

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528793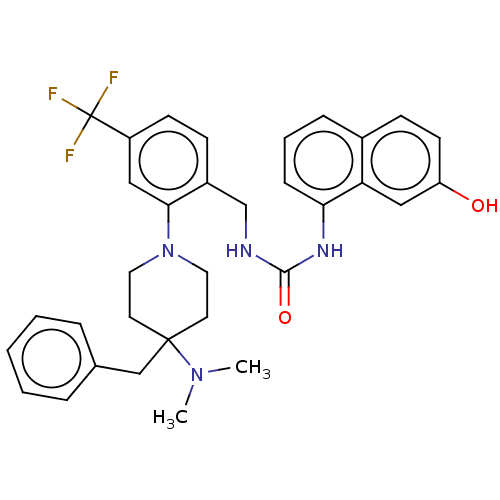 (CHEMBL4471708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528804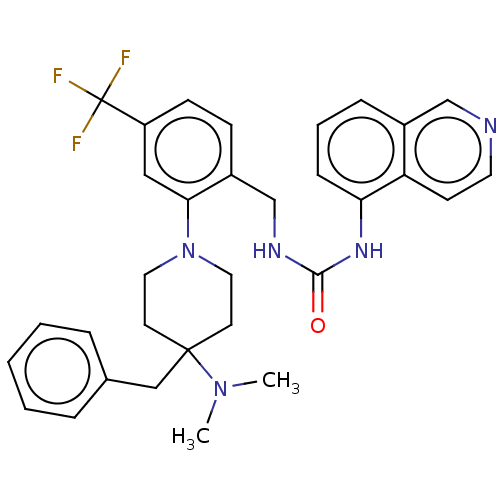 (CHEMBL4553702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528797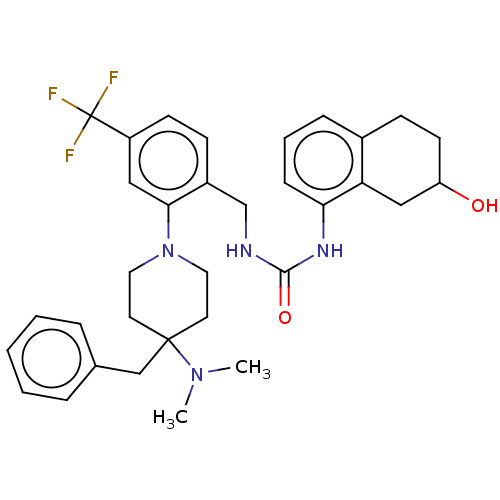 (CHEMBL4438643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528802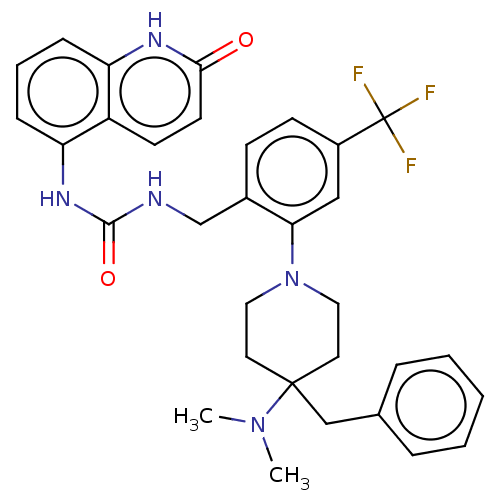 (CHEMBL4562769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528800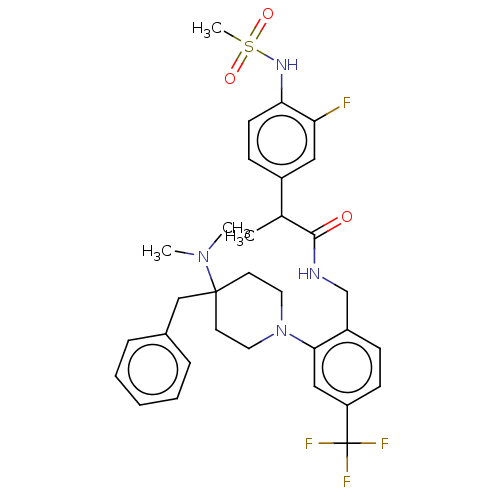 (CHEMBL4586277) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528807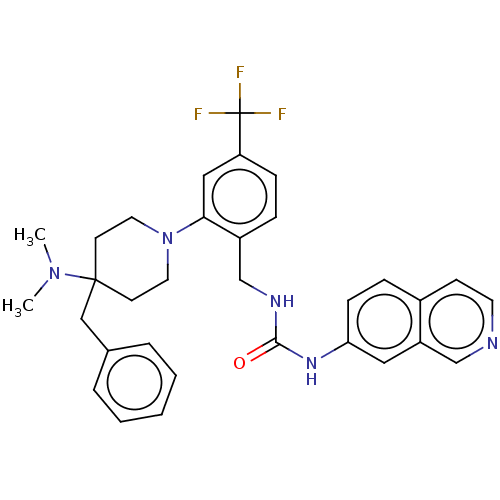 (CHEMBL4453980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528792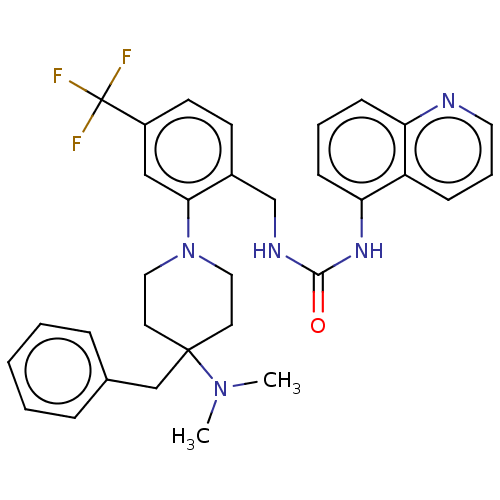 (CHEMBL4438974) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528795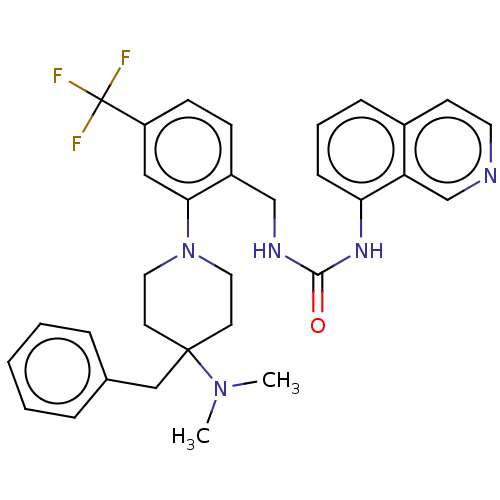 (CHEMBL4442473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528796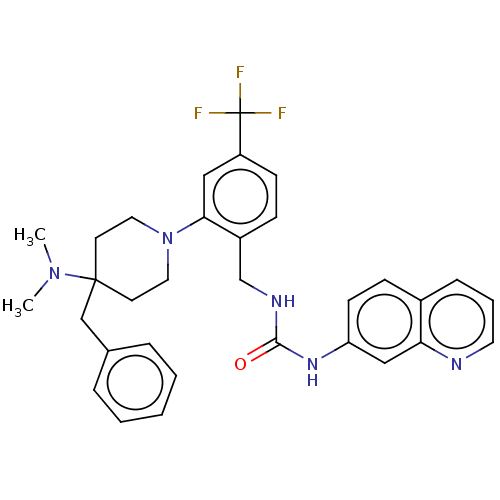 (CHEMBL4459611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528798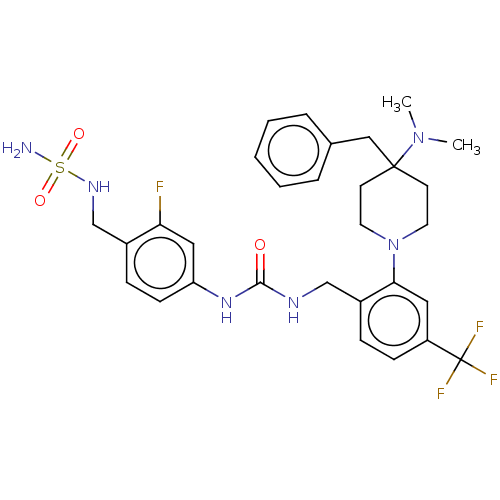 (CHEMBL4536869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528799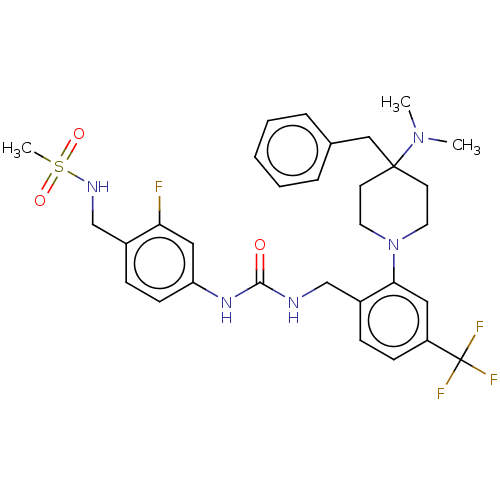 (CHEMBL4571845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528803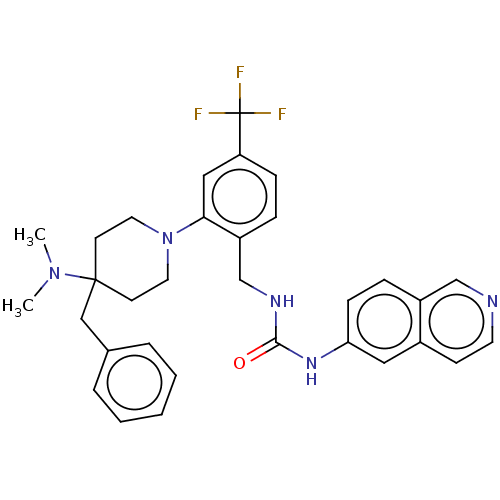 (CHEMBL4457304) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528805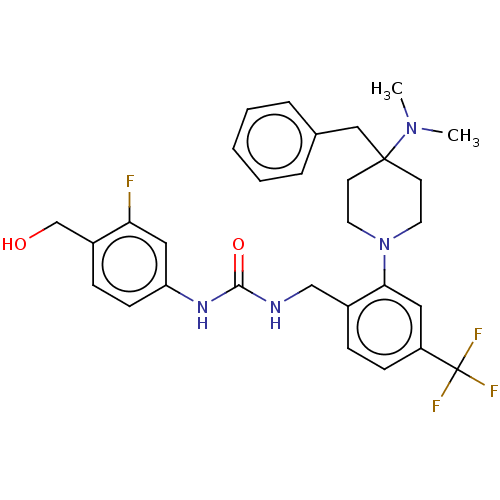 (CHEMBL4555508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528792 (CHEMBL4438974) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528806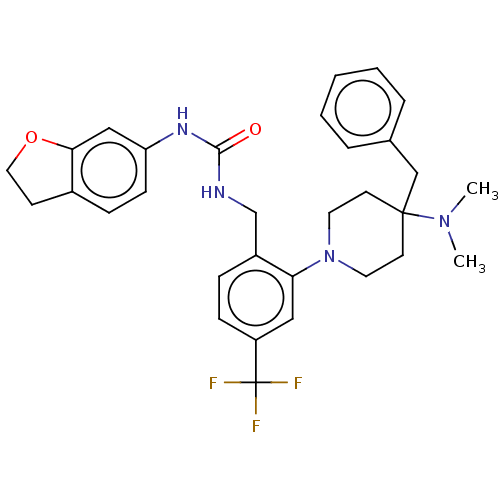 (CHEMBL4457595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528803 (CHEMBL4457304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528798 (CHEMBL4536869) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528808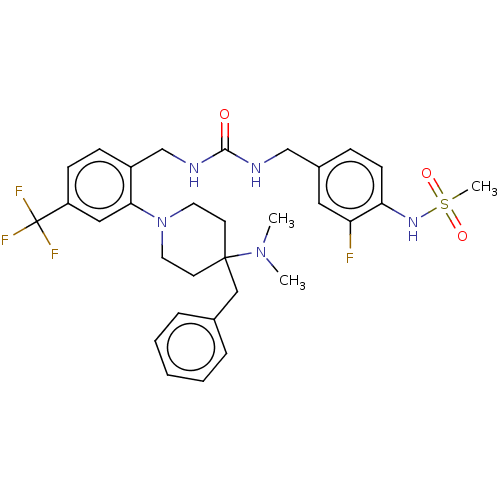 (CHEMBL4476530) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528801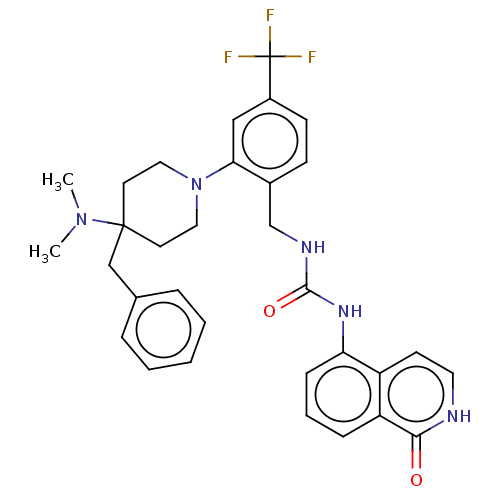 (CHEMBL4448866) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528801 (CHEMBL4448866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528793 (CHEMBL4471708) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528796 (CHEMBL4459611) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528807 (CHEMBL4453980) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528795 (CHEMBL4442473) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528806 (CHEMBL4457595) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528805 (CHEMBL4555508) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528802 (CHEMBL4562769) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528797 (CHEMBL4438643) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 261 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528808 (CHEMBL4476530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528799 (CHEMBL4571845) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528794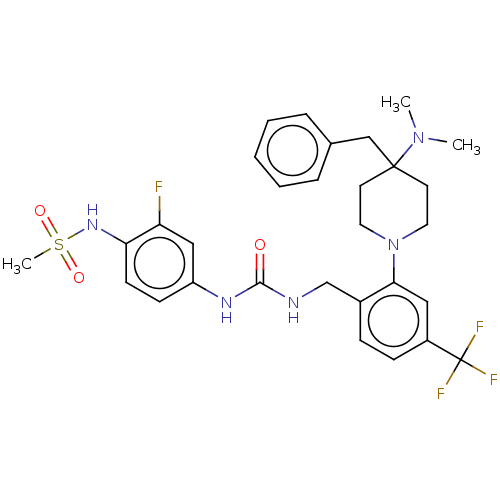 (CHEMBL4483060) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528800 (CHEMBL4586277) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 428 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50528804 (CHEMBL4553702) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 507 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50528794 (CHEMBL4483060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111634 BindingDB Entry DOI: 10.7270/Q2765JR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50068224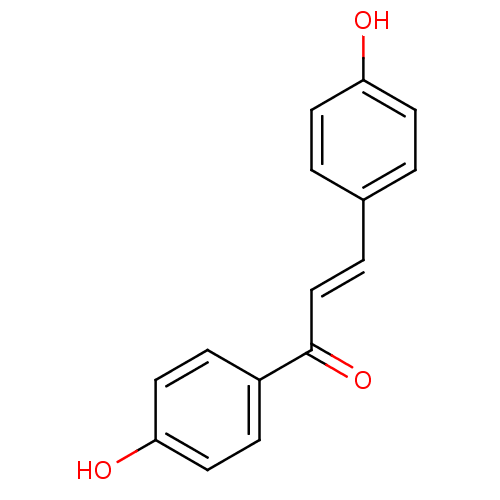 ((E)-1,3-Bis-(4-hydroxy-phenyl)-propenone | 1,3-bis...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174839 (1-(4-aminophenyl)-3-(4-hydroxyphenyl)prop-2-en-1-o...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50316855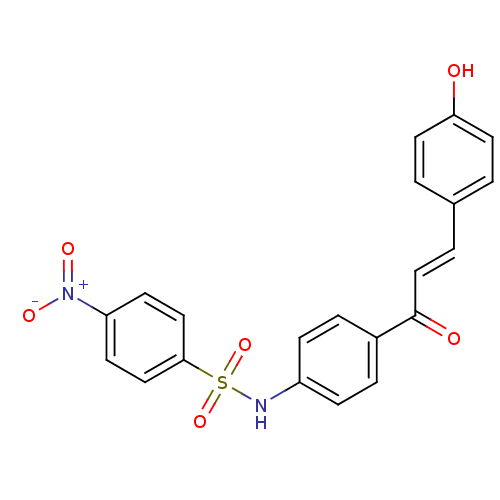 (4'-(4-Nitrobenzensulfonamide)-4-hydroxychalcone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50174835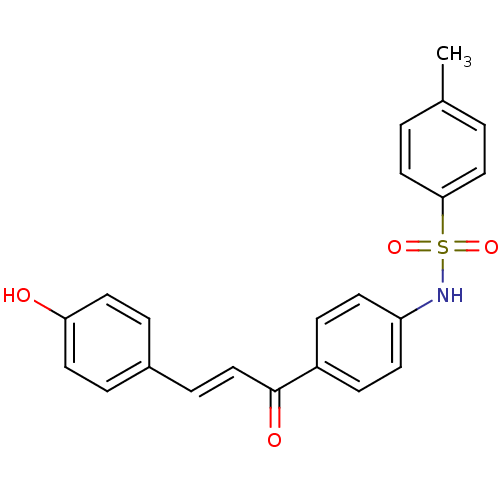 (4'-(4-toluenesulfonamido)-4-hydroxychalcone | 4'-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50316853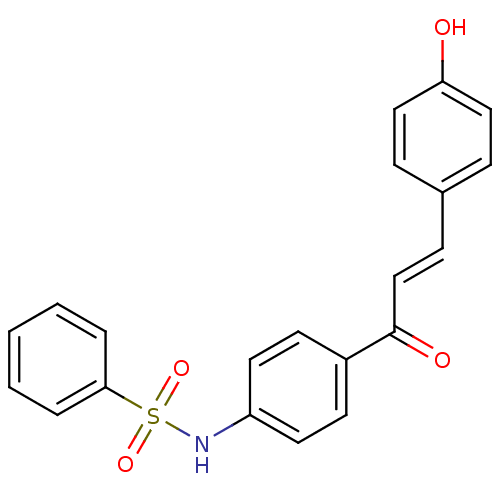 (4'-(Benzensulfonamide)-4-hydroxychalcone | CHEMBL1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50316857 (4'-(4-Fluorobenzensulfonamide)-4-hydroxychalcone |...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50316856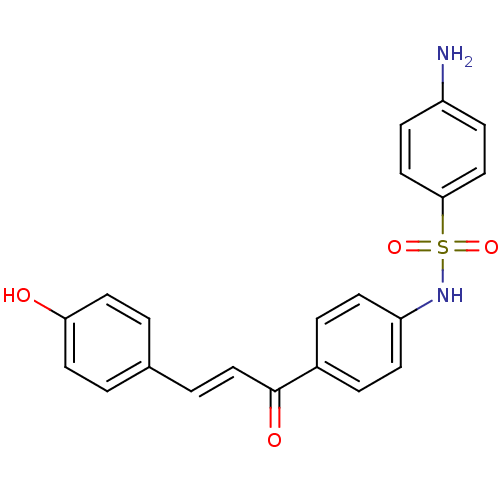 (4'-(4-Aminobenzensulfonamide)-4-hydroxychalcone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50316854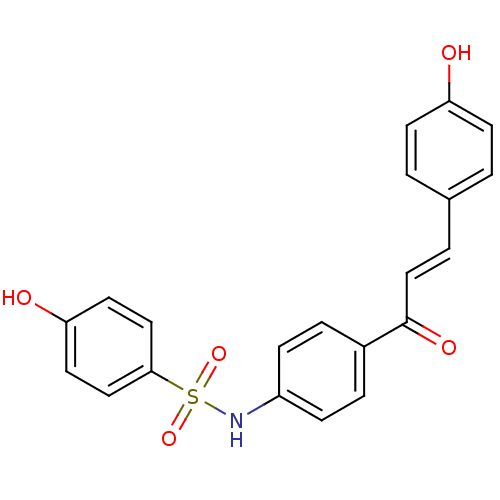 (4'-(4-Hydroxylbenzensulfonamide)-4-hydroxychalcone...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Functional Crop Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using as L-tyrosine substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 45: 2010-7 (2010) Article DOI: 10.1016/j.ejmech.2010.01.049 BindingDB Entry DOI: 10.7270/Q2H70FZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347952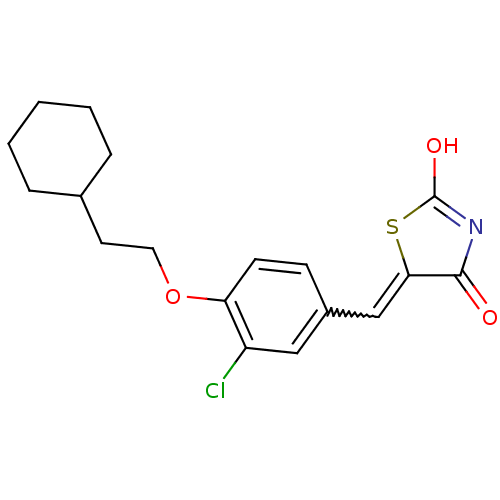 (CHEMBL1800139 | US8637558, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human 15-PGDH expressed in Escherichia coli using PGE2 as substrate and NAD+ as coenzyme assessed as formation of NADH by spectrophotom... | J Med Chem 54: 5260-4 (2011) Article DOI: 10.1021/jm200390u BindingDB Entry DOI: 10.7270/Q2B56K33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347956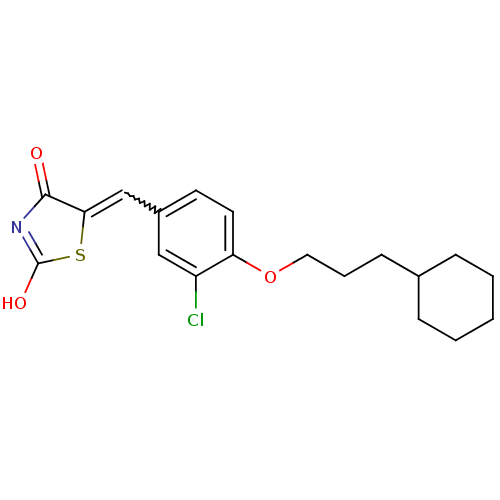 (CHEMBL1800143 | US8637558, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human 15-PGDH expressed in Escherichia coli using PGE2 as substrate and NAD+ as coenzyme assessed as formation of NADH by spectrophotom... | J Med Chem 54: 5260-4 (2011) Article DOI: 10.1021/jm200390u BindingDB Entry DOI: 10.7270/Q2B56K33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50545002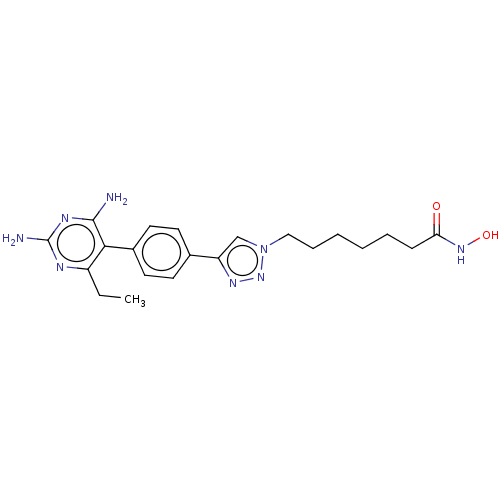 (CHEMBL4635479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347953 (CHEMBL1800140 | US8637558, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human 15-PGDH expressed in Escherichia coli using PGE2 as substrate and NAD+ as coenzyme assessed as formation of NADH by spectrophotom... | J Med Chem 54: 5260-4 (2011) Article DOI: 10.1021/jm200390u BindingDB Entry DOI: 10.7270/Q2B56K33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50545003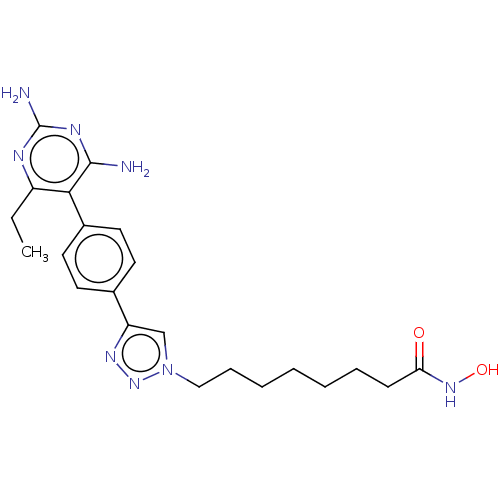 (CHEMBL4636452) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using fluorogenic HDAC substrate 3 preincubated for 30 mins followed by substrate addition and measured after 1... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115345 BindingDB Entry DOI: 10.7270/Q2PR80K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347957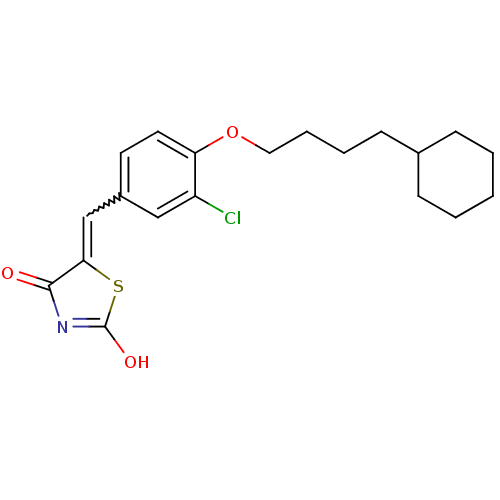 (CHEMBL1800144 | US8637558, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human 15-PGDH expressed in Escherichia coli using PGE2 as substrate and NAD+ as coenzyme assessed as formation of NADH by spectrophotom... | J Med Chem 54: 5260-4 (2011) Article DOI: 10.1021/jm200390u BindingDB Entry DOI: 10.7270/Q2B56K33 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218 total ) | Next | Last >> |