

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16292 (3-N-[(2S,3S,5R)-3-amino-5-[(4-fluorophenyl)carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 26 | -42.9 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16291 (3-N-[(2S,3S,5R)-3-amino-5-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16286 (3-N-[(1R,3S,4S)-1-{[(1S)-1-(benzylcarbamoyl)-2-met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | -40.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16287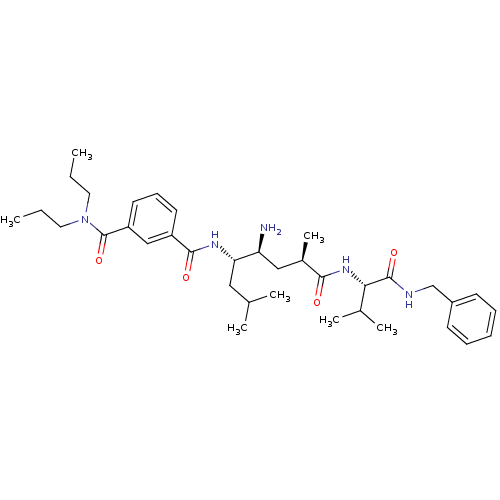 (3-N-[(1R,3S,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16289 (3-N-[(1R,3S,4S)-1-[(4-fluorophenyl)carbamoyl]-3-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16290 (3-N-[(1R,3S,4S)-3-amino-1-[(4-fluorophenyl)carbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [22-454] (Homo sapiens (Human)) | BDBM16288 (3-N-[(1R,3R,4S)-3-amino-1-{[(1S)-1-(benzylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 22 |
Sunesis | Assay Description Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ... | J Med Chem 49: 839-42 (2006) Article DOI: 10.1021/jm0509142 BindingDB Entry DOI: 10.7270/Q2PV6HM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381823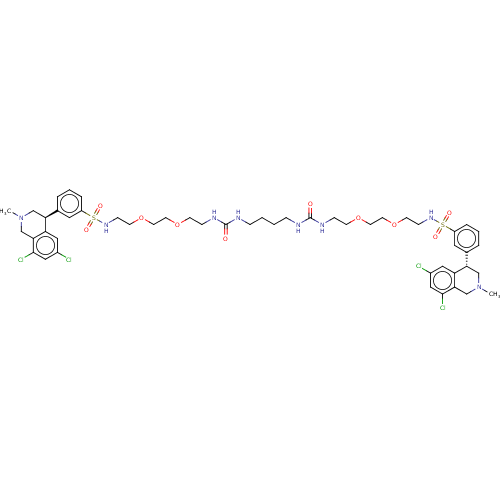 (CVD-0019453 | US10272079, Compound 002 | US1027207...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381804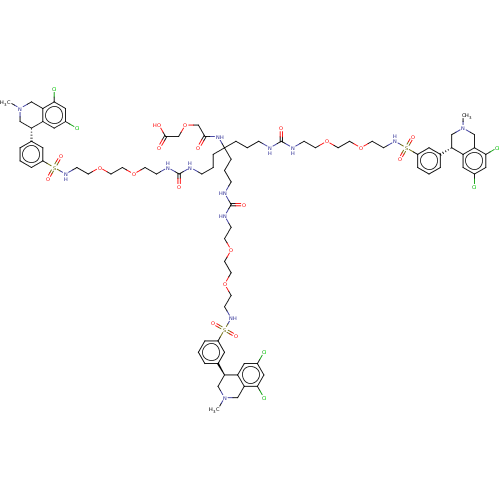 (US10272079, Compound 161) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381783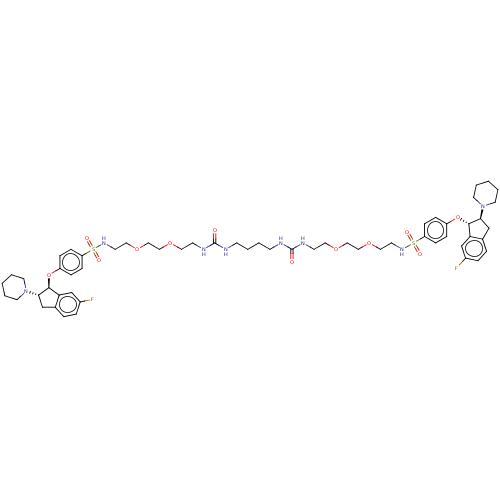 (US10272079, Compound 140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50601173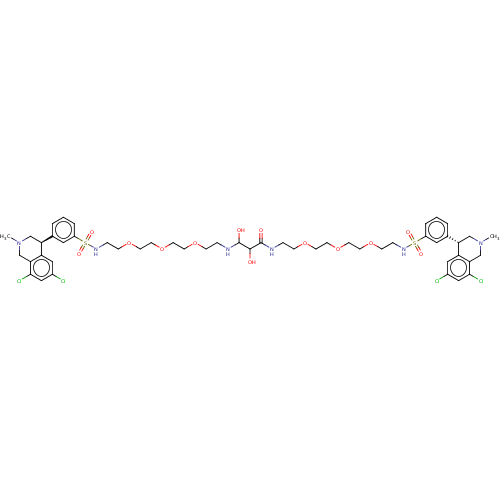 (CHEMBL5176968) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381691 (US10272079, Compound 48 | US10272079, Compound 65) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381826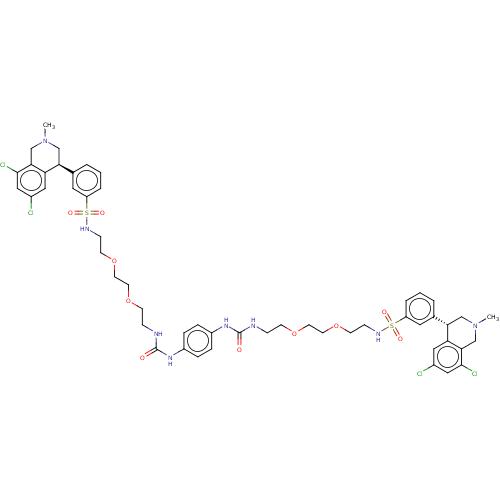 (US10272079, Compound 003) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50601180 (CHEMBL5170002) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381795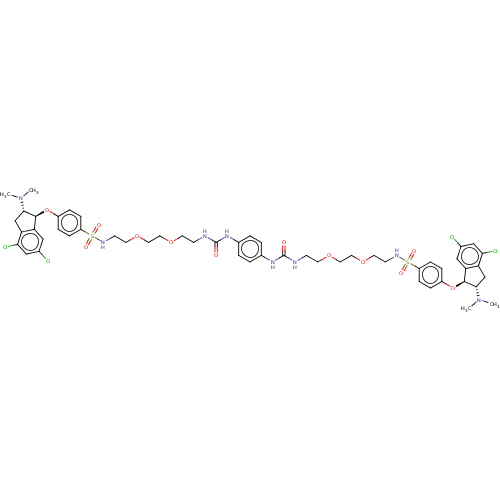 (US10272079, Compound 152) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381710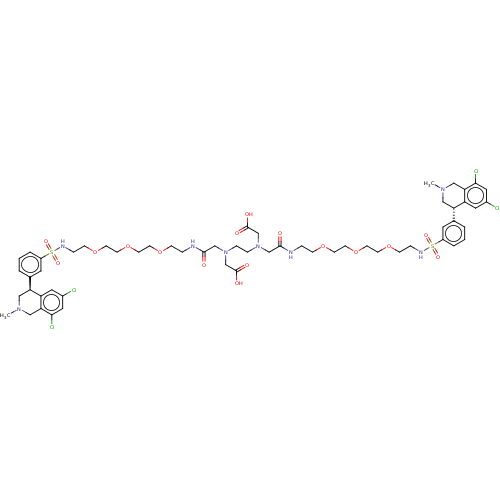 (US10272079, Compound 67) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50601178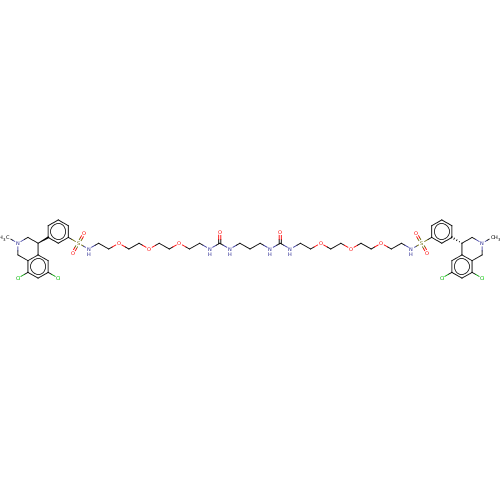 (CHEMBL5202545) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381762 (US10272079, Compound 119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381826 (US10272079, Compound 003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381742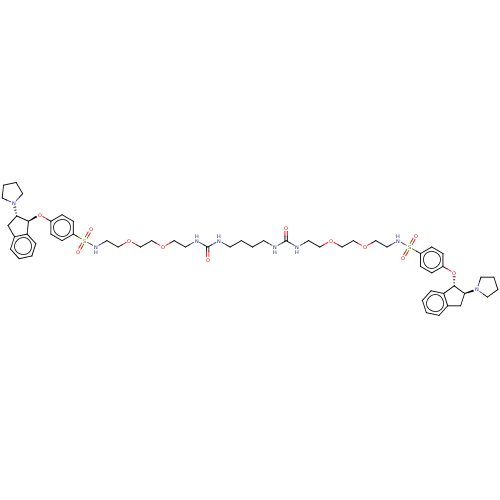 (US10272079, Compound 99) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381793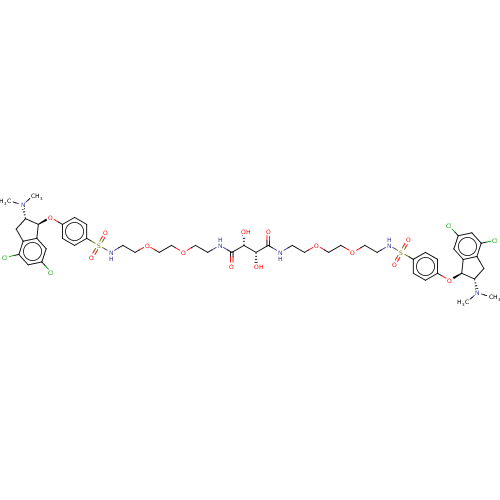 (US10272079, Compound 150) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381826 (US10272079, Compound 003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381817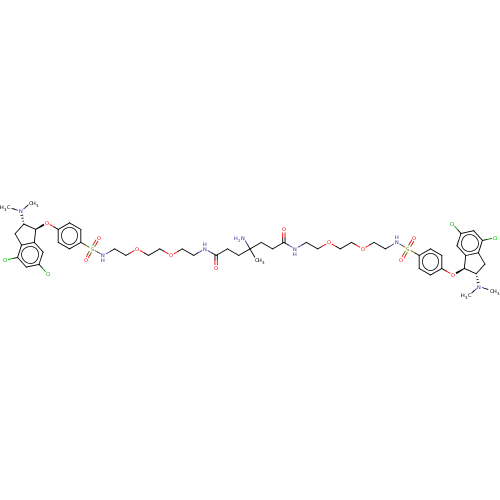 (US10272079, Compound 174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381667 (US10272079, Compound 24) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381758 (US10272079, Compound 115) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381666 (US10272079, Compound 23) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381817 (US10272079, Compound 174) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381740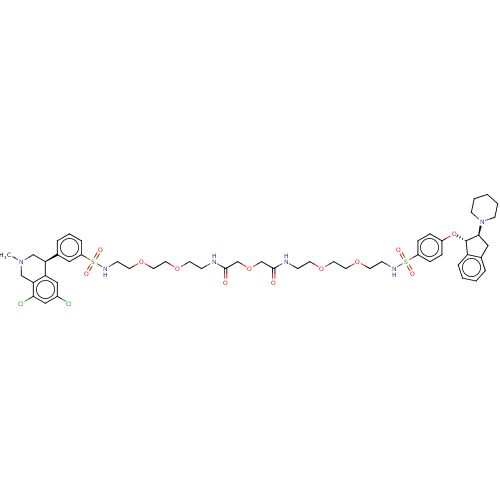 (US10272079, Compound 97) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM426615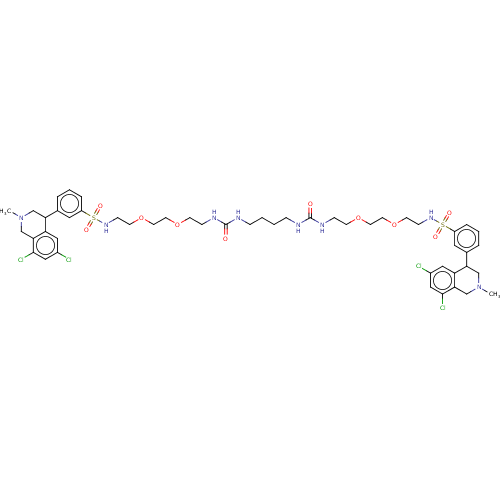 (N,N′-(10,17-dioxo-3,6,21,24-tetraoxa-9,11,16...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381778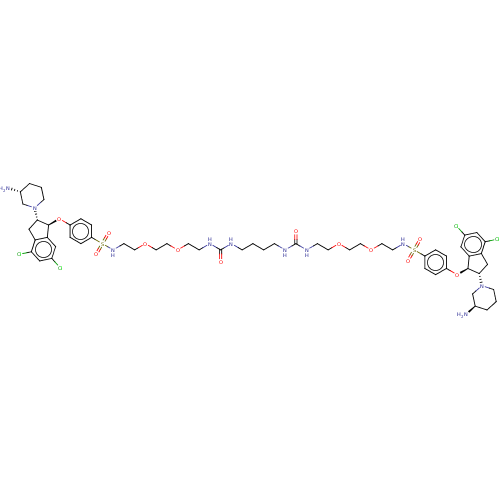 (US10272079, Compound 135) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381823 (CVD-0019453 | US10272079, Compound 002 | US1027207...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The ability of compounds to inhibit Rat NHE3-mediated Na+-dependent H+ antiport after application and washout was measured using a modification of th... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381809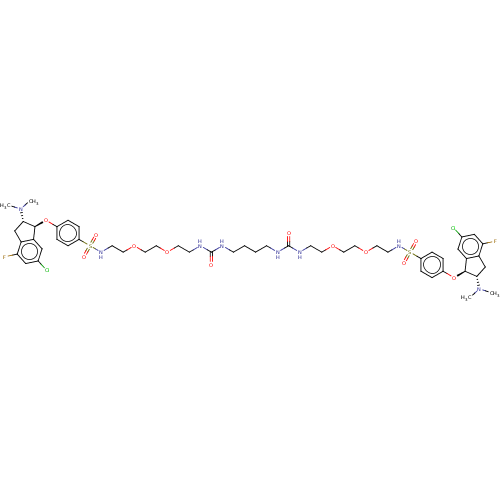 (US10272079, Compound 166) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381791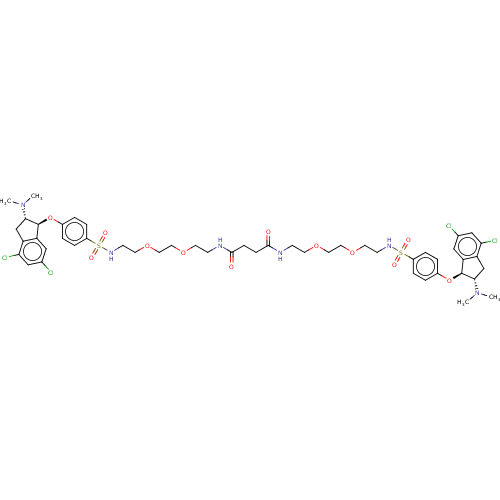 (US10272079, Compound 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381675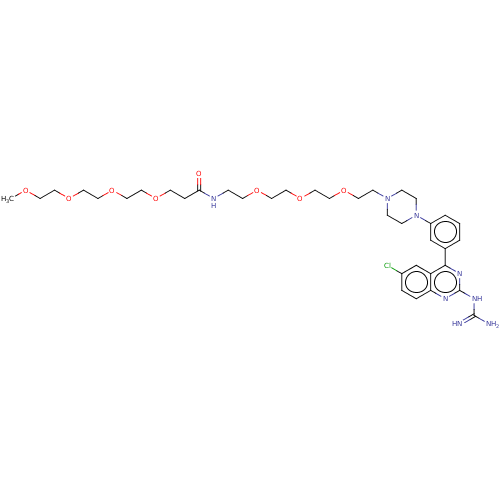 (US10272079, Compound 32) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381673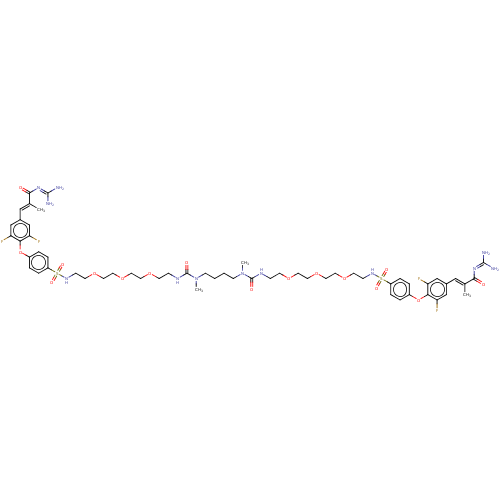 (US10272079, Compound 30) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381670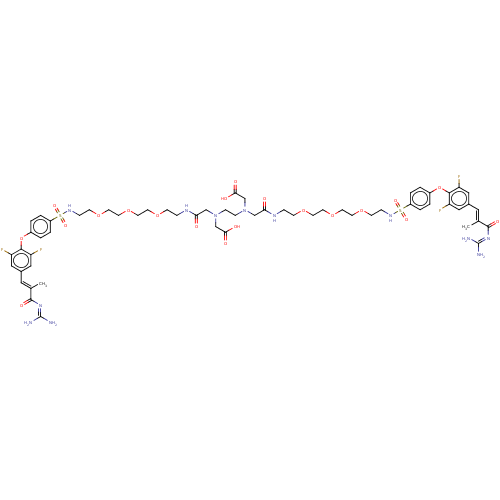 (US10272079, Compound 27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381741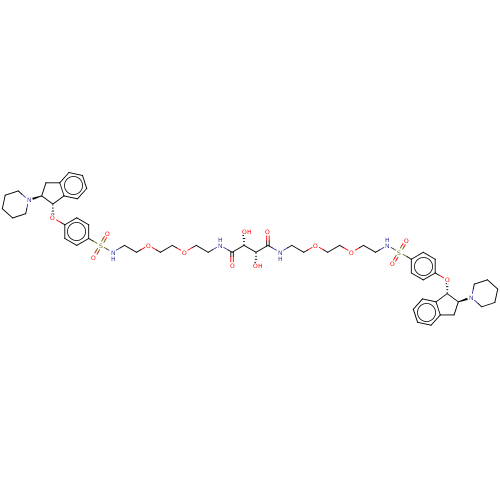 (US10272079, Compound 98) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381751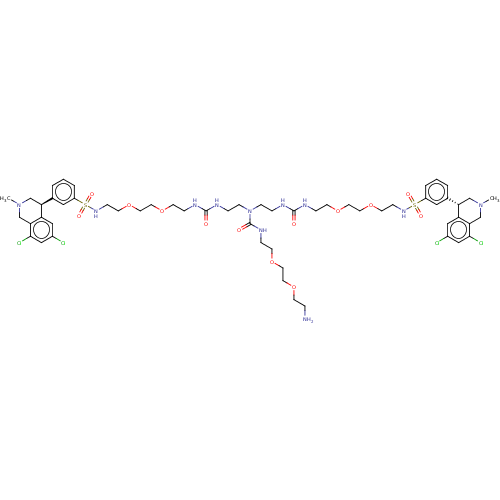 (US10272079, Compound 108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM26333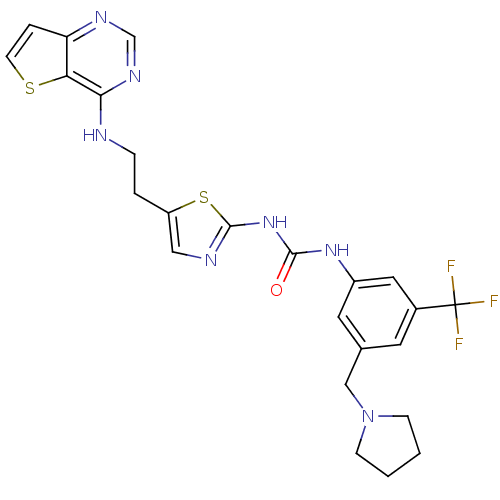 (1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals | Assay Description Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... | Bioorg Med Chem Lett 18: 4880-4 (2008) Article DOI: 10.1016/j.bmcl.2008.07.073 BindingDB Entry DOI: 10.7270/Q279430N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM50601172 (CHEMBL5183104) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00037 BindingDB Entry DOI: 10.7270/Q2DB85X0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381810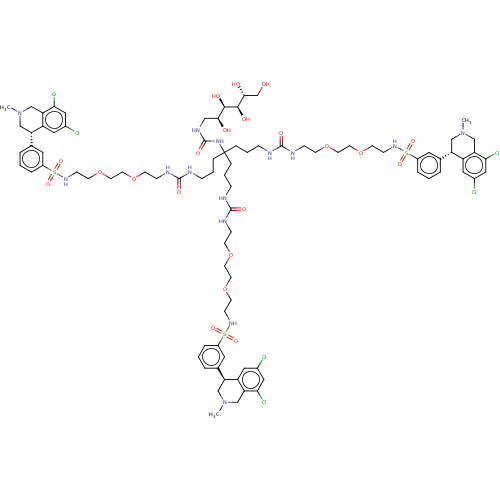 (US10272079, Compound 167) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381690 (US10272079, Compound 47 | US10272079, Compound 66) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381789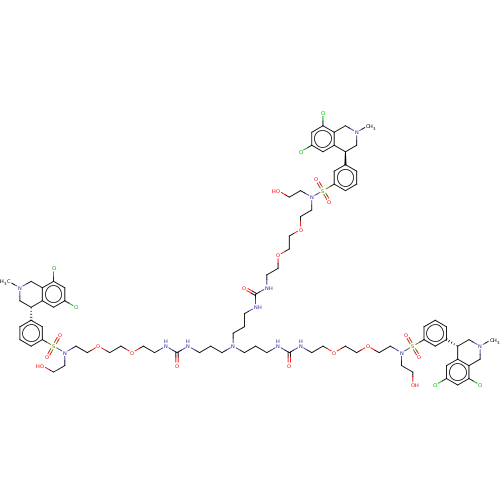 (US10272079, Compound 146) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381648 (US10272079, Compound 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381690 (US10272079, Compound 47 | US10272079, Compound 66) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50310621 (CHEMBL1079538 | N-(4-(6-(trifluoromethyl)-3H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Aurora B after 60 mins | Bioorg Med Chem Lett 19: 5158-61 (2009) Article DOI: 10.1016/j.bmcl.2009.07.016 BindingDB Entry DOI: 10.7270/Q2T72HK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381739 (US10272079, Compound 96) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381743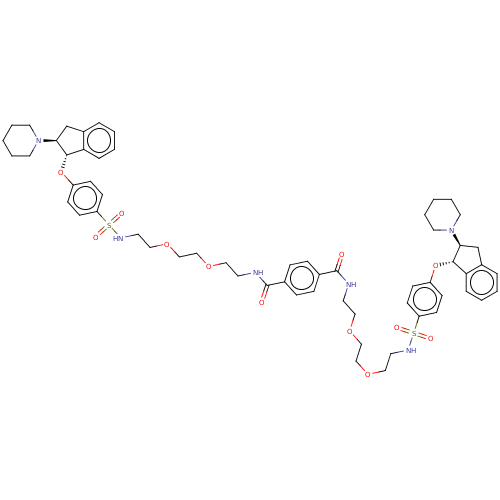 (US10272079, Compound 100) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Homo sapiens (Human)) | BDBM381678 (US10272079, Compound 35) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/hydrogen exchanger 3 (Rattus norvegicus) | BDBM381794 (US10272079, Compound 151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description Cell-based activity under Prompt Conditions. Rat or human NHE3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensiti... | J Med Chem 51: 5663-79 (2008) BindingDB Entry DOI: 10.7270/Q2251MHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 721 total ) | Next | Last >> |