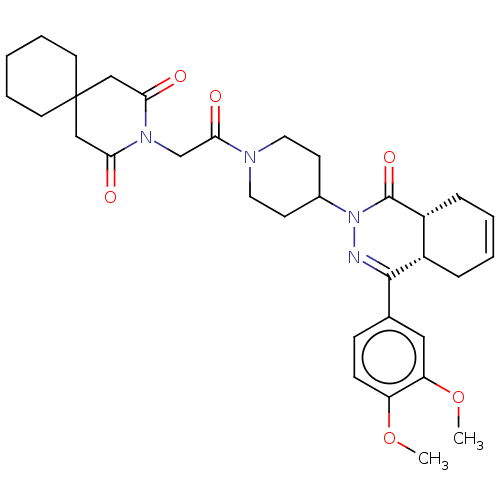 (CHEMBL4453005) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527531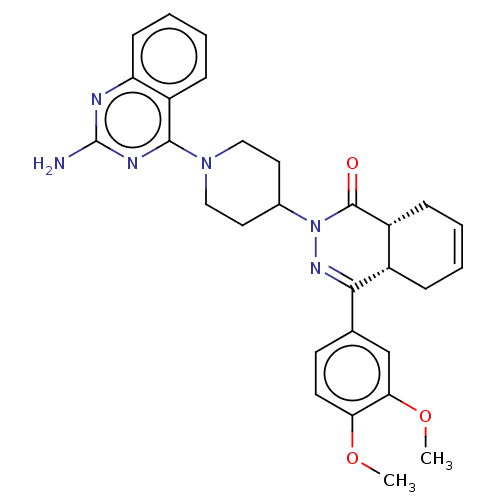 (CHEMBL4524402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50115642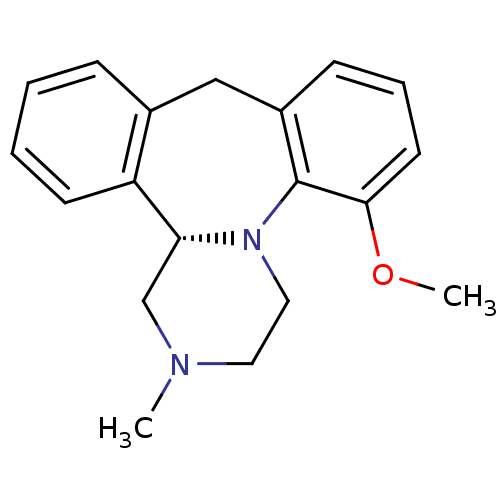 ((+)-65-Methoxy-2-methyl-1,2,3,4,9,13b-hexahydro-2,...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy Curated by ChEMBL | Assay Description The binding affinity of the compound at 5-hydroxytryptamine 2C receptor was determined using [3H]-mesulergine | J Med Chem 45: 3280-5 (2002) BindingDB Entry DOI: 10.7270/Q28051ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527528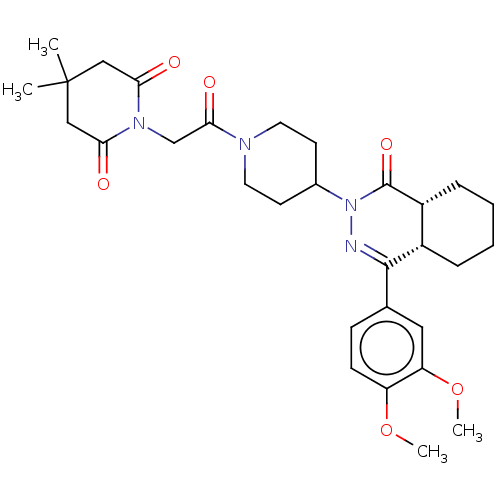 (CHEMBL4441871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527550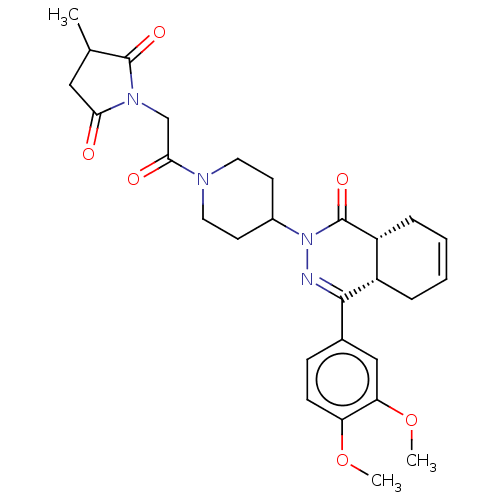 (CHEMBL4461456) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50068071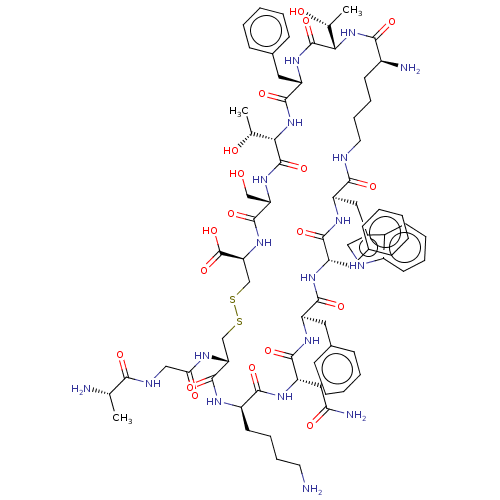 (CHEMBL2370908 | SRIF) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University Curated by ChEMBL | Assay Description In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand | J Med Chem 41: 4693-705 (1998) Article DOI: 10.1021/jm980118e BindingDB Entry DOI: 10.7270/Q2TT4RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50115643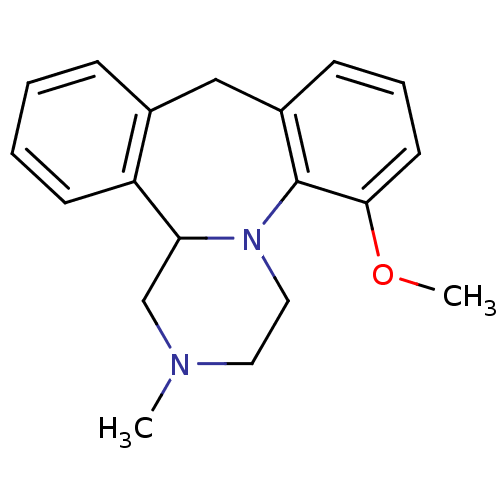 ((+/-)-6 5-Methoxy-2-methyl-1,2,3,4,9,13b-hexahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy Curated by ChEMBL | Assay Description The binding affinity of the compound at 5-hydroxytryptamine 2C receptor was determined using [3H]-mesulergine | J Med Chem 45: 3280-5 (2002) BindingDB Entry DOI: 10.7270/Q28051ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527535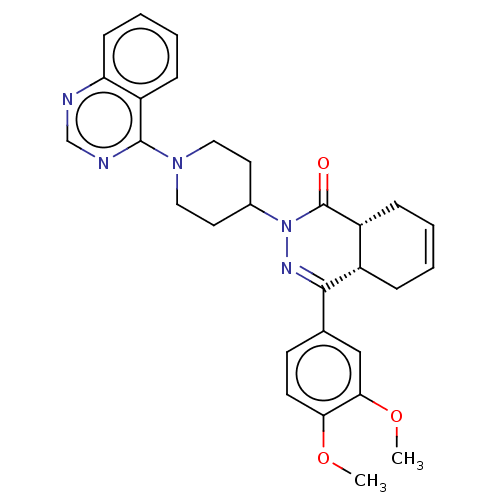 (CHEMBL4589927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527533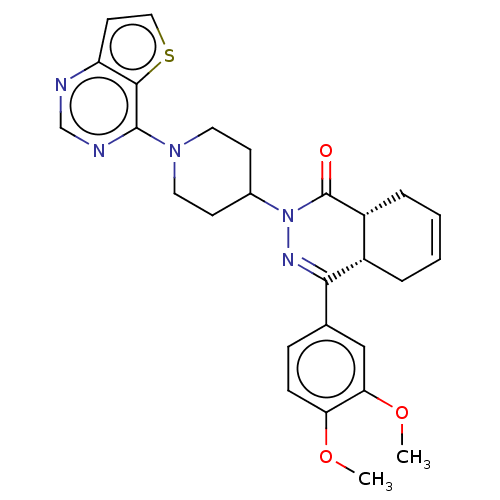 (CHEMBL4592553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631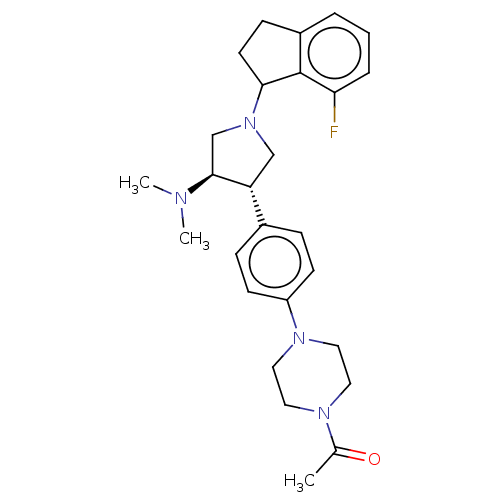 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235630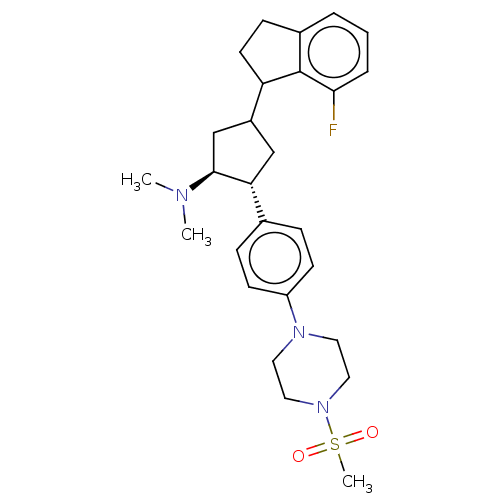 (CHEMBL4093096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50068063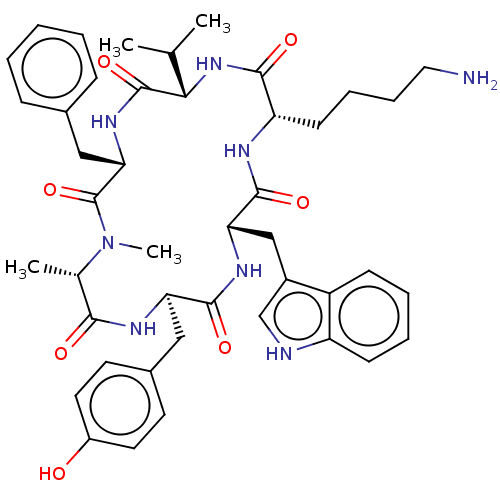 ((12S,15R,18S)-9-(4-Amino-butyl)-3-benzyl-15-(4-hyd...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University Curated by ChEMBL | Assay Description In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand | J Med Chem 41: 4693-705 (1998) Article DOI: 10.1021/jm980118e BindingDB Entry DOI: 10.7270/Q2TT4RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50272772 (10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeast Louisiana University Curated by ChEMBL | Assay Description In vitro binding affinity at somatostatin 2 receptor in transfected HEK 293 cell using [125 I]Tyr11-SRIF-14 as radioligand | J Med Chem 41: 4693-705 (1998) Article DOI: 10.1021/jm980118e BindingDB Entry DOI: 10.7270/Q2TT4RNG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527555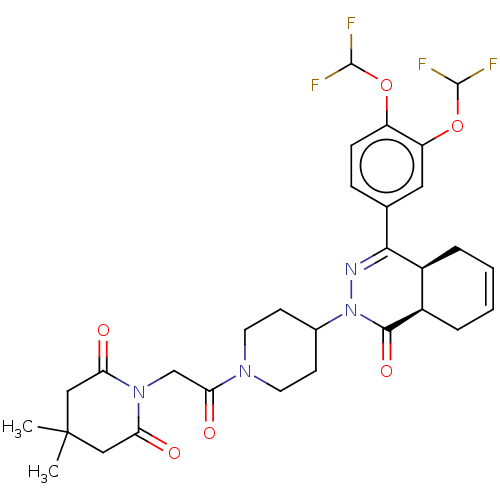 (CHEMBL4530777) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527560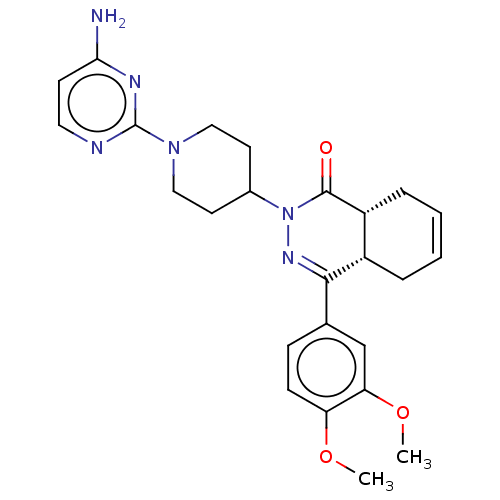 (CHEMBL4444578) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527567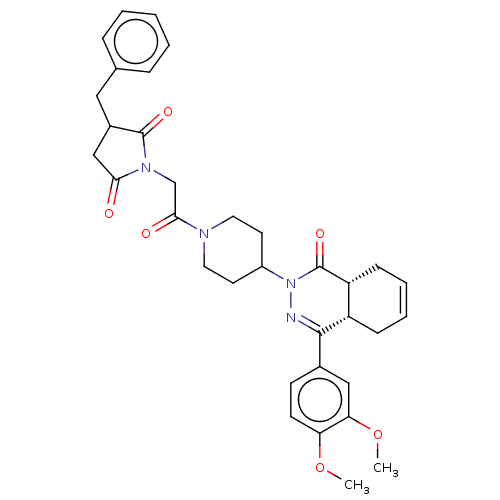 (CHEMBL4473426) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527554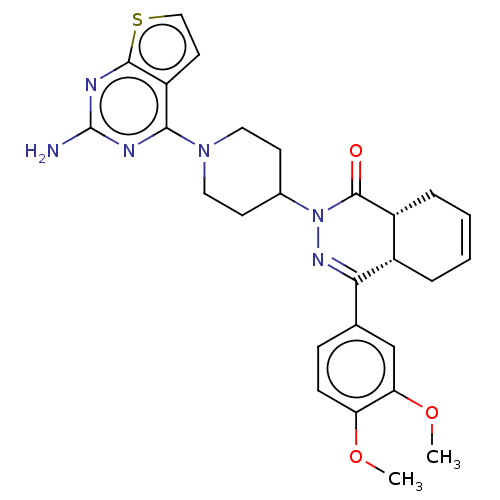 (CHEMBL4460623) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin D2 receptor 2 (Mus musculus (mouse)) | BDBM50356677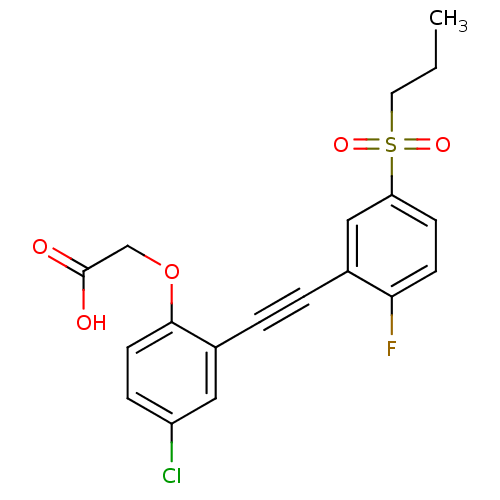 (CHEMBL1917592) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono S.A. Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from mouse CRTH2 expressed in human HEK cells by liquid scintillation counting | J Med Chem 54: 7299-317 (2011) Article DOI: 10.1021/jm200866y BindingDB Entry DOI: 10.7270/Q2N58MS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527559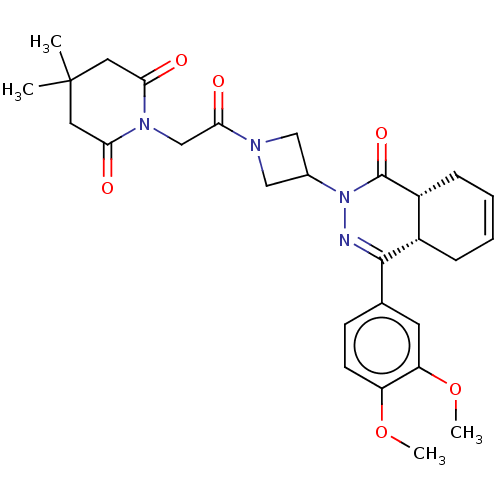 (CHEMBL4591859) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50115640 ((-)-6 5-Methoxy-2-methyl-1,2,3,4,9,13b-hexahydro-2...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy Curated by ChEMBL | Assay Description The binding affinity of the compound at 5-hydroxytryptamine 2C receptor was determined using [3H]-mesulergine | J Med Chem 45: 3280-5 (2002) BindingDB Entry DOI: 10.7270/Q28051ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235643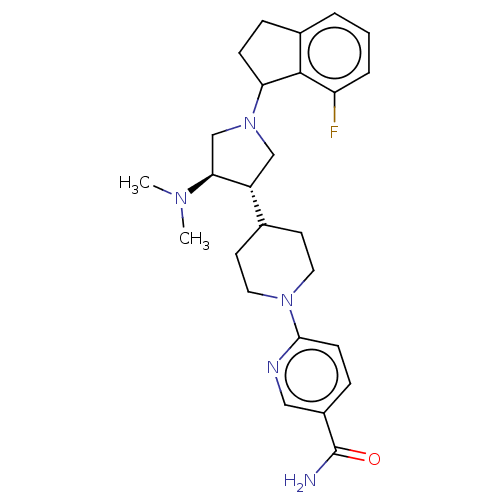 (CHEMBL4076017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. | J Med Chem 35: 4911-3 (1993) BindingDB Entry DOI: 10.7270/Q2GF0SG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. | J Med Chem 35: 4911-3 (1993) BindingDB Entry DOI: 10.7270/Q2GF0SG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096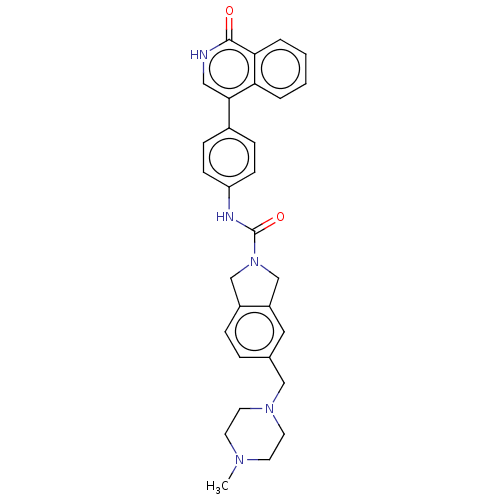 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50597579 (CHEMBL5197201) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00044f BindingDB Entry DOI: 10.7270/Q21C21X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50461413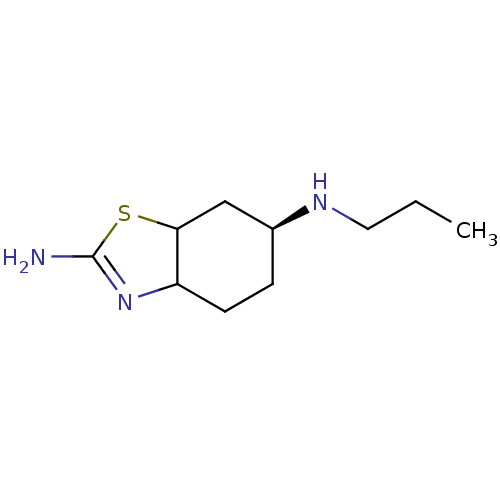 (CHEMBL1201343) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Illinois University Edwardsville Curated by ChEMBL | Assay Description Binding affinity to human dopamine D3 receptor | Bioorg Med Chem Lett 28: 1897-1902 (2018) Article DOI: 10.1016/j.bmcl.2018.03.084 BindingDB Entry DOI: 10.7270/Q2F47RS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50597587 (CHEMBL5174969) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00044f BindingDB Entry DOI: 10.7270/Q21C21X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50597581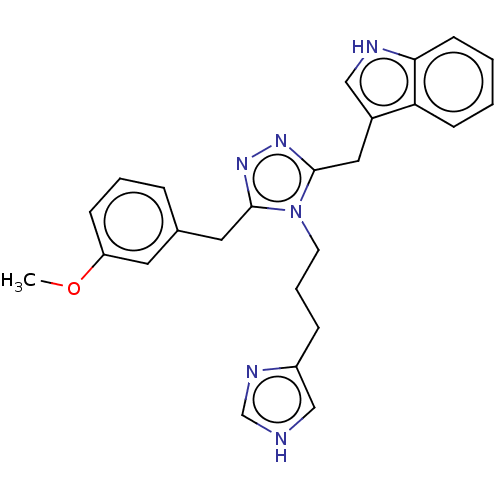 (CHEMBL5183772) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00044f BindingDB Entry DOI: 10.7270/Q21C21X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527525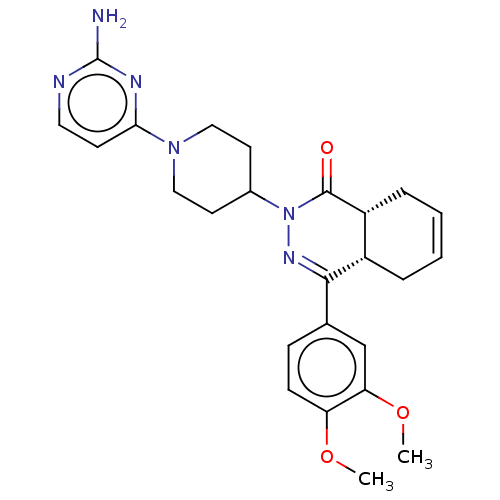 (CHEMBL4522249) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50115642 ((+)-65-Methoxy-2-methyl-1,2,3,4,9,13b-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy Curated by ChEMBL | Assay Description The binding affinity of the compound at 5-hydroxytryptamine 2A receptor was determined using [3H]-ketanserin | J Med Chem 45: 3280-5 (2002) BindingDB Entry DOI: 10.7270/Q28051ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 1 of Calcitonin receptor (1) (Homo sapiens (Human)) | BDBM50110273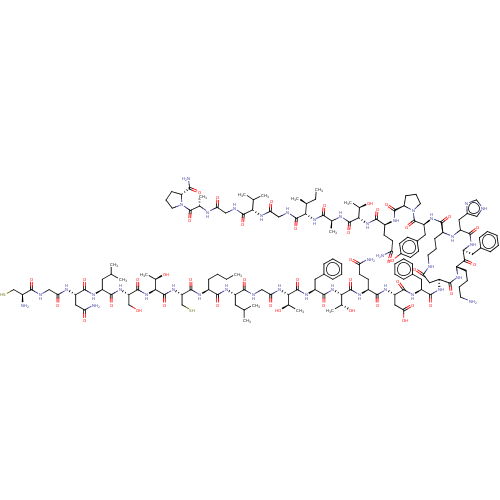 (CGNLSTCMLGTYTQDFc[DKFHK]FPQTAIGVGAP-amide | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rutgers University Curated by ChEMBL | Assay Description Inhibition of [125I]-salmon calcitonin (sCT) binding to human calcitonin receptor I1 expressed in HEK293 cells | J Med Chem 45: 1108-21 (2002) BindingDB Entry DOI: 10.7270/Q2N015V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527529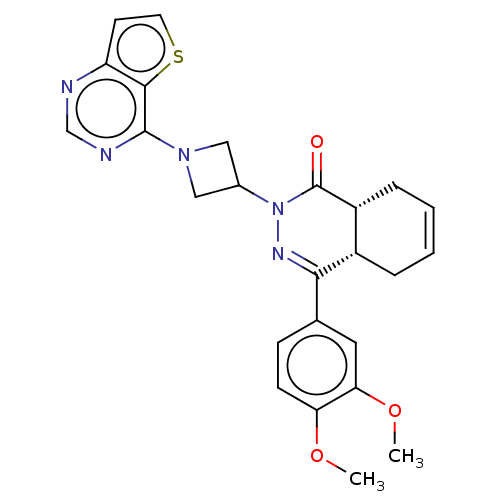 (CHEMBL4454600) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50099628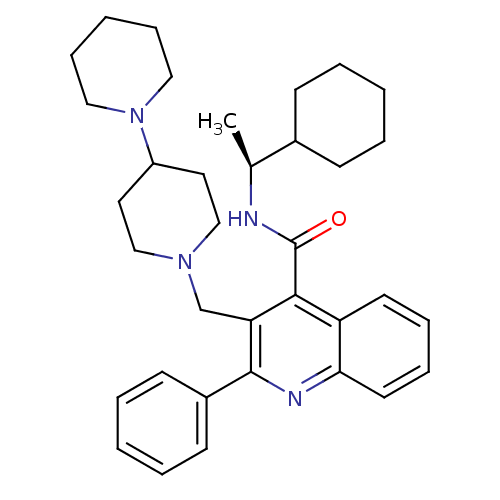 (3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes | J Med Chem 44: 1675-89 (2001) BindingDB Entry DOI: 10.7270/Q29C6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50040237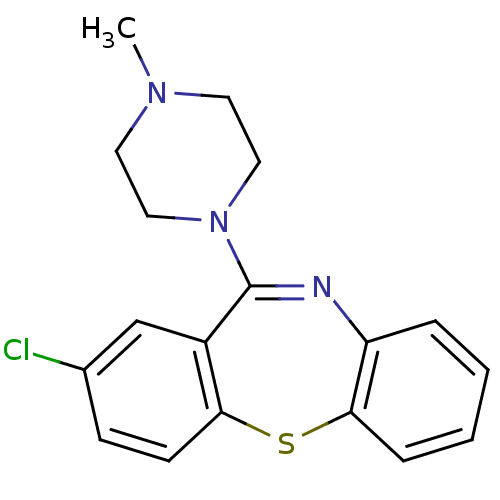 (2-Chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liège Curated by ChEMBL | Assay Description In vitro binding affinity against rat 5-hydroxytryptamine 2 receptor. | J Med Chem 36: 2107-14 (1993) BindingDB Entry DOI: 10.7270/Q22B8X37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50597590 (CHEMBL5185547) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00044f BindingDB Entry DOI: 10.7270/Q21C21X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50427452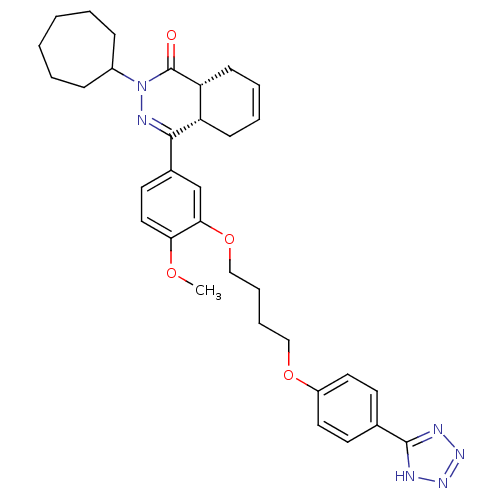 (CHEMBL2326941) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE4B1 expressed in Sf21 insect cells using cAMP as substrate by scintillation proximity assay | J Med Chem 61: 3870-3888 (2018) Article DOI: 10.1021/acs.jmedchem.7b01670 BindingDB Entry DOI: 10.7270/Q2P84FF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM523801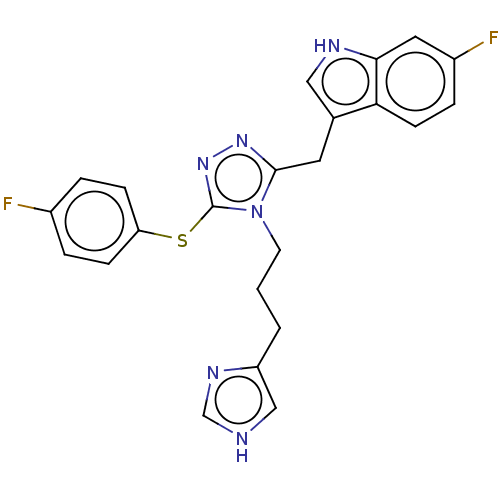 (US11136312, Compound SK-I-128) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.632 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive radioligand binding experiments were performed using MEMBRANE TARGET Systems (Perkin-Elmer, Boston, Mass.) for human somatostatin recepto... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM523803 (US11136312, Compound SK-I-132) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive radioligand binding experiments were performed using MEMBRANE TARGET Systems (Perkin-Elmer, Boston, Mass.) for human somatostatin recepto... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DB851B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235658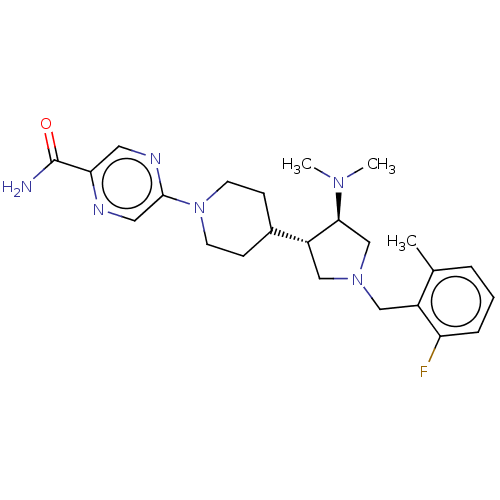 (CHEMBL4073166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. | J Med Chem 35: 4911-3 (1993) BindingDB Entry DOI: 10.7270/Q2GF0SG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000040 (((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the Tachykinin receptor 1 in human IM-9 cell using [125I]-BH-SP of the compound. | J Med Chem 35: 4911-3 (1993) BindingDB Entry DOI: 10.7270/Q2GF0SG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11925 total ) | Next | Last >> |