 Found 343 hits with Last Name = 'jain' and Initial = 'hd'
Found 343 hits with Last Name = 'jain' and Initial = 'hd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mycobacterium avium) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium avium DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver DHFR using dihydrofolic acid substrate and NADPH cofactor |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mycobacterium avium) | BDBM50310338

(CHEMBL596672 | N-[4-[(2,4-Diamino-5-methyl-furo[2,...)Show SMILES Cc1c(Sc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)oc2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-13-14(20)23-19(21)24-16(13)30-18(8)31-10-4-2-9(3-5-10)15(27)22-11(17(28)29)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,28,29)(H4,20,21,23,24)/t11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium avium DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50158423

(CHEMBL224868 | N-[4-[(2-amino-6-mtehyl-3,4-dihydro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(Cl)c1 |r| Show InChI InChI=1S/C19H18ClN5O6S/c1-7-14(13-15(22-7)24-19(21)25-17(13)29)32-8-2-3-9(10(20)6-8)16(28)23-11(18(30)31)4-5-12(26)27/h2-3,6,11H,4-5H2,1H3,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50158422
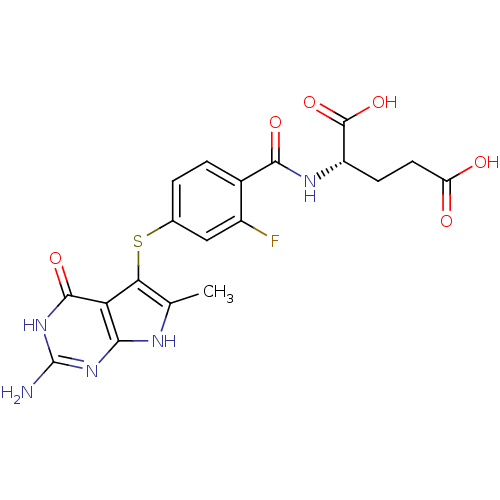
(CHEMBL388340 | N-[4-[(2-amino-6-methyl-3,4-dihydro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c1 |r| Show InChI InChI=1S/C19H18FN5O6S/c1-7-14(13-15(22-7)24-19(21)25-17(13)29)32-8-2-3-9(10(20)6-8)16(28)23-11(18(30)31)4-5-12(26)27/h2-3,6,11H,4-5H2,1H3,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18797
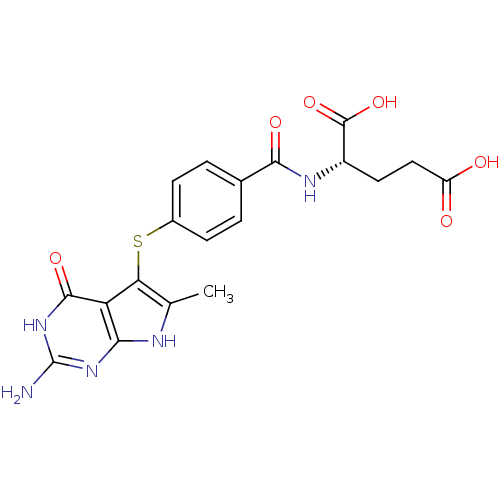
((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18797

((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262603
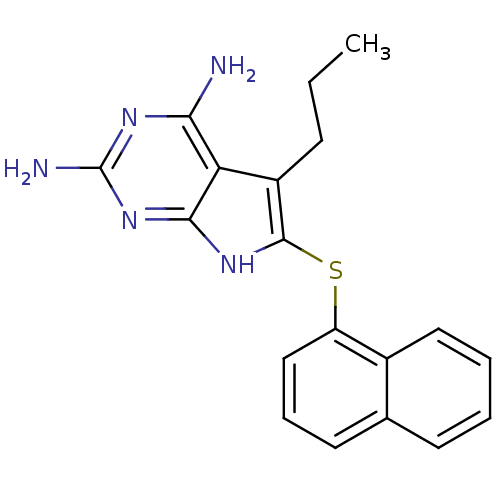
(2,4-Diamino-5-propyl-6-(1'-naphthylsulfanyl)-7H-py...)Show InChI InChI=1S/C19H19N5S/c1-2-6-13-15-16(20)22-19(21)24-17(15)23-18(13)25-14-10-5-8-11-7-3-4-9-12(11)14/h3-5,7-10H,2,6H2,1H3,(H5,20,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50377948
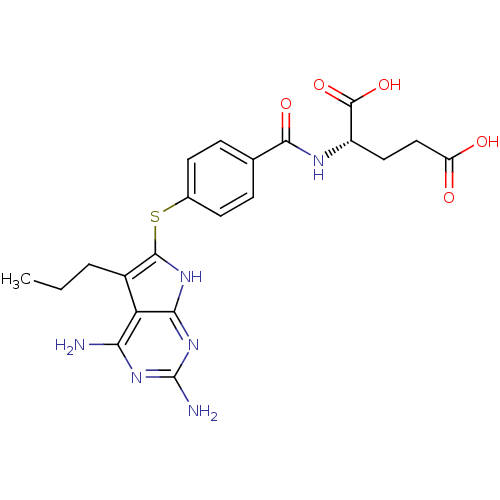
(CHEMBL476548)Show SMILES CCCc1c(Sc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)[nH]c2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C21H24N6O5S/c1-2-3-12-15-16(22)25-21(23)27-17(15)26-19(12)33-11-6-4-10(5-7-11)18(30)24-13(20(31)32)8-9-14(28)29/h4-7,13H,2-3,8-9H2,1H3,(H,24,30)(H,28,29)(H,31,32)(H5,22,23,25,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver DHFR using dihydrofolic acid substrate and NADPH cofactor |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262763
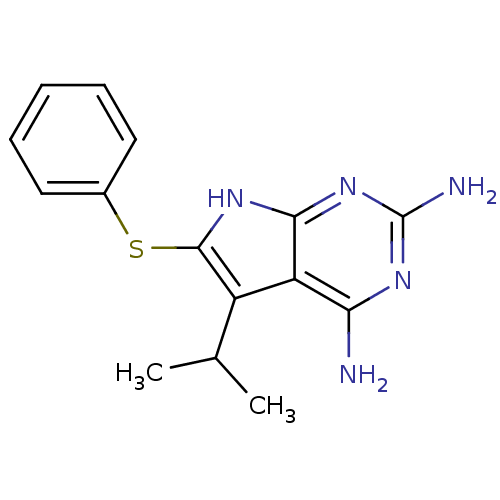
(2,4-Diamino-5-isopropyl-6-(phenylsulfanyl)-7H-pyrr...)Show InChI InChI=1S/C15H17N5S/c1-8(2)10-11-12(16)18-15(17)20-13(11)19-14(10)21-9-6-4-3-5-7-9/h3-8H,1-2H3,(H5,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50041943
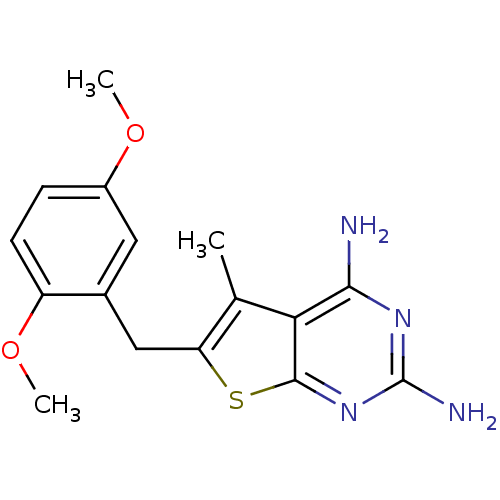
(6-(2,5-Dimethoxy-benzyl)-5-methyl-thieno[2,3-d]pyr...)Show InChI InChI=1S/C16H18N4O2S/c1-8-12(23-15-13(8)14(17)19-16(18)20-15)7-9-6-10(21-2)4-5-11(9)22-3/h4-6H,7H2,1-3H3,(H4,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Pneumocystis carinii) | BDBM50310338

(CHEMBL596672 | N-[4-[(2,4-Diamino-5-methyl-furo[2,...)Show SMILES Cc1c(Sc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)oc2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-13-14(20)23-19(21)24-16(13)30-18(8)31-10-4-2-9(3-5-10)15(27)22-11(17(28)29)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,28,29)(H4,20,21,23,24)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Pneumocystis carinii DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18798
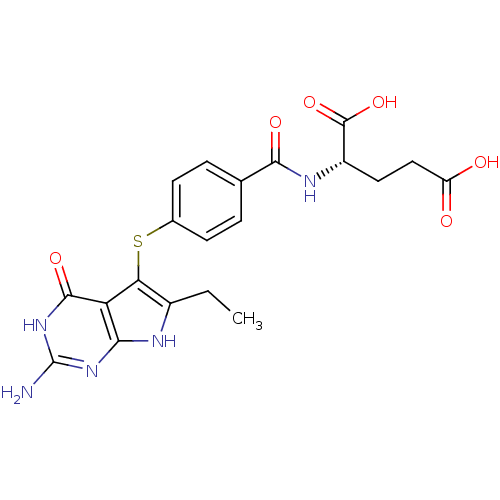
((2S)-2-{[4-({2-amino-6-ethyl-4-oxo-3H,4H,7H-pyrrol...)Show SMILES CCc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H21N5O6S/c1-2-11-15(14-16(22-11)24-20(21)25-18(14)29)32-10-5-3-9(4-6-10)17(28)23-12(19(30)31)7-8-13(26)27/h3-6,12H,2,7-8H2,1H3,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262599

(2,4-Diamino-5-propyl-6-(phenylsulfanyl)-7H-pyrrolo...)Show InChI InChI=1S/C15H17N5S/c1-2-6-10-11-12(16)18-15(17)20-13(11)19-14(10)21-9-7-4-3-5-8-9/h3-5,7-8H,2,6H2,1H3,(H5,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262805

(2,4-Diamino-5-isopropyl-6-(1'-naphthylsulfanyl)-7H...)Show SMILES CC(C)c1c(Sc2cccc3ccccc23)[nH]c2nc(N)nc(N)c12 Show InChI InChI=1S/C19H19N5S/c1-10(2)14-15-16(20)22-19(21)24-17(15)23-18(14)25-13-9-5-7-11-6-3-4-8-12(11)13/h3-10H,1-2H3,(H5,20,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18808
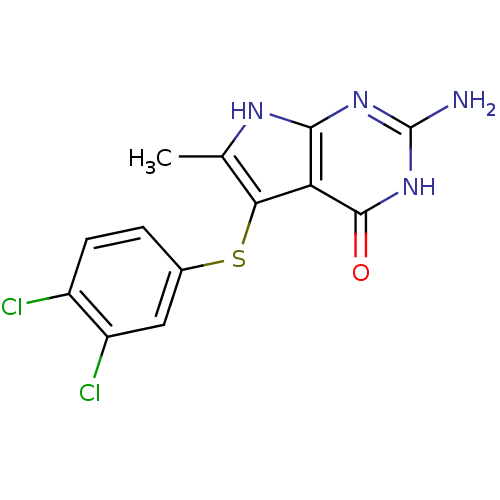
(2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C13H10Cl2N4OS/c1-5-10(21-6-2-3-7(14)8(15)4-6)9-11(17-5)18-13(16)19-12(9)20/h2-4H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18808

(2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-methyl-...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C13H10Cl2N4OS/c1-5-10(21-6-2-3-7(14)8(15)4-6)9-11(17-5)18-13(16)19-12(9)20/h2-4H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18807
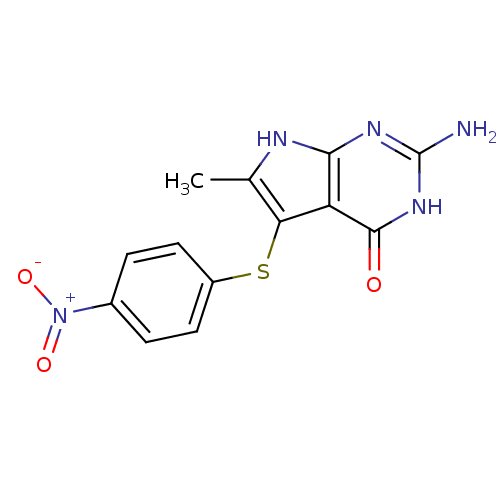
(2-amino-6-methyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C13H11N5O3S/c1-6-10(9-11(15-6)16-13(14)17-12(9)19)22-8-4-2-7(3-5-8)18(20)21/h2-5H,1H3,(H4,14,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18807

(2-amino-6-methyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C13H11N5O3S/c1-6-10(9-11(15-6)16-13(14)17-12(9)19)22-8-4-2-7(3-5-8)18(20)21/h2-5H,1H3,(H4,14,15,16,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262653

(2,4-Diamino-5-propyl-6-(2'-naphthylsulfanyl)-7H-py...)Show InChI InChI=1S/C19H19N5S/c1-2-5-14-15-16(20)22-19(21)24-17(15)23-18(14)25-13-9-8-11-6-3-4-7-12(11)10-13/h3-4,6-10H,2,5H2,1H3,(H5,20,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
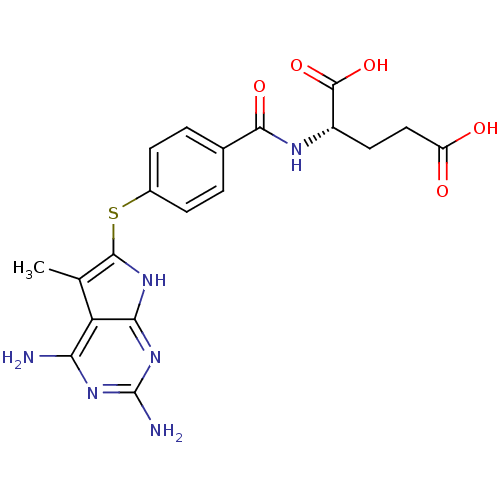
(Toxoplasma gondii) | BDBM18245
((2S)-2-{[4-({2,4-diamino-5-methyl-7H-pyrrolo[2,3-d...)Show SMILES Cc1c(Sc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)[nH]c2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C19H20N6O5S/c1-8-13-14(20)23-19(21)25-15(13)24-17(8)31-10-4-2-9(3-5-10)16(28)22-11(18(29)30)6-7-12(26)27/h2-5,11H,6-7H2,1H3,(H,22,28)(H,26,27)(H,29,30)(H5,20,21,23,24,25)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50377949

(CHEMBL476751)Show SMILES CC(C)c1c(Sc2ccc(CC(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2)[nH]c2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C22H26N6O5S/c1-10(2)16-17-18(23)26-22(24)28-19(17)27-20(16)34-12-5-3-11(4-6-12)9-14(29)25-13(21(32)33)7-8-15(30)31/h3-6,10,13H,7-9H2,1-2H3,(H,25,29)(H,30,31)(H,32,33)(H5,23,24,26,27,28)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver DHFR using dihydrofolic acid substrate and NADPH cofactor |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18245

((2S)-2-{[4-({2,4-diamino-5-methyl-7H-pyrrolo[2,3-d...)Show SMILES Cc1c(Sc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)[nH]c2nc(N)nc(N)c12 |r| Show InChI InChI=1S/C19H20N6O5S/c1-8-13-14(20)23-19(21)25-15(13)24-17(8)31-10-4-2-9(3-5-10)16(28)22-11(18(29)30)6-7-12(26)27/h2-5,11H,6-7H2,1H3,(H,22,28)(H,26,27)(H,29,30)(H5,20,21,23,24,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50165444
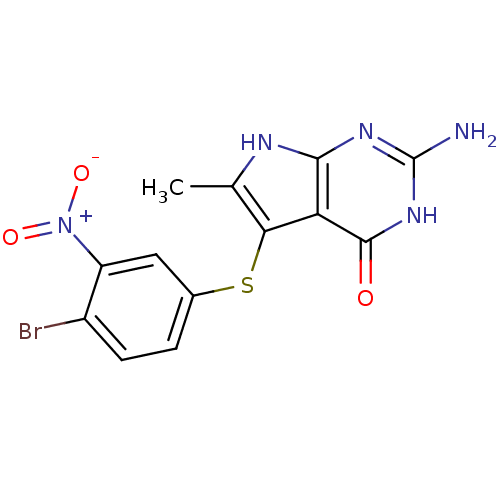
(2-Amino-5-(4-bromo-3-nitro-phenylsulfanyl)-6-methy...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Br)c(c1)[N+]([O-])=O Show InChI InChI=1S/C13H10BrN5O3S/c1-5-10(9-11(16-5)17-13(15)18-12(9)20)23-6-2-3-7(14)8(4-6)19(21)22/h2-4H,1H3,(H4,15,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50030819

((S)-2-(4-(2-(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)...)Show SMILES Nc1nc(N)c2c(CCc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)coc2n1 Show InChI InChI=1S/C20H21N5O6/c21-16-15-12(9-31-18(15)25-20(22)24-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(29)30)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,29,30)(H4,21,22,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50165450
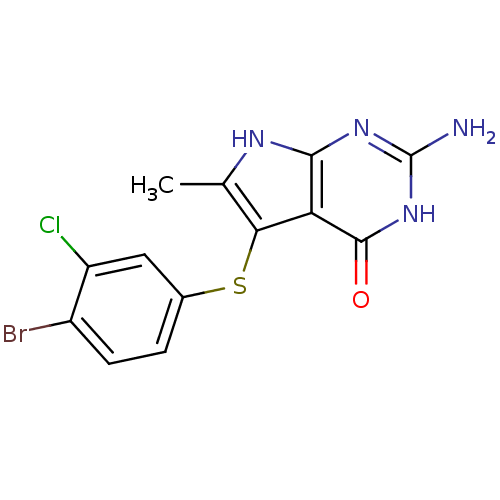
(2-Amino-5-(4-bromo-3-chloro-phenylsulfanyl)-6-meth...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Br)c(Cl)c1 Show InChI InChI=1S/C13H10BrClN4OS/c1-5-10(21-6-2-3-7(14)8(15)4-6)9-11(17-5)18-13(16)19-12(9)20/h2-4H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50030820
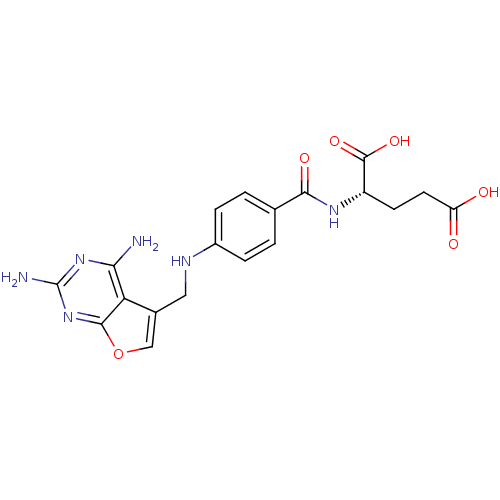
((S)-2-(4-((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)me...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)coc2n1 Show InChI InChI=1S/C19H20N6O6/c20-15-14-10(8-31-17(14)25-19(21)24-15)7-22-11-3-1-9(2-4-11)16(28)23-12(18(29)30)5-6-13(26)27/h1-4,8,12,22H,5-7H2,(H,23,28)(H,26,27)(H,29,30)(H4,20,21,24,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50091145
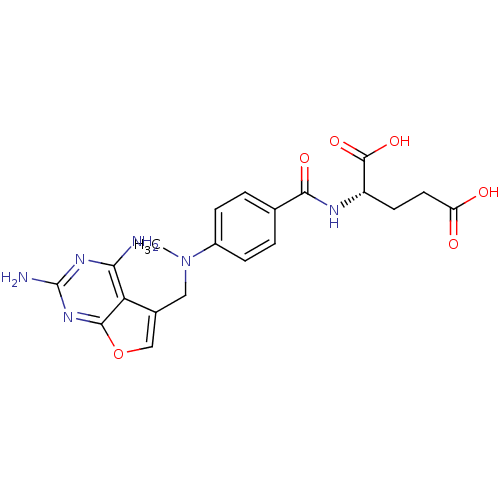
((S)-2-(4-(((2,4-diaminofuro[2,3-d]pyrimidin-5-yl)m...)Show SMILES CN(Cc1coc2nc(N)nc(N)c12)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N6O6/c1-26(8-11-9-32-18-15(11)16(21)24-20(22)25-18)12-4-2-10(3-5-12)17(29)23-13(19(30)31)6-7-14(27)28/h2-5,9,13H,6-8H2,1H3,(H,23,29)(H,27,28)(H,30,31)(H4,21,22,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18799
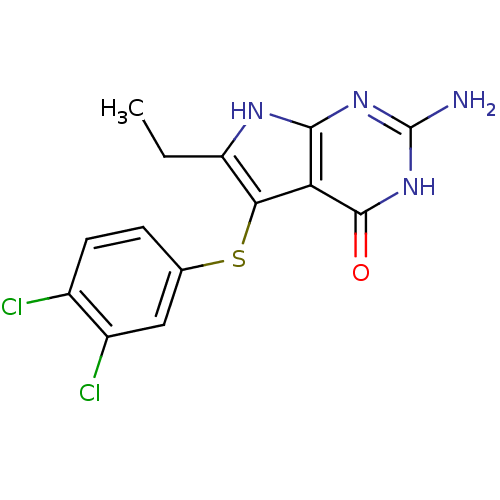
(2-amino-5-[(3,4-dichlorophenyl)sulfanyl]-6-ethyl-3...)Show SMILES CCc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H12Cl2N4OS/c1-2-9-11(22-6-3-4-7(15)8(16)5-6)10-12(18-9)19-14(17)20-13(10)21/h3-5H,2H2,1H3,(H4,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262600
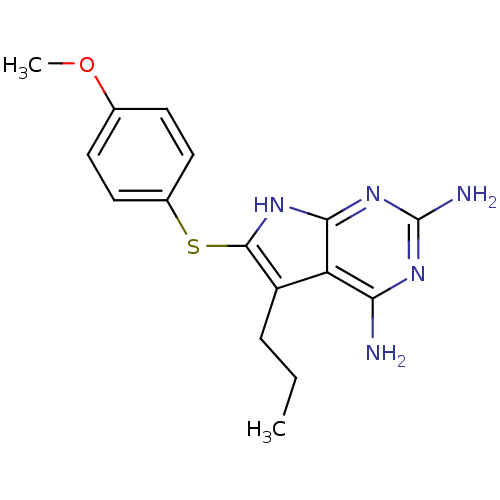
(2,4-Diamino-5-propyl-6-(4'-methoxyphenylsulfanyl)-...)Show InChI InChI=1S/C16H19N5OS/c1-3-4-11-12-13(17)19-16(18)21-14(12)20-15(11)23-10-7-5-9(22-2)6-8-10/h5-8H,3-4H2,1-2H3,(H5,17,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18800
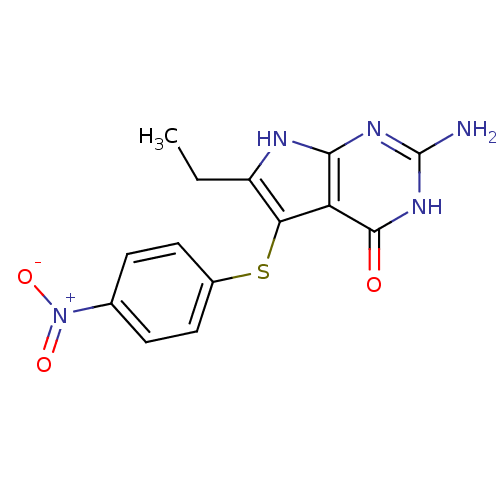
(2-amino-6-ethyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H,...)Show SMILES CCc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C14H13N5O3S/c1-2-9-11(10-12(16-9)17-14(15)18-13(10)20)23-8-5-3-7(4-6-8)19(21)22/h3-6H,2H2,1H3,(H4,15,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM18797

((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 49: 1055-65 (2006)
Article DOI: 10.1021/jm058276a
BindingDB Entry DOI: 10.7270/Q2833Q90 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM18797

((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50165454
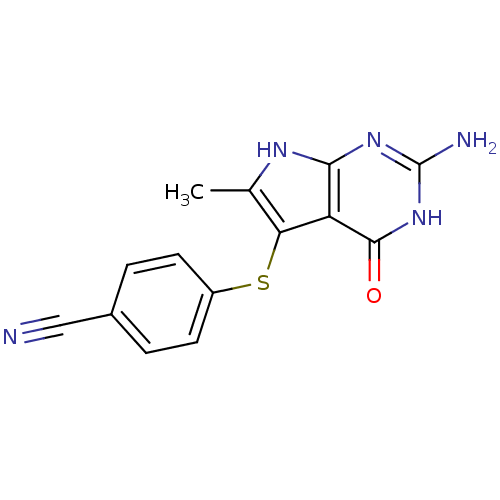
(4-(2-Amino-6-methyl-4-oxo-4,7-dihydro-3H-pyrrolo[2...)Show InChI InChI=1S/C14H11N5OS/c1-7-11(21-9-4-2-8(6-15)3-5-9)10-12(17-7)18-14(16)19-13(10)20/h2-5H,1H3,(H4,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mycobacterium avium) | BDBM18069

(5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...)Show InChI InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium avium DHFR at 37 degC by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50262762
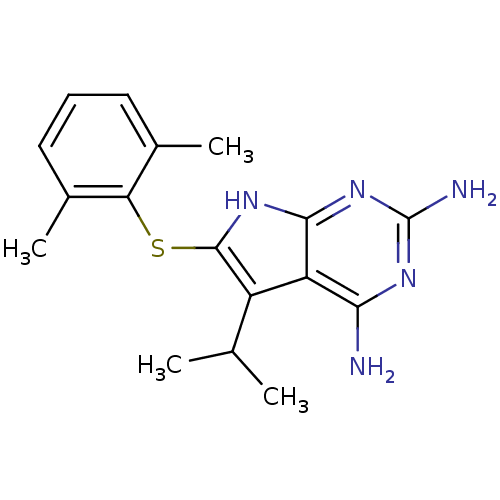
(2,4-Diamino-5-isopropyl-6-(2',6'-dimethylphenylsul...)Show InChI InChI=1S/C17H21N5S/c1-8(2)11-12-14(18)20-17(19)22-15(12)21-16(11)23-13-9(3)6-5-7-10(13)4/h5-8H,1-4H3,(H5,18,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii dihydrofolate reductase |
J Med Chem 51: 4589-600 (2008)
Article DOI: 10.1021/jm800244v
BindingDB Entry DOI: 10.7270/Q2ZW1MTV |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50054514

(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3,7-dihy...)Show InChI InChI=1S/C12H11N5OS/c1-6-9(19-7-2-4-14-5-3-7)8-10(15-6)16-12(13)17-11(8)18/h2-5H,1H3,(H4,13,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795
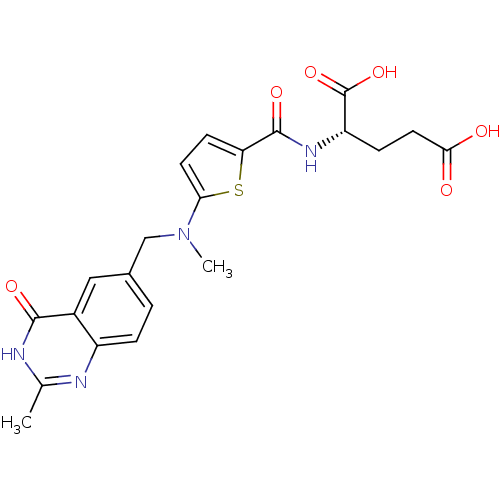
((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase at 30 degC under pH 7.4 by spectrophotometry |
Bioorg Med Chem 18: 953-61 (2010)
Article DOI: 10.1016/j.bmc.2009.11.029
BindingDB Entry DOI: 10.7270/Q25B03FP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795

((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidylate synthase |
Bioorg Med Chem Lett 15: 2225-30 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.029
BindingDB Entry DOI: 10.7270/Q2ZW1MQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18795

((2S)-2-[(5-{methyl[(2-methyl-4-oxo-1,4-dihydroquin...)Show SMILES CN(Cc1ccc2nc(C)[nH]c(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 47: 6730-9 (2004)
Article DOI: 10.1021/jm040144e
BindingDB Entry DOI: 10.7270/Q23N247N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data