 Found 537 hits with Last Name = 'jacobs' and Initial = 'c'
Found 537 hits with Last Name = 'jacobs' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097624
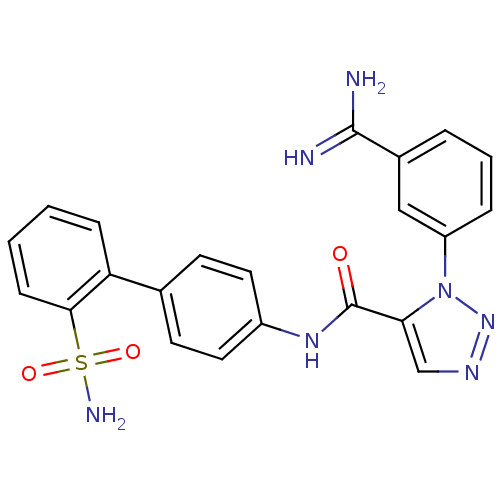
(3-(3-Carbamimidoyl-phenyl)-3H-[1,2,3]triazole-4-ca...)Show SMILES NC(=N)c1cccc(c1)-n1nncc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H19N7O3S/c23-21(24)15-4-3-5-17(12-15)29-19(13-26-28-29)22(30)27-16-10-8-14(9-11-16)18-6-1-2-7-20(18)33(25,31)32/h1-13H,(H3,23,24)(H,27,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626
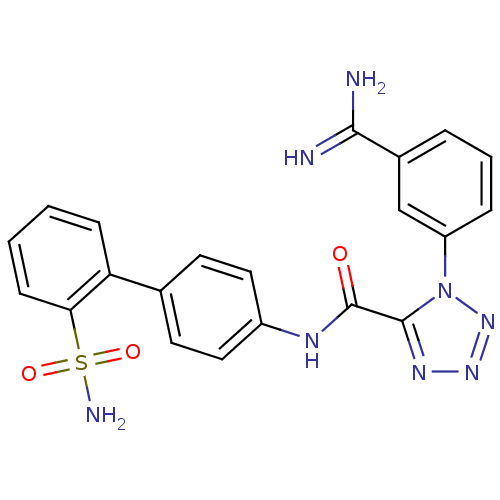
(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50097625
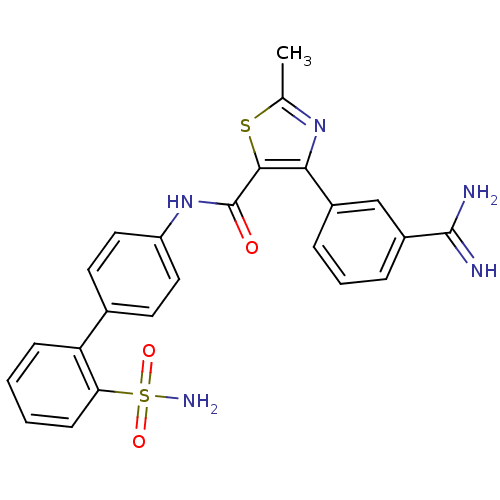
(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097625

(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12659
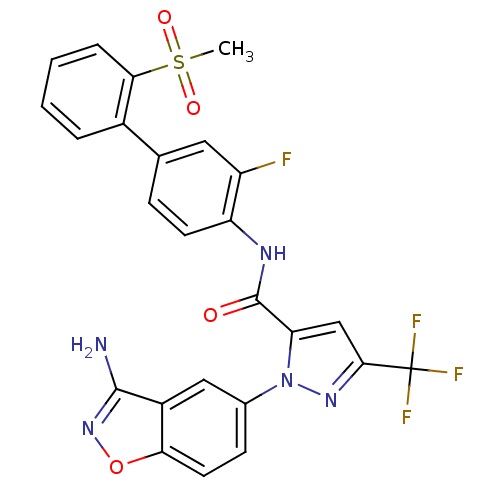
(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H17F4N5O4S/c1-39(36,37)21-5-3-2-4-15(21)13-6-8-18(17(26)10-13)31-24(35)19-12-22(25(27,28)29)32-34(19)14-7-9-20-16(11-14)23(30)33-38-20/h2-12H,1H3,(H2,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50087533
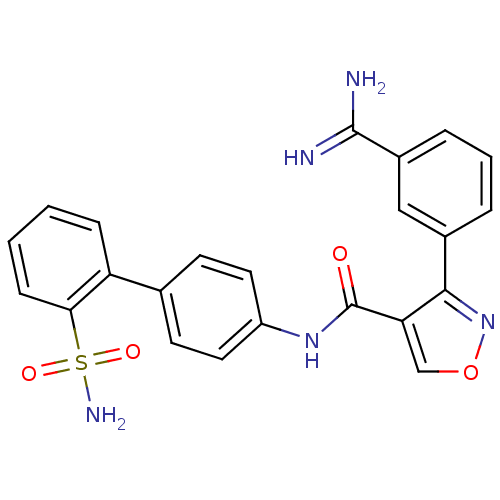
(3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...)Show SMILES NC(=N)c1cccc(c1)-c1nocc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H19N5O4S/c24-22(25)16-5-3-4-15(12-16)21-19(13-32-28-21)23(29)27-17-10-8-14(9-11-17)18-6-1-2-7-20(18)33(26,30)31/h1-13H,(H3,24,25)(H,27,29)(H2,26,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096095
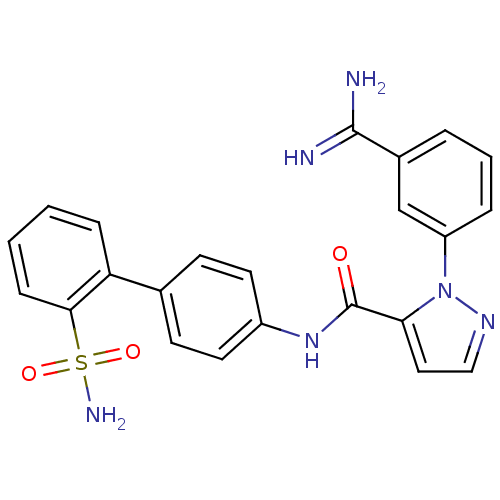
(2-(3-Carbamimidoyl-phenyl)-2H-pyrazole-3-carboxyli...)Show SMILES NC(=N)c1cccc(c1)-n1nccc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H20N6O3S/c24-22(25)16-4-3-5-18(14-16)29-20(12-13-27-29)23(30)28-17-10-8-15(9-11-17)19-6-1-2-7-21(19)33(26,31)32/h1-14H,(H3,24,25)(H,28,30)(H2,26,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224712

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-oxopyridi...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)-n2ccccc2=O)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C25H19N7O4/c26-23-17-12-16(5-7-21(17)36-29-23)32-20(13-18(28-32)24(27)34)25(35)31-10-8-14-11-15(4-6-19(14)31)30-9-2-1-3-22(30)33/h1-7,9,11-13H,8,10H2,(H2,26,29)(H2,27,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097620
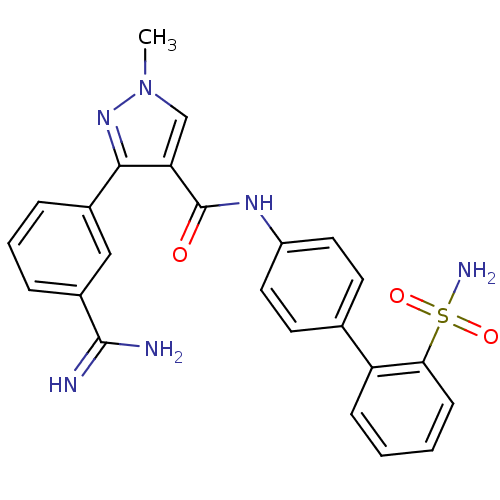
(3-(3-Carbamimidoyl-phenyl)-1-methyl-1H-pyrazole-4-...)Show SMILES Cn1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)c(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-30-14-20(22(29-30)16-5-4-6-17(13-16)23(25)26)24(31)28-18-11-9-15(10-12-18)19-7-2-3-8-21(19)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097619
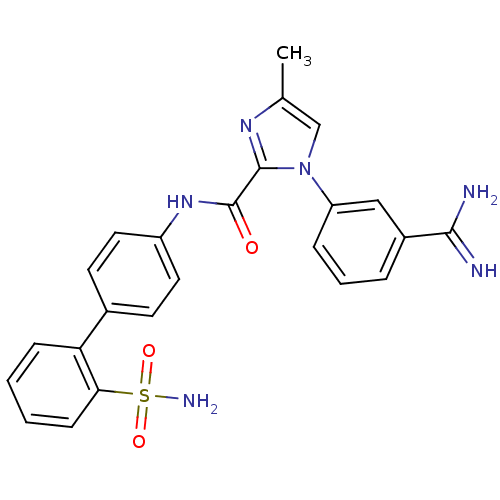
(1-(3-Carbamimidoyl-phenyl)-4-methyl-1H-imidazole-2...)Show SMILES Cc1cn(c(n1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-14-30(19-6-4-5-17(13-19)22(25)26)23(28-15)24(31)29-18-11-9-16(10-12-18)20-7-2-3-8-21(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,29,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097621
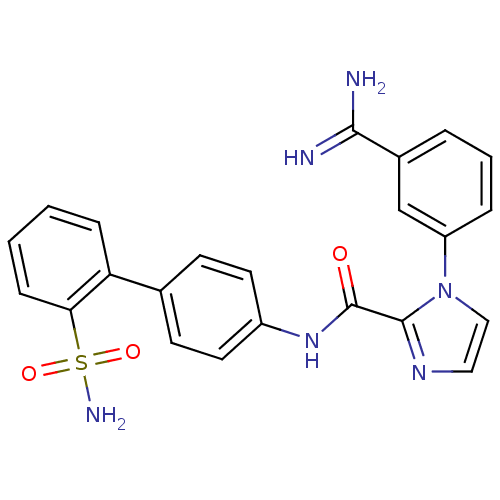
(1-(3-Carbamimidoyl-phenyl)-1H-imidazole-2-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1ccnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H20N6O3S/c24-21(25)16-4-3-5-18(14-16)29-13-12-27-22(29)23(30)28-17-10-8-15(9-11-17)19-6-1-2-7-20(19)33(26,31)32/h1-14H,(H3,24,25)(H,28,30)(H2,26,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097632
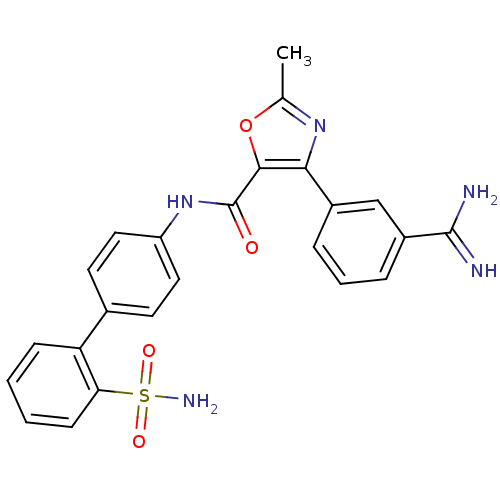
(4-(3-Carbamimidoyl-phenyl)-2-methyl-oxazole-5-carb...)Show SMILES Cc1nc(c(o1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O4S/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50087533

(3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...)Show SMILES NC(=N)c1cccc(c1)-c1nocc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H19N5O4S/c24-22(25)16-5-3-4-15(12-16)21-19(13-32-28-21)23(29)27-17-10-8-14(9-11-17)18-6-1-2-7-20(18)33(26,30)31/h1-13H,(H3,24,25)(H,27,29)(H2,26,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-8 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50069218
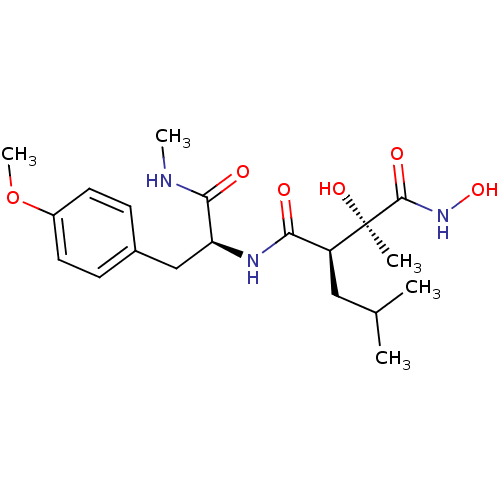
((2R,3R)-2,N*1*-Dihydroxy-3-isobutyl-N*4*-[(S)-2-(4...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@@](C)(O)C(=O)NO Show InChI InChI=1S/C20H31N3O6/c1-12(2)10-15(20(3,27)19(26)23-28)17(24)22-16(18(25)21-4)11-13-6-8-14(29-5)9-7-13/h6-9,12,15-16,27-28H,10-11H2,1-5H3,(H,21,25)(H,22,24)(H,23,26)/t15-,16-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloproteinase-1 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224704
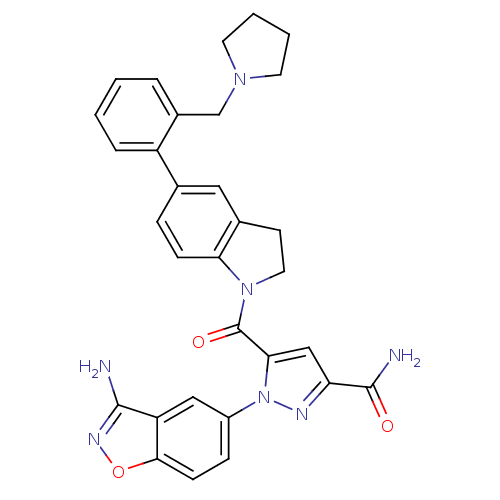
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-(pyrrolid...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)-c2ccccc2CN2CCCC2)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C31H29N7O3/c32-29-24-16-22(8-10-28(24)41-35-29)38-27(17-25(34-38)30(33)39)31(40)37-14-11-20-15-19(7-9-26(20)37)23-6-2-1-5-21(23)18-36-12-3-4-13-36/h1-2,5-10,15-17H,3-4,11-14,18H2,(H2,32,35)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096084
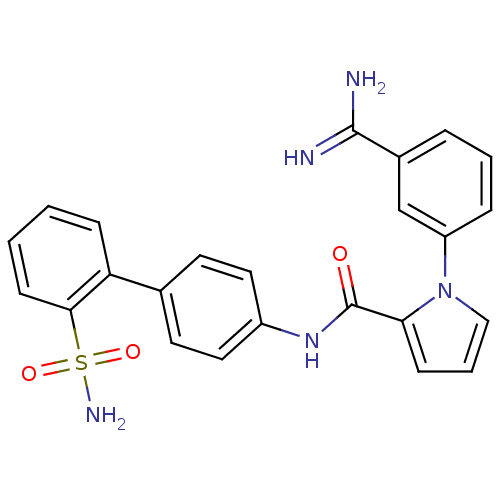
(1-(3-Carbamimidoyl-phenyl)-1H-pyrrole-2-carboxylic...)Show SMILES NC(=N)c1cccc(c1)-n1cccc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C24H21N5O3S/c25-23(26)17-5-3-6-19(15-17)29-14-4-8-21(29)24(30)28-18-12-10-16(11-13-18)20-7-1-2-9-22(20)33(27,31)32/h1-15H,(H3,25,26)(H,28,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-7 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097631

(4-(3-Carbamimidoyl-phenyl)-thiazole-5-carboxylic a...)Show SMILES NC(=N)c1cccc(c1)-c1ncsc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H19N5O3S2/c24-22(25)16-5-3-4-15(12-16)20-21(32-13-27-20)23(29)28-17-10-8-14(9-11-17)18-6-1-2-7-19(18)33(26,30)31/h1-13H,(H3,24,25)(H,28,29)(H2,26,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224711

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-(methylsu...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C27H22N6O5S/c1-39(36,37)24-5-3-2-4-18(24)15-6-8-21-16(12-15)10-11-32(21)27(35)22-14-20(26(29)34)30-33(22)17-7-9-23-19(13-17)25(28)31-38-23/h2-9,12-14H,10-11H2,1H3,(H2,28,31)(H2,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069214

((2R,3R)-N*4*-((S)-5-Amino-1-methylcarbamoyl-pentyl...)Show SMILES CNC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCc1ccccc1)[C@@](C)(O)C(=O)NO Show InChI InChI=1S/C21H34N4O5/c1-21(29,20(28)25-30)16(12-8-11-15-9-4-3-5-10-15)18(26)24-17(19(27)23-2)13-6-7-14-22/h3-5,9-10,16-17,29-30H,6-8,11-14,22H2,1-2H3,(H,23,27)(H,24,26)(H,25,28)/t16-,17-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloprotease-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076993

((2S,11S,12R)-12-Isobutyl-13-oxo-1,7diaza-cyclotrid...)Show SMILES CNC(=O)[C@@H]1CCCCNCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C18H34N4O4/c1-12(2)11-14-13(17(24)22-26)7-6-10-20-9-5-4-8-15(18(25)19-3)21-16(14)23/h12-15,20,26H,4-11H2,1-3H3,(H,19,25)(H,21,23)(H,22,24)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102631
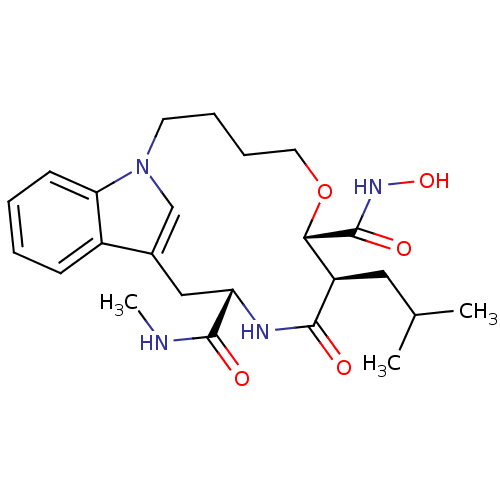
(8-Isobutyl-9-oxo-6-oxa-1,10-diaza-tricyclo[11.6.1....)Show SMILES CNC(=O)[C@@H]1Cc2cn(CCCCO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)c1ccccc21 Show InChI InChI=1S/C24H34N4O5/c1-15(2)12-18-21(24(31)27-32)33-11-7-6-10-28-14-16(17-8-4-5-9-20(17)28)13-19(23(30)25-3)26-22(18)29/h4-5,8-9,14-15,18-19,21,32H,6-7,10-13H2,1-3H3,(H,25,30)(H,26,29)(H,27,31)/t18-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50076991
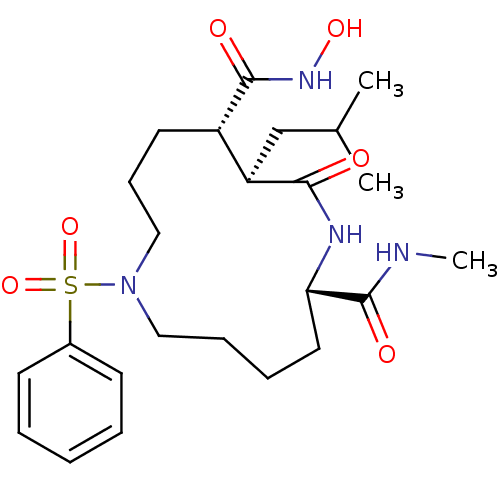
((2S,11S,12R)-7-Benzenesulfonyl-12-isobutyl-13-oxo-...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H38N4O6S/c1-17(2)16-20-19(23(30)27-32)12-9-15-28(35(33,34)18-10-5-4-6-11-18)14-8-7-13-21(24(31)25-3)26-22(20)29/h4-6,10-11,17,19-21,32H,7-9,12-16H2,1-3H3,(H,25,31)(H,26,29)(H,27,30)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50064340
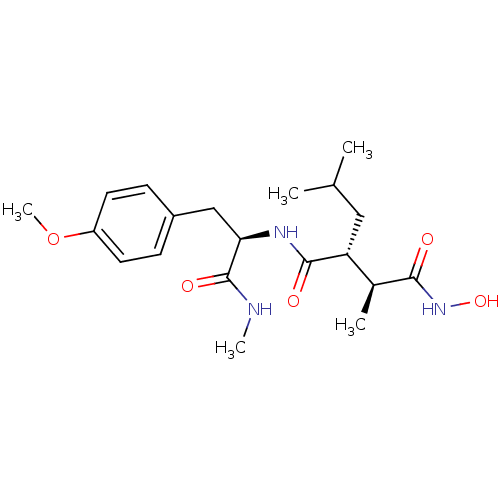
((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50064340

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-[(R)-2-(4-met...)Show SMILES CNC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C20H31N3O5/c1-12(2)10-16(13(3)18(24)23-27)19(25)22-17(20(26)21-4)11-14-6-8-15(28-5)9-7-14/h6-9,12-13,16-17,27H,10-11H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102600

(12-Isobutyl-4-methyl-3,11-dioxo-1-oxa-4,10-diaza-c...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)CO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C18H32N4O6/c1-11(2)9-12-15(18(26)21-27)28-10-14(23)22(4)8-6-5-7-13(17(25)19-3)20-16(12)24/h11-13,15,27H,5-10H2,1-4H3,(H,19,25)(H,20,24)(H,21,26)/t12-,13+,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
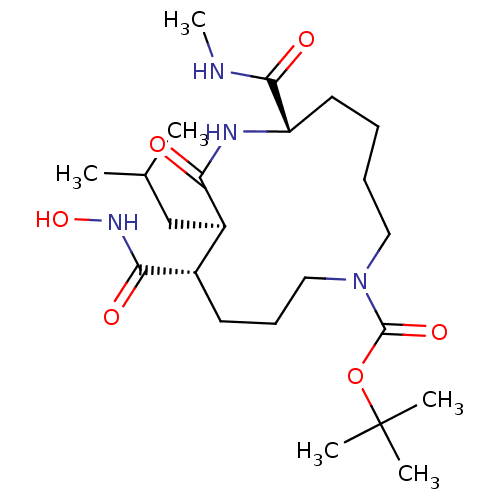
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50076995
((6S,9R,10S)-10-Hydroxycarbamoyl-9-isobutyl-6-methy...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H42N4O6/c1-15(2)14-17-16(20(29)26-32)10-9-13-27(22(31)33-23(3,4)5)12-8-7-11-18(21(30)24-6)25-19(17)28/h15-18,32H,7-14H2,1-6H3,(H,24,30)(H,25,28)(H,26,29)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076991

((2S,11S,12R)-7-Benzenesulfonyl-12-isobutyl-13-oxo-...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H38N4O6S/c1-17(2)16-20-19(23(30)27-32)12-9-15-28(35(33,34)18-10-5-4-6-11-18)14-8-7-13-21(24(31)25-3)26-22(20)29/h4-6,10-11,17,19-21,32H,7-9,12-16H2,1-3H3,(H,25,31)(H,26,29)(H,27,30)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
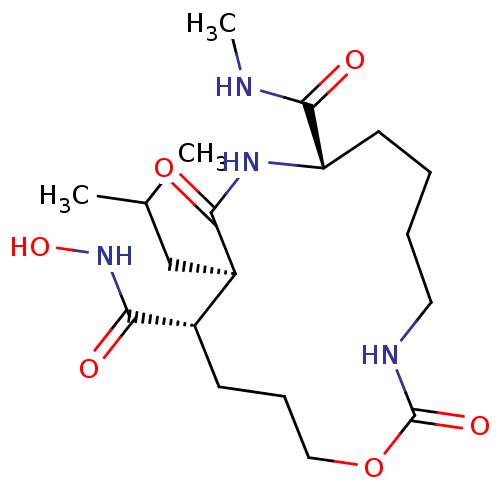
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102604
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CNC(=O)[C@@H]1CCCCNC(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C19H34N4O6/c1-12(2)11-14-13(17(25)23-28)7-6-10-29-19(27)21-9-5-4-8-15(18(26)20-3)22-16(14)24/h12-15,28H,4-11H2,1-3H3,(H,20,26)(H,21,27)(H,22,24)(H,23,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
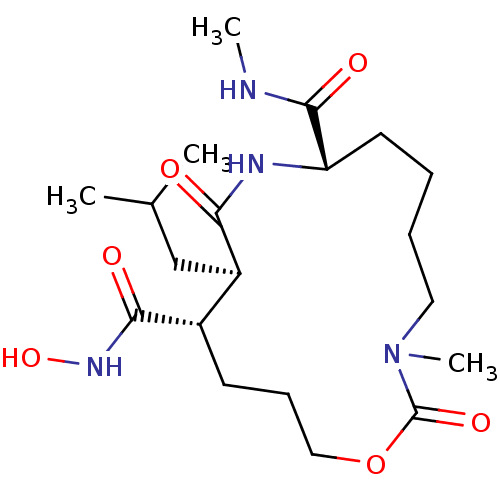
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50102630
(11-Isobutyl-3-methyl-2,10-dioxo-1-oxa-3,9-diaza-cy...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H36N4O6/c1-13(2)12-15-14(18(26)23-29)8-7-11-30-20(28)24(4)10-6-5-9-16(19(27)21-3)22-17(15)25/h13-16,29H,5-12H2,1-4H3,(H,21,27)(H,22,25)(H,23,26)/t14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-9. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102630

(11-Isobutyl-3-methyl-2,10-dioxo-1-oxa-3,9-diaza-cy...)Show SMILES CNC(=O)[C@@H]1CCCCN(C)C(=O)OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H36N4O6/c1-13(2)12-15-14(18(26)23-29)8-7-11-30-20(28)24(4)10-6-5-9-16(19(27)21-3)22-17(15)25/h13-16,29H,5-12H2,1-4H3,(H,21,27)(H,22,25)(H,23,26)/t14-,15+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069218

((2R,3R)-2,N*1*-Dihydroxy-3-isobutyl-N*4*-[(S)-2-(4...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)[C@@](C)(O)C(=O)NO Show InChI InChI=1S/C20H31N3O6/c1-12(2)10-15(20(3,27)19(26)23-28)17(24)22-16(18(25)21-4)11-13-6-8-14(29-5)9-7-13/h6-9,12,15-16,27-28H,10-11H2,1-5H3,(H,21,25)(H,22,24)(H,23,26)/t15-,16-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloprotease-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
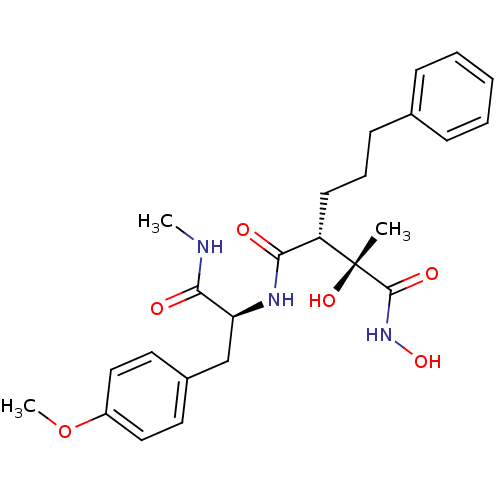
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069228
((2R,3R)-2,N*1*-Dihydroxy-N*4*-[(S)-2-(4-methoxy-ph...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CCCc1ccccc1)[C@@](C)(O)C(=O)NO Show InChI InChI=1S/C25H33N3O6/c1-25(32,24(31)28-33)20(11-7-10-17-8-5-4-6-9-17)22(29)27-21(23(30)26-2)16-18-12-14-19(34-3)15-13-18/h4-6,8-9,12-15,20-21,32-33H,7,10-11,16H2,1-3H3,(H,26,30)(H,27,29)(H,28,31)/t20-,21-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloprotease-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069221

((2R,3R)-N*4*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccccc1)[C@@](C)(O)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C21H33N3O5/c1-20(2,3)16(18(26)22-5)23-17(25)15(21(4,28)19(27)24-29)13-9-12-14-10-7-6-8-11-14/h6-8,10-11,15-16,28-29H,9,12-13H2,1-5H3,(H,22,26)(H,23,25)(H,24,27)/t15-,16+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloprotease-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097627

(2-(3-Carbamimidoyl-phenyl)-N-(2'-sulfamoyl-bipheny...)Show SMILES NC(=N)c1cccc(c1)-c1ncccc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H21N5O3S/c26-24(27)18-6-3-5-17(15-18)23-21(8-4-14-29-23)25(31)30-19-12-10-16(11-13-19)20-7-1-2-9-22(20)34(28,32)33/h1-15H,(H3,26,27)(H,30,31)(H2,28,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224697
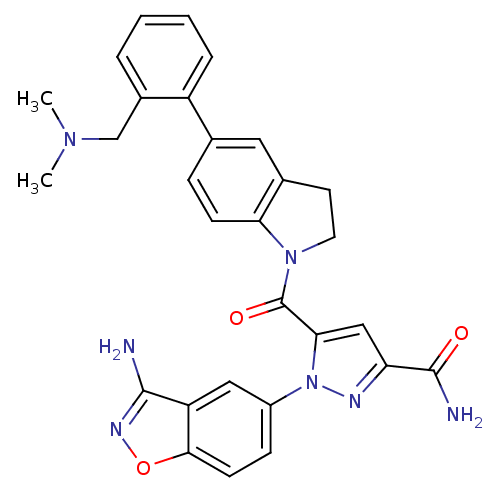
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-((dimethy...)Show SMILES CN(C)Cc1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C29H27N7O3/c1-34(2)16-19-5-3-4-6-21(19)17-7-9-24-18(13-17)11-12-35(24)29(38)25-15-23(28(31)37)32-36(25)20-8-10-26-22(14-20)27(30)33-39-26/h3-10,13-15H,11-12,16H2,1-2H3,(H2,30,33)(H2,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069224
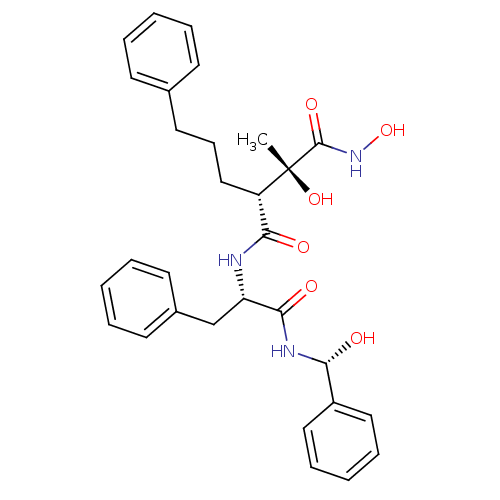
((2R,3R)-2,N*1*-Dihydroxy-N*4*-{(S)-1-[((R)-hydroxy...)Show SMILES C[C@@](O)([C@@H](CCCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C30H35N3O6/c1-30(38,29(37)33-39)24(19-11-16-21-12-5-2-6-13-21)27(35)31-25(20-22-14-7-3-8-15-22)28(36)32-26(34)23-17-9-4-10-18-23/h2-10,12-15,17-18,24-26,34,38-39H,11,16,19-20H2,1H3,(H,31,35)(H,32,36)(H,33,37)/t24-,25-,26+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloproteinase-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50069213

((2R,3R)-N*4*-((S)-5-Amino-1-phenethylcarbamoyl-pen...)Show SMILES C[C@@](O)([C@@H](CCCc1ccccc1)C(=O)N[C@@H](CCCCN)C(=O)NCCc1ccccc1)C(=O)NO Show InChI InChI=1S/C28H40N4O5/c1-28(36,27(35)32-37)23(16-10-15-21-11-4-2-5-12-21)25(33)31-24(17-8-9-19-29)26(34)30-20-18-22-13-6-3-7-14-22/h2-7,11-14,23-24,36-37H,8-10,15-20,29H2,1H3,(H,30,34)(H,31,33)(H,32,35)/t23-,24-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against matrix metalloproteinase-9 |
Bioorg Med Chem Lett 8: 837-42 (1999)
BindingDB Entry DOI: 10.7270/Q29022WN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50102610
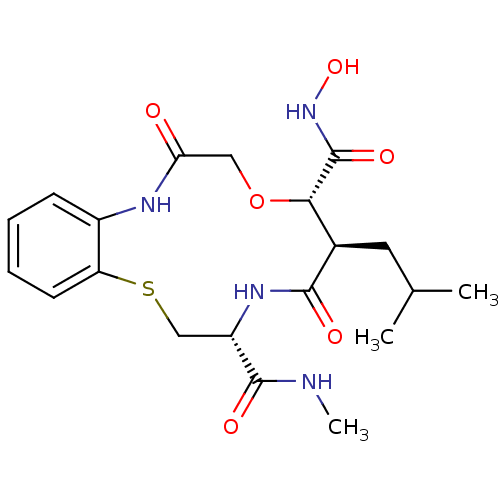
(10-Isobutyl-6,11-dioxo-6,7,9,10,11,12,13,14-octahy...)Show SMILES CNC(=O)[C@@H]1CSc2ccccc2NC(=O)CO[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO Show InChI InChI=1S/C20H28N4O6S/c1-11(2)8-12-17(20(28)24-29)30-9-16(25)22-13-6-4-5-7-15(13)31-10-14(19(27)21-3)23-18(12)26/h4-7,11-12,14,17,29H,8-10H2,1-3H3,(H,21,27)(H,22,25)(H,23,26)(H,24,28)/t12-,14+,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50076995

((6S,9R,10S)-10-Hydroxycarbamoyl-9-isobutyl-6-methy...)Show SMILES CNC(=O)[C@@H]1CCCCN(CCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H42N4O6/c1-15(2)14-17-16(20(29)26-32)10-9-13-27(22(31)33-23(3,4)5)12-8-7-11-18(21(30)24-6)25-19(17)28/h15-18,32H,7-14H2,1-6H3,(H,24,30)(H,25,28)(H,26,29)/t16-,17+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro inhibition of human MMP-1. |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102608
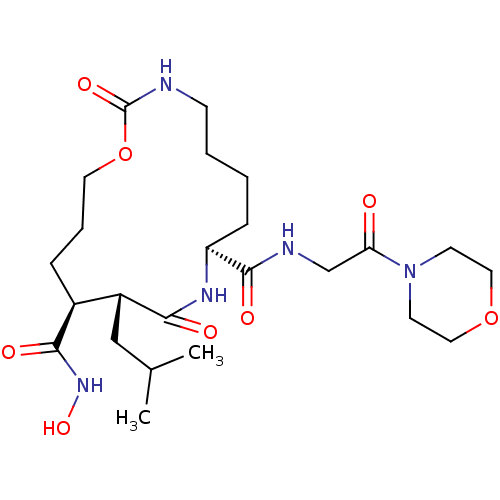
(11-Isobutyl-2,10-dioxo-1-oxa-3,9-diaza-cyclopentad...)Show SMILES CC(C)C[C@@H]1[C@H](CCCOC(=O)NCCCC[C@H](NC1=O)C(=O)NCC(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C24H41N5O8/c1-16(2)14-18-17(22(32)28-35)6-5-11-37-24(34)25-8-4-3-7-19(27-21(18)31)23(33)26-15-20(30)29-9-12-36-13-10-29/h16-19,35H,3-15H2,1-2H3,(H,25,34)(H,26,33)(H,27,31)(H,28,32)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097630
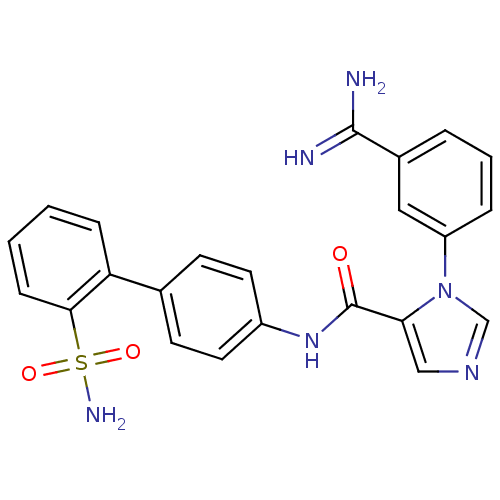
(3-(3-Carbamimidoyl-phenyl)-3H-imidazole-4-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1cncc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C23H20N6O3S/c24-22(25)16-4-3-5-18(12-16)29-14-27-13-20(29)23(30)28-17-10-8-15(9-11-17)19-6-1-2-7-21(19)33(26,31)32/h1-14H,(H3,24,25)(H,28,30)(H2,26,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102594

(7-Isobutyl-8-oxo-2-oxa-9-aza-bicyclo[10.2.2]hexade...)Show SMILES CNC(=O)CNC(=O)[C@@H]1Cc2ccc(OCCC[C@@H]([C@@H](CC(C)C)C(=O)N1)C(=O)NO)cc2 Show InChI InChI=1S/C23H34N4O6/c1-14(2)11-18-17(22(30)27-32)5-4-10-33-16-8-6-15(7-9-16)12-19(26-21(18)29)23(31)25-13-20(28)24-3/h6-9,14,17-19,32H,4-5,10-13H2,1-3H3,(H,24,28)(H,25,31)(H,26,29)(H,27,30)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-2 |
J Med Chem 44: 2636-60 (2001)
BindingDB Entry DOI: 10.7270/Q2FB53NR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data