 Found 2788 hits with Last Name = 'jiang' and Initial = 'q'
Found 2788 hits with Last Name = 'jiang' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(CALF) | BDBM85393
(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85396
(CACO)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27N3O6/c1-29-14-13-26-23-17-6-9-20(35-2)24(23)36-25(26)19(31)11-12-27(26,21(29)15-17)28-22(32)10-5-16-3-7-18(8-4-16)30(33)34/h3-10,21,25H,11-15H2,1-2H3,(H,28,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Opioid receptor delta 1
(Bos taurus) | BDBM85393

(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85394
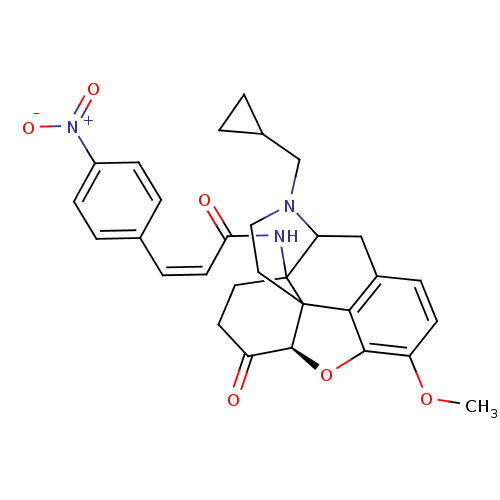
(N-CPM-CACO)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31N3O6/c1-38-23-10-7-20-16-24-30(31-25(35)11-6-18-4-8-21(9-5-18)33(36)37)13-12-22(34)28-29(30,26(20)27(23)39-28)14-15-32(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,31,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(CALF) | BDBM85393

(14β-(p-chlorocinnamoylamino)-7,8-dihydrocodei...)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27ClN2O4/c1-30-14-13-26-23-17-6-9-20(33-2)24(23)34-25(26)19(31)11-12-27(26,21(30)15-17)29-22(32)10-5-16-3-7-18(28)8-4-16/h3-10,21,25H,11-15H2,1-2H3,(H,29,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(CALF) | BDBM85395
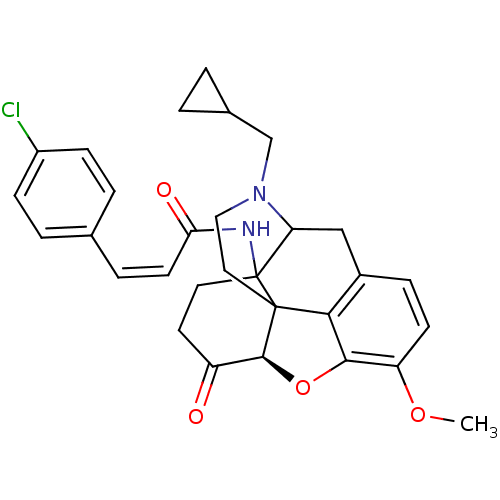
(MC-CAM)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31ClN2O4/c1-36-23-10-7-20-16-24-30(32-25(35)11-6-18-4-8-21(31)9-5-18)13-12-22(34)28-29(30,26(20)27(23)37-28)14-15-33(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,32,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85788
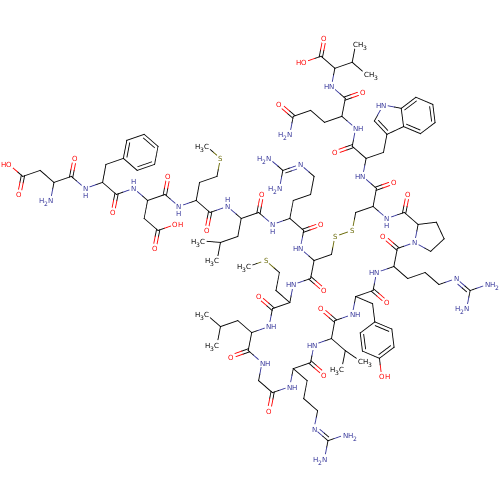
(MCH | hMCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C105H160N30O26S4/c1-53(2)42-70-86(144)118-50-80(138)119-64(24-16-36-114-103(108)109)90(148)133-83(55(5)6)100(158)130-73(45-58-28-30-60(136)31-29-58)93(151)124-69(26-18-38-116-105(112)113)101(159)135-39-19-27-78(135)99(157)132-77(98(156)128-74(46-59-49-117-63-23-15-14-22-61(59)63)95(153)121-66(32-33-79(107)137)91(149)134-84(56(7)8)102(160)161)52-165-164-51-76(97(155)123-68(35-41-163-10)88(146)126-70)131-87(145)65(25-17-37-115-104(110)111)120-92(150)71(43-54(3)4)127-89(147)67(34-40-162-9)122-96(154)75(48-82(141)142)129-94(152)72(44-57-20-12-11-13-21-57)125-85(143)62(106)47-81(139)140/h11-15,20-23,28-31,49,53-56,62,64-78,83-84,117,136H,16-19,24-27,32-48,50-52,106H2,1-10H3,(H2,107,137)(H,118,144)(H,119,138)(H,120,150)(H,121,153)(H,122,154)(H,123,155)(H,124,151)(H,125,143)(H,126,146)(H,127,147)(H,128,156)(H,129,152)(H,130,158)(H,131,145)(H,132,157)(H,133,148)(H,134,149)(H,139,140)(H,141,142)(H,160,161)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816
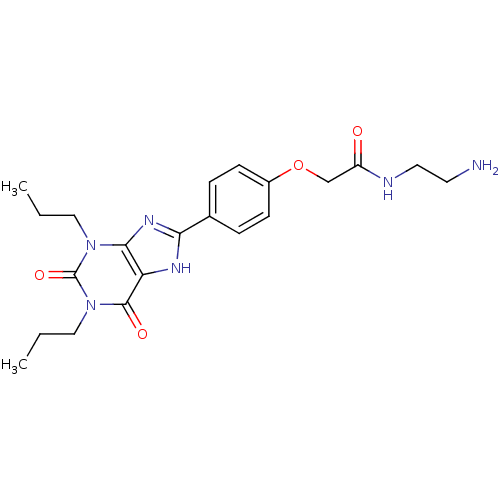
(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(CALF) | BDBM85394

(N-CPM-CACO)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31N3O6/c1-38-23-10-7-20-16-24-30(31-25(35)11-6-18-4-8-21(9-5-18)33(36)37)13-12-22(34)28-29(30,26(20)27(23)39-28)14-15-32(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,31,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Opioid receptor delta 1
(Bos taurus) | BDBM85395

(MC-CAM)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31ClN2O4/c1-36-23-10-7-20-16-24-30(32-25(35)11-6-18-4-8-21(31)9-5-18)13-12-22(34)28-29(30,26(20)27(23)37-28)14-15-33(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,32,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(CALF) | BDBM85395

(MC-CAM)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(Cl)cc1 |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31ClN2O4/c1-36-23-10-7-20-16-24-30(32-25(35)11-6-18-4-8-21(31)9-5-18)13-12-22(34)28-29(30,26(20)27(23)37-28)14-15-33(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,32,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Opioid receptor delta 1
(Bos taurus) | BDBM85394

(N-CPM-CACO)Show SMILES COc1ccc2CC3N(CC4CC4)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:25:24:19.5.6:8.14.13,9:8:24:19.5.6,THB:18:19:24:8.14.13| Show InChI InChI=1S/C30H31N3O6/c1-38-23-10-7-20-16-24-30(31-25(35)11-6-18-4-8-21(9-5-18)33(36)37)13-12-22(34)28-29(30,26(20)27(23)39-28)14-15-32(24)17-19-2-3-19/h4-11,19,24,28H,2-3,12-17H2,1H3,(H,31,35)/b11-6-/t24?,28-,29?,30?/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(CALF) | BDBM85396

(CACO)Show SMILES COc1ccc2CC3N(C)CCC45[C@@H](Oc1c24)C(=O)CCC35NC(=O)\C=C/c1ccc(cc1)[N+]([O-])=O |r,TLB:22:21:16.5.6:8.11.10,9:8:21:16.5.6,THB:15:16:21:8.11.10| Show InChI InChI=1S/C27H27N3O6/c1-29-14-13-26-23-17-6-9-20(35-2)24(23)36-25(26)19(31)11-12-27(26,21(29)15-17)28-22(32)10-5-16-3-7-18(8-4-16)30(33)34/h3-10,21,25H,11-15H2,1-2H3,(H,28,32)/b10-5-/t21?,25-,26?,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rochester
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 304-11 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8XJC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50452520
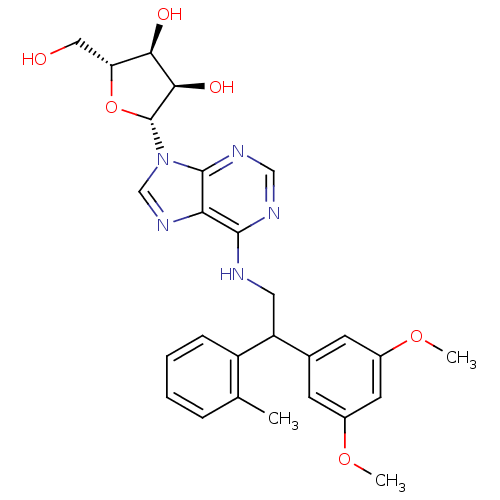
(CHEMBL2094089 | N6-[2-(3,5-dimethoxyphenyl)-2-(2-m...)Show SMILES COc1cc(OC)cc(c1)C(CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)c1ccccc1C |r| Show InChI InChI=1S/C27H31N5O6/c1-15-6-4-5-7-19(15)20(16-8-17(36-2)10-18(9-16)37-3)11-28-25-22-26(30-13-29-25)32(14-31-22)27-24(35)23(34)21(12-33)38-27/h4-10,13-14,20-21,23-24,27,33-35H,11-12H2,1-3H3,(H,28,29,30)/t20?,21-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50452520

(CHEMBL2094089 | N6-[2-(3,5-dimethoxyphenyl)-2-(2-m...)Show SMILES COc1cc(OC)cc(c1)C(CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)c1ccccc1C |r| Show InChI InChI=1S/C27H31N5O6/c1-15-6-4-5-7-19(15)20(16-8-17(36-2)10-18(9-16)37-3)11-28-25-22-26(30-13-29-25)32(14-31-22)27-24(35)23(34)21(12-33)38-27/h4-10,13-14,20-21,23-24,27,33-35H,11-12H2,1-3H3,(H,28,29,30)/t20?,21-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50452520

(CHEMBL2094089 | N6-[2-(3,5-dimethoxyphenyl)-2-(2-m...)Show SMILES COc1cc(OC)cc(c1)C(CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)c1ccccc1C |r| Show InChI InChI=1S/C27H31N5O6/c1-15-6-4-5-7-19(15)20(16-8-17(36-2)10-18(9-16)37-3)11-28-25-22-26(30-13-29-25)32(14-31-22)27-24(35)23(34)21(12-33)38-27/h4-10,13-14,20-21,23-24,27,33-35H,11-12H2,1-3H3,(H,28,29,30)/t20?,21-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50207816

(CHEMBL273094 | N-(2-Amino-ethyl)-2-[4-(2,6-dioxo-1...)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCN)cc1 Show InChI InChI=1S/C21H28N6O4/c1-3-11-26-19-17(20(29)27(12-4-2)21(26)30)24-18(25-19)14-5-7-15(8-6-14)31-13-16(28)23-10-9-22/h5-8H,3-4,9-13,22H2,1-2H3,(H,23,28)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50432670
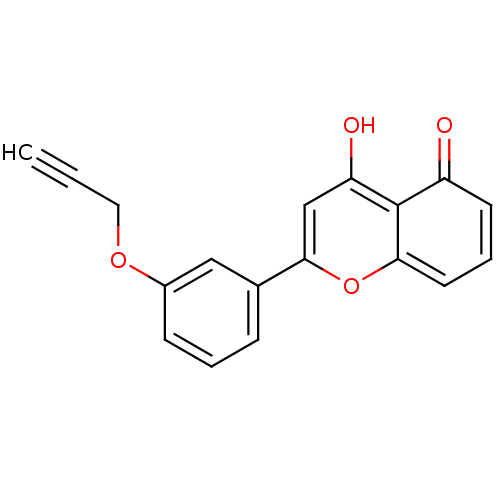
(CHEMBL2347914)Show InChI InChI=1S/C18H12O4/c1-2-9-21-13-6-3-5-12(10-13)17-11-15(20)18-14(19)7-4-8-16(18)22-17/h1,3-8,10-11,20H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Irreversible inhibition of CYP1A2 (unknown origin)-mediated demethylation of resorufin methyl ether after 5 mins by spectrofluorimetric analysis |
J Med Chem 56: 4082-92 (2013)
Article DOI: 10.1021/jm4003654
BindingDB Entry DOI: 10.7270/Q2CN758W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50432670

(CHEMBL2347914)Show InChI InChI=1S/C18H12O4/c1-2-9-21-13-6-3-5-12(10-13)17-11-15(20)18-14(19)7-4-8-16(18)22-17/h1,3-8,10-11,20H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Irreversible inhibition of CYP1A1 (unknown origin)-mediated deethylation of resorufin ethyl ether after 5 mins by spectrofluorimetric analysis |
J Med Chem 56: 4082-92 (2013)
Article DOI: 10.1021/jm4003654
BindingDB Entry DOI: 10.7270/Q2CN758W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85790

(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181669
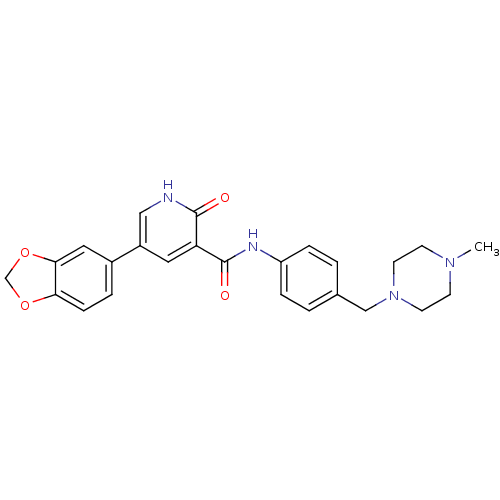
(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 788 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against ALK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM16173
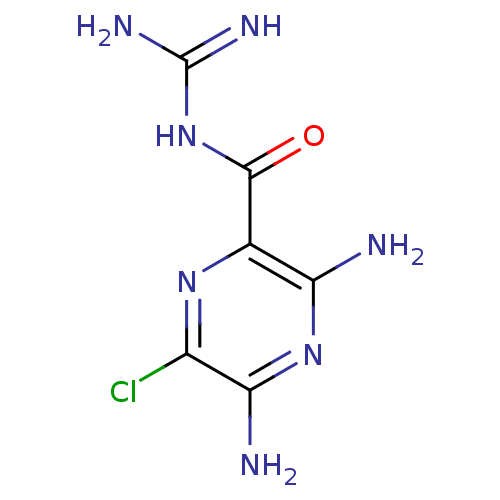
(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181669

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C25H26N4O4/c1-28-8-10-29(11-9-28)15-17-2-5-20(6-3-17)27-25(31)21-12-19(14-26-24(21)30)18-4-7-22-23(13-18)33-16-32-22/h2-7,12-14H,8-11,15-16H2,1H3,(H,26,30)(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181670

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2cc(c[nH]c2=O)-c2ccc3OCOc3c2)cc1 Show InChI InChI=1S/C24H24N4O4/c1-27-8-10-28(11-9-27)19-5-3-18(4-6-19)26-24(30)20-12-17(14-25-23(20)29)16-2-7-21-22(13-16)32-15-31-21/h2-7,12-14H,8-11,15H2,1H3,(H,25,29)(H,26,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50181675

(5-benzo[1,3]dioxol-5-yl-2-oxo-1,2-dihydro-pyridine...)Show SMILES CN1CCN(CCCc2ccc(NC(=O)c3cc(c[nH]c3=O)-c3ccc4OCOc4c3)cc2)CC1 Show InChI InChI=1S/C27H30N4O4/c1-30-11-13-31(14-12-30)10-2-3-19-4-7-22(8-5-19)29-27(33)23-15-21(17-28-26(23)32)20-6-9-24-25(16-20)35-18-34-24/h4-9,15-17H,2-3,10-14,18H2,1H3,(H,28,32)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ChemBridge Research Laboratories and ChemBridge Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against IRK |
J Med Chem 49: 1006-15 (2006)
Article DOI: 10.1021/jm050824x
BindingDB Entry DOI: 10.7270/Q2H994T6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM16173

(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM16173

(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50049391
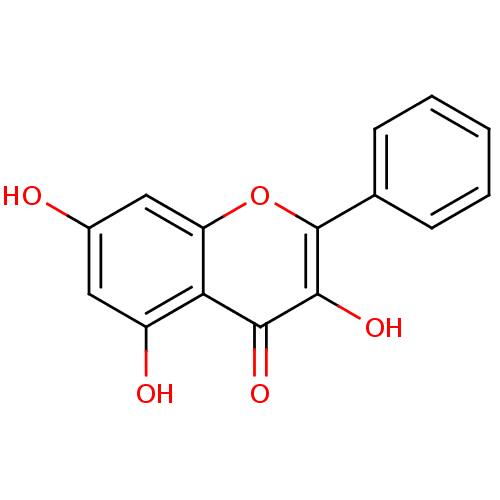
(3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...)Show InChI InChI=1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50049391

(3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...)Show InChI InChI=1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50336640

((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...)Show SMILES COC(=O)C1C(C(C(=O)OC)=C(C)N=C1C)c1ccccc1[N+]([O-])=O |c:13,t:10| Show InChI InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,13,15H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50336640

((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...)Show SMILES COC(=O)C1C(C(C(=O)OC)=C(C)N=C1C)c1ccccc1[N+]([O-])=O |c:13,t:10| Show InChI InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,13,15H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50049391

(3,5,7-Trihydroxyflavone | 3,5,7-triOH-flavone | 3,...)Show InChI InChI=1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048454
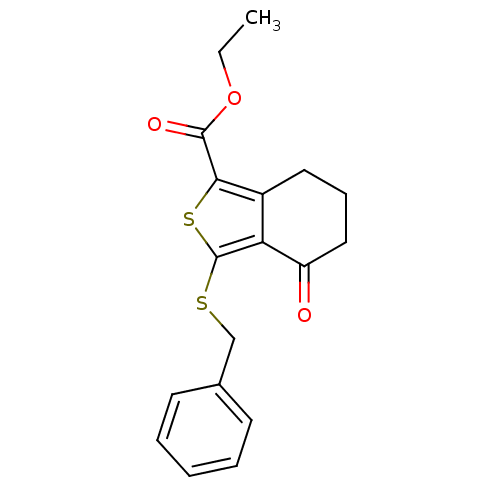
(3-Benzylsulfanyl-4-oxo-4,5,6,7-tetrahydro-benzo[c]...)Show InChI InChI=1S/C18H18O3S2/c1-2-21-17(20)16-13-9-6-10-14(19)15(13)18(23-16)22-11-12-7-4-3-5-8-12/h3-5,7-8H,2,6,9-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells |
J Med Chem 40: 2588-95 (1997)
Article DOI: 10.1021/jm970084v
BindingDB Entry DOI: 10.7270/Q27M08MC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data