 Found 873 hits with Last Name = 'jin' and Initial = 'b'
Found 873 hits with Last Name = 'jin' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204090

(CHEMBL3958859)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1cc(-[#8])cc(-[#16]-c2ccccc2-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C33H42O4S/c1-23(2)11-8-12-24(3)13-9-14-25(4)15-10-16-26(5)19-20-27-21-28(34)22-31(32(27)35)38-30-18-7-6-17-29(30)33(36)37/h6-7,11,13,15,17-19,21-22,34-35H,8-10,12,14,16,20H2,1-5H3,(H,36,37)/b24-13+,25-15+,26-19+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204086
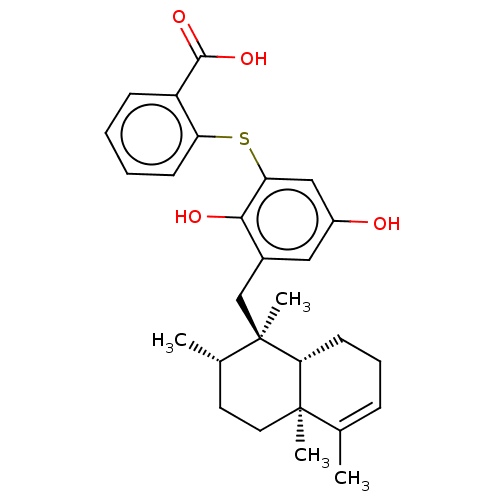
(Avarol-3''-Thiosalicylate | CHEMBL238756)Show SMILES [H][C@@]12CCC=C(C)[C@@]1(C)CC[C@H](C)[C@@]2(C)Cc1cc(O)cc(Sc2ccccc2C(O)=O)c1O |r,t:4| Show InChI InChI=1S/C28H34O4S/c1-17-8-7-11-24-27(17,3)13-12-18(2)28(24,4)16-19-14-20(29)15-23(25(19)30)33-22-10-6-5-9-21(22)26(31)32/h5-6,8-10,14-15,18,24,29-30H,7,11-13,16H2,1-4H3,(H,31,32)/t18-,24+,27+,28+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50204087
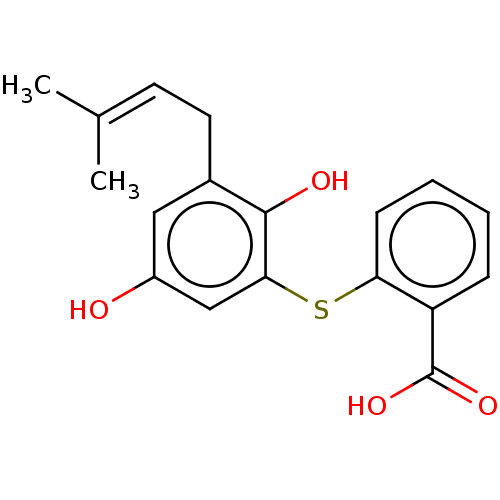
(CHEMBL3920392)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-c1cc(-[#8])cc(-[#16]-c2ccccc2-[#6](-[#8])=O)c1-[#8] Show InChI InChI=1S/C18H18O4S/c1-11(2)7-8-12-9-13(19)10-16(17(12)20)23-15-6-4-3-5-14(15)18(21)22/h3-7,9-10,19-20H,8H2,1-2H3,(H,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate by Dixon plot analysis |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223
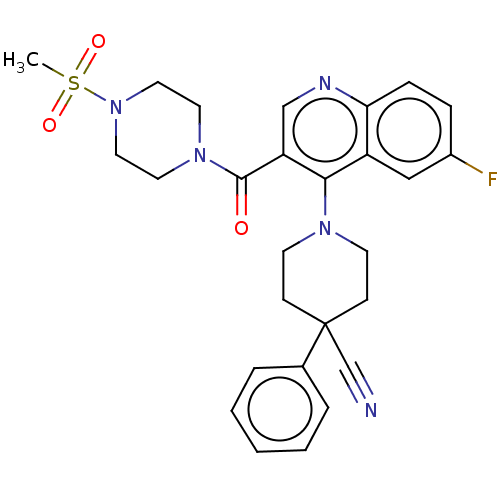
(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456222
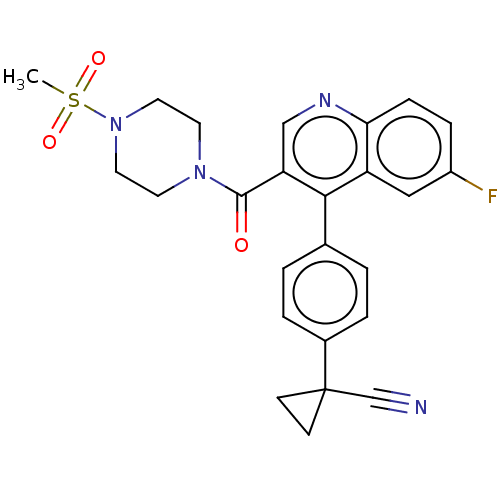
(CHEMBL4206272)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23FN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using propionaldehyde as substrate and varied concentration of NAD+ as cofactor preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456222

(CHEMBL4206272)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23FN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223

(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+ as cofactor and varied concentration of propionaldehyde as substrate preincubated for 15 mins followed by subs... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50606398
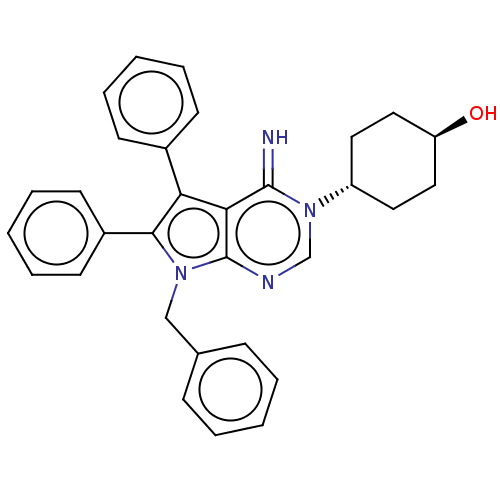
(ML-246 | Metarrestin | Ml-246)Show SMILES O[C@H]1CC[C@@H](CC1)n1cnc2n(Cc3ccccc3)c(c(-c3ccccc3)c2c1=N)-c1ccccc1 |wU:4.7,wD:1.0,(20.99,-11.86,;22.33,-11.09,;23.66,-11.86,;25,-11.09,;25,-9.55,;23.66,-8.78,;22.33,-9.55,;26.33,-8.78,;26.33,-7.24,;27.66,-6.47,;29,-7.24,;30.46,-6.76,;30.94,-5.3,;29.91,-4.15,;30.38,-2.69,;29.35,-1.55,;27.85,-1.87,;27.37,-3.33,;28.4,-4.47,;31.37,-8.01,;30.46,-9.26,;30.94,-10.72,;32.44,-11.04,;32.92,-12.5,;31.89,-13.65,;30.38,-13.33,;29.91,-11.86,;29,-8.78,;27.66,-9.55,;27.66,-11.09,;32.91,-8.01,;33.68,-6.68,;35.22,-6.68,;35.99,-8.01,;35.22,-9.34,;33.68,-9.34,)| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00204
BindingDB Entry DOI: 10.7270/Q2FN1B9R |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50606398

(ML-246 | Metarrestin | Ml-246)Show SMILES O[C@H]1CC[C@@H](CC1)n1cnc2n(Cc3ccccc3)c(c(-c3ccccc3)c2c1=N)-c1ccccc1 |wU:4.7,wD:1.0,(20.99,-11.86,;22.33,-11.09,;23.66,-11.86,;25,-11.09,;25,-9.55,;23.66,-8.78,;22.33,-9.55,;26.33,-8.78,;26.33,-7.24,;27.66,-6.47,;29,-7.24,;30.46,-6.76,;30.94,-5.3,;29.91,-4.15,;30.38,-2.69,;29.35,-1.55,;27.85,-1.87,;27.37,-3.33,;28.4,-4.47,;31.37,-8.01,;30.46,-9.26,;30.94,-10.72,;32.44,-11.04,;32.92,-12.5,;31.89,-13.65,;30.38,-13.33,;29.91,-11.86,;29,-8.78,;27.66,-9.55,;27.66,-11.09,;32.91,-8.01,;33.68,-6.68,;35.22,-6.68,;35.99,-8.01,;35.22,-9.34,;33.68,-9.34,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00204
BindingDB Entry DOI: 10.7270/Q2FN1B9R |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50606398

(ML-246 | Metarrestin | Ml-246)Show SMILES O[C@H]1CC[C@@H](CC1)n1cnc2n(Cc3ccccc3)c(c(-c3ccccc3)c2c1=N)-c1ccccc1 |wU:4.7,wD:1.0,(20.99,-11.86,;22.33,-11.09,;23.66,-11.86,;25,-11.09,;25,-9.55,;23.66,-8.78,;22.33,-9.55,;26.33,-8.78,;26.33,-7.24,;27.66,-6.47,;29,-7.24,;30.46,-6.76,;30.94,-5.3,;29.91,-4.15,;30.38,-2.69,;29.35,-1.55,;27.85,-1.87,;27.37,-3.33,;28.4,-4.47,;31.37,-8.01,;30.46,-9.26,;30.94,-10.72,;32.44,-11.04,;32.92,-12.5,;31.89,-13.65,;30.38,-13.33,;29.91,-11.86,;29,-8.78,;27.66,-9.55,;27.66,-11.09,;32.91,-8.01,;33.68,-6.68,;35.22,-6.68,;35.99,-8.01,;35.22,-9.34,;33.68,-9.34,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00204
BindingDB Entry DOI: 10.7270/Q2FN1B9R |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50606398

(ML-246 | Metarrestin | Ml-246)Show SMILES O[C@H]1CC[C@@H](CC1)n1cnc2n(Cc3ccccc3)c(c(-c3ccccc3)c2c1=N)-c1ccccc1 |wU:4.7,wD:1.0,(20.99,-11.86,;22.33,-11.09,;23.66,-11.86,;25,-11.09,;25,-9.55,;23.66,-8.78,;22.33,-9.55,;26.33,-8.78,;26.33,-7.24,;27.66,-6.47,;29,-7.24,;30.46,-6.76,;30.94,-5.3,;29.91,-4.15,;30.38,-2.69,;29.35,-1.55,;27.85,-1.87,;27.37,-3.33,;28.4,-4.47,;31.37,-8.01,;30.46,-9.26,;30.94,-10.72,;32.44,-11.04,;32.92,-12.5,;31.89,-13.65,;30.38,-13.33,;29.91,-11.86,;29,-8.78,;27.66,-9.55,;27.66,-11.09,;32.91,-8.01,;33.68,-6.68,;35.22,-6.68,;35.99,-8.01,;35.22,-9.34,;33.68,-9.34,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00204
BindingDB Entry DOI: 10.7270/Q2FN1B9R |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606398

(ML-246 | Metarrestin | Ml-246)Show SMILES O[C@H]1CC[C@@H](CC1)n1cnc2n(Cc3ccccc3)c(c(-c3ccccc3)c2c1=N)-c1ccccc1 |wU:4.7,wD:1.0,(20.99,-11.86,;22.33,-11.09,;23.66,-11.86,;25,-11.09,;25,-9.55,;23.66,-8.78,;22.33,-9.55,;26.33,-8.78,;26.33,-7.24,;27.66,-6.47,;29,-7.24,;30.46,-6.76,;30.94,-5.3,;29.91,-4.15,;30.38,-2.69,;29.35,-1.55,;27.85,-1.87,;27.37,-3.33,;28.4,-4.47,;31.37,-8.01,;30.46,-9.26,;30.94,-10.72,;32.44,-11.04,;32.92,-12.5,;31.89,-13.65,;30.38,-13.33,;29.91,-11.86,;29,-8.78,;27.66,-9.55,;27.66,-11.09,;32.91,-8.01,;33.68,-6.68,;35.22,-6.68,;35.99,-8.01,;35.22,-9.34,;33.68,-9.34,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00204
BindingDB Entry DOI: 10.7270/Q2FN1B9R |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Klebsiella pneumoniae) | BDBM50548231
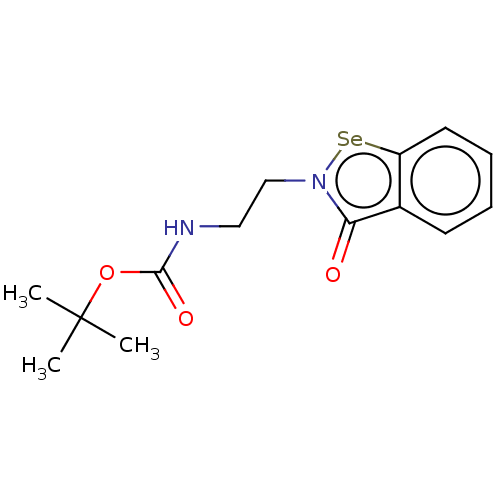
(CHEMBL4753599)Show SMILES [#6]C([#6])([#6])[#8]-[#6](=O)-[#7]-[#6]-[#6]-n1[se;v2]c2ccccc2c1=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type N-terminal His6-tagged Klebsiella pneumoniae NDM-1 expressed in Escherichia coli BL21 assessed as inhibition constant using n... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2018.06.007
BindingDB Entry DOI: 10.7270/Q2SB49CC |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355
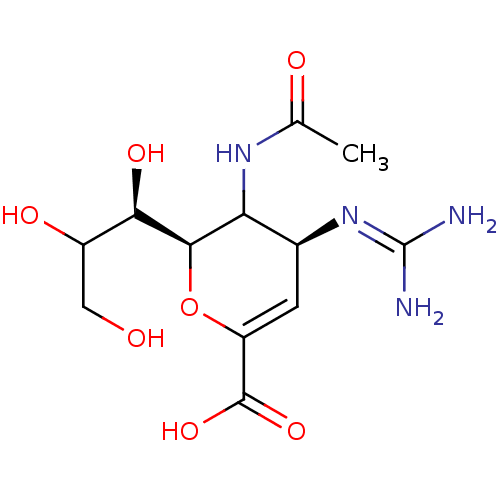
((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6?,8?,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against neuraminidase of influenza A/Mem/Bel/71 (H3N1) virus starin; (n=5) |
J Med Chem 48: 2964-71 (2005)
Article DOI: 10.1021/jm040891b
BindingDB Entry DOI: 10.7270/Q2CJ8D10 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Chimica Biomolecolare (ICB)
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate incubated for 15 mins followed by substrate addition by Ellman's method |
Eur J Med Chem 122: 326-338 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.036
BindingDB Entry DOI: 10.7270/Q2QJ7K8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355

((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6?,8?,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.48 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against neuraminidase of influenza A/PR/8/34 (H1N1) virus starin; (n=5) |
J Med Chem 48: 2964-71 (2005)
Article DOI: 10.1021/jm040891b
BindingDB Entry DOI: 10.7270/Q2CJ8D10 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337934

((R)-3-(2,3-dihydroxypropoxy)-6-fluoro-5-(2-fluoro-...)Show SMILES Cn1c2ncn(OC[C@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)c(F)c1=O |r| Show InChI InChI=1S/C17H15F2IN4O5/c1-23-15-12(16(27)24(7-21-15)29-6-9(26)5-25)14(13(19)17(23)28)22-11-3-2-8(20)4-10(11)18/h2-4,7,9,22,25-26H,5-6H2,1H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337929
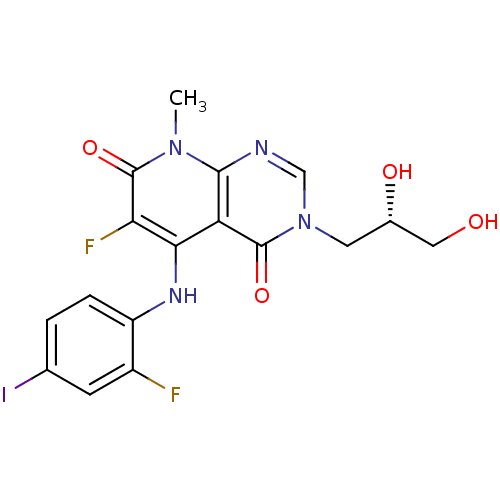
((S)-3-(2,3-dihydroxypropyl)-6-fluoro-5-(2-fluoro-4...)Show SMILES Cn1c2ncn(C[C@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)c(F)c1=O |r| Show InChI InChI=1S/C17H15F2IN4O4/c1-23-15-12(16(27)24(7-21-15)5-9(26)6-25)14(13(19)17(23)28)22-11-3-2-8(20)4-10(11)18/h2-4,7,9,22,25-26H,5-6H2,1H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337926
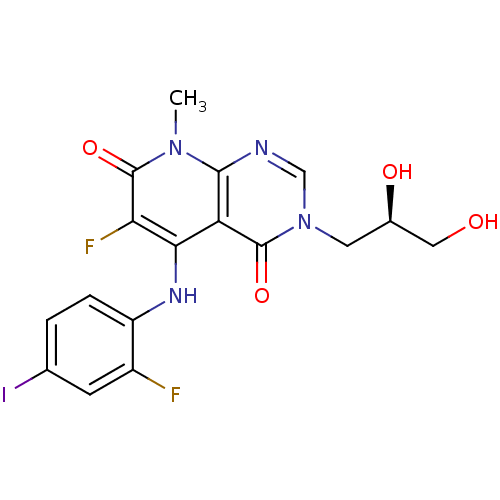
((R)-3-(2,3-dihydroxypropyl)-6-fluoro-5-(2-fluoro-4...)Show SMILES Cn1c2ncn(C[C@@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)c(F)c1=O |r| Show InChI InChI=1S/C17H15F2IN4O4/c1-23-15-12(16(27)24(7-21-15)5-9(26)6-25)14(13(19)17(23)28)22-11-3-2-8(20)4-10(11)18/h2-4,7,9,22,25-26H,5-6H2,1H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165355

((2R,4S)-3-(acetylamino)-4-{[(E)-amino(imino)methyl...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6?,8?,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against neuraminidase of influenza A/Chicken/Vietnam/8/2004(H5N1) virus starin; (n=5) |
J Med Chem 48: 2964-71 (2005)
Article DOI: 10.1021/jm040891b
BindingDB Entry DOI: 10.7270/Q2CJ8D10 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337933
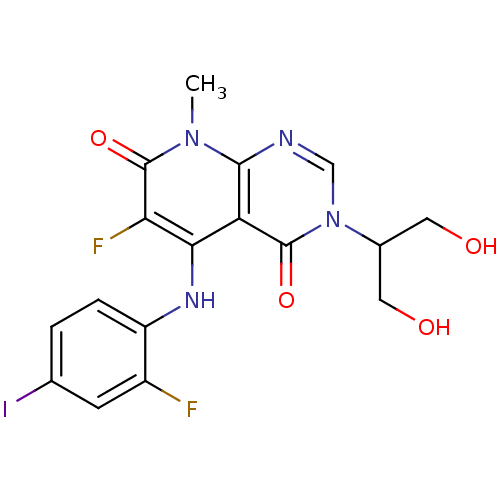
(3-(1,3-dihydroxypropan-2-yl)-6-fluoro-5-(2-fluoro-...)Show SMILES Cn1c2ncn(C(CO)CO)c(=O)c2c(Nc2ccc(I)cc2F)c(F)c1=O Show InChI InChI=1S/C17H15F2IN4O4/c1-23-15-12(16(27)24(7-21-15)9(5-25)6-26)14(13(19)17(23)28)22-11-3-2-8(20)4-10(11)18/h2-4,7,9,22,25-26H,5-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM1711
(CHEMBL1684067 | US8470837, 8)Show SMILES Cc1c(Nc2ccc(I)cc2F)c2c(ncn(C[C@@H](O)CO)c2=O)n(C)c1=O |r| Show InChI InChI=1S/C18H18FIN4O4/c1-9-15(22-13-4-3-10(20)5-12(13)19)14-16(23(2)17(9)27)21-8-24(18(14)28)6-11(26)7-25/h3-5,8,11,22,25-26H,6-7H2,1-2H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337932
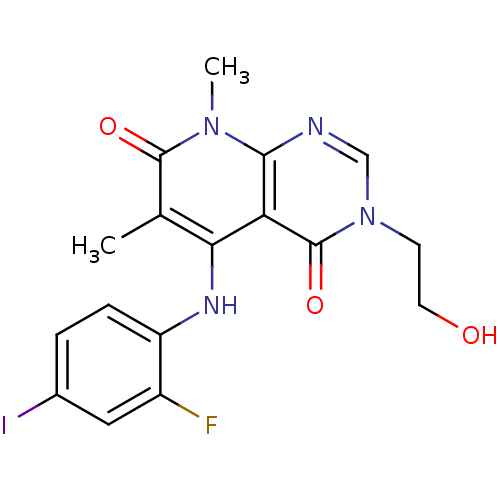
(5-(2-fluoro-4-iodophenylamino)-3-(2-hydroxyethyl)-...)Show SMILES Cc1c(Nc2ccc(I)cc2F)c2c(ncn(CCO)c2=O)n(C)c1=O Show InChI InChI=1S/C17H16FIN4O3/c1-9-14(21-12-4-3-10(19)7-11(12)18)13-15(22(2)16(9)25)20-8-23(5-6-24)17(13)26/h3-4,7-8,21,24H,5-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337920

((R)-3-(2,3-dihydroxypropyl)-5-(2-fluoro-4-iodophen...)Show SMILES Cn1c2ncn(C[C@@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)cc1=O |r| Show InChI InChI=1S/C17H16FIN4O4/c1-22-14(26)5-13(21-12-3-2-9(19)4-11(12)18)15-16(22)20-8-23(17(15)27)6-10(25)7-24/h2-5,8,10,21,24-25H,6-7H2,1H3/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337928
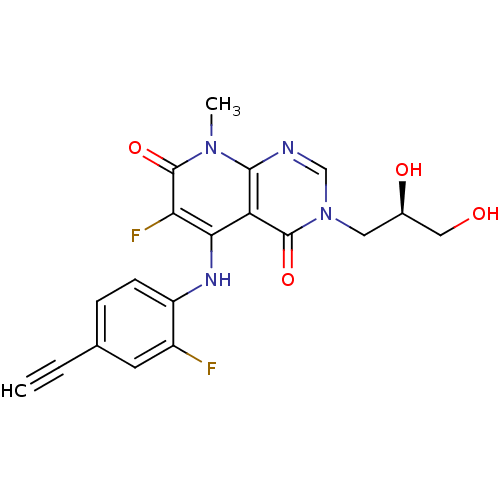
((R)-3-(2,3-dihydroxypropyl)-5-(4-ethynyl-2-fluorop...)Show SMILES Cn1c2ncn(C[C@@H](O)CO)c(=O)c2c(Nc2ccc(cc2F)C#C)c(F)c1=O |r| Show InChI InChI=1S/C19H16F2N4O4/c1-3-10-4-5-13(12(20)6-10)23-16-14-17(24(2)19(29)15(16)21)22-9-25(18(14)28)7-11(27)8-26/h1,4-6,9,11,23,26-27H,7-8H2,2H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337937

(5-(2-fluoro-4-iodophenylamino)-3-(3-hydroxypropyl)...)Show InChI InChI=1S/C17H16FIN4O3/c1-22-14(25)8-13(21-12-4-3-10(19)7-11(12)18)15-16(22)20-9-23(17(15)26)5-2-6-24/h3-4,7-9,21,24H,2,5-6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
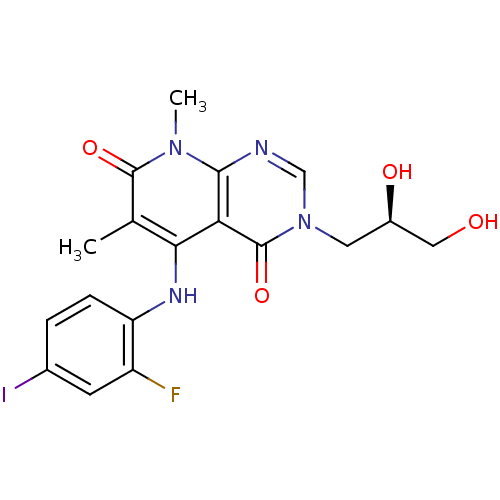
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM1714
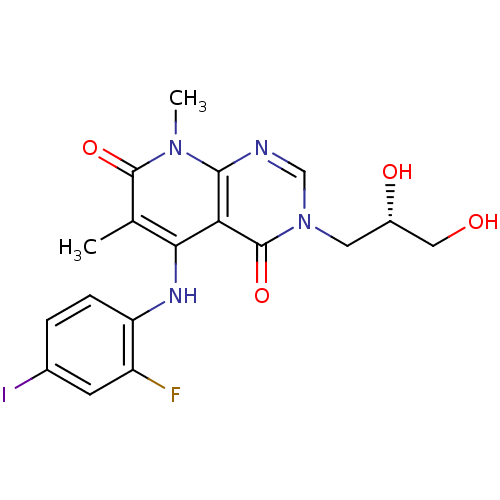
(CHEMBL1684071 | US8470837, 13)Show SMILES Cc1c(Nc2ccc(I)cc2F)c2c(ncn(C[C@H](O)CO)c2=O)n(C)c1=O |r| Show InChI InChI=1S/C18H18FIN4O4/c1-9-15(22-13-4-3-10(20)5-12(13)19)14-16(23(2)17(9)27)21-8-24(18(14)28)6-11(26)7-25/h3-5,8,11,22,25-26H,6-7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456309

(CHEMBL4207222)Show SMILES COc1ccc2c(-c3ccc(cc3)C3(CC3)C#N)c(cnc2c1)C(=O)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C26H26N4O4S/c1-34-20-7-8-21-23(15-20)28-16-22(25(31)29-11-13-30(14-12-29)35(2,32)33)24(21)18-3-5-19(6-4-18)26(17-27)9-10-26/h3-8,15-16H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50337924
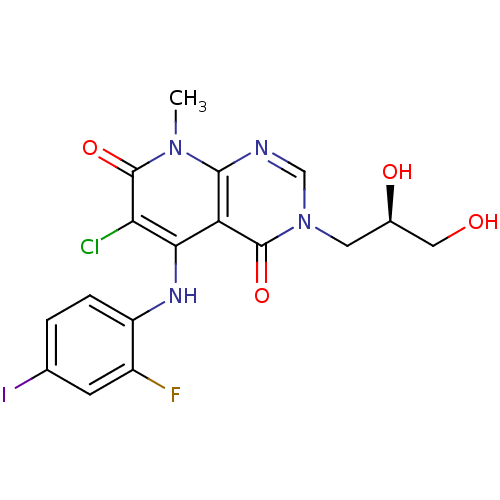
((R)-6-chloro-3-(2,3-dihydroxypropyl)-5-(2-fluoro-4...)Show SMILES Cn1c2ncn(C[C@@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)c(Cl)c1=O |r| Show InChI InChI=1S/C17H15ClFIN4O4/c1-23-15-12(16(27)24(7-21-15)5-9(26)6-25)14(13(18)17(23)28)22-11-3-2-8(20)4-10(11)19/h2-4,7,9,22,25-26H,5-6H2,1H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456228

(CHEMBL4215704)Show SMILES CC(C)(C#N)c1ccc(cc1)-c1c(cnc2ccc(F)cc12)C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C28H27FN4O2/c1-28(2,17-30)20-7-5-18(6-8-20)25-22-15-21(29)9-10-24(22)31-16-23(25)27(35)33-13-11-32(12-14-33)26(34)19-3-4-19/h5-10,15-16,19H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456233

(CHEMBL4211904)Show SMILES Fc1ccc2ncc(C(=O)N3CCN(CC3)C(=O)C3CC3)c(-c3ccc(cc3)C3(CC3)C#N)c2c1 Show InChI InChI=1S/C28H25FN4O2/c29-21-7-8-24-22(15-21)25(18-3-5-20(6-4-18)28(17-30)9-10-28)23(16-31-24)27(35)33-13-11-32(12-14-33)26(34)19-1-2-19/h3-8,15-16,19H,1-2,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456224
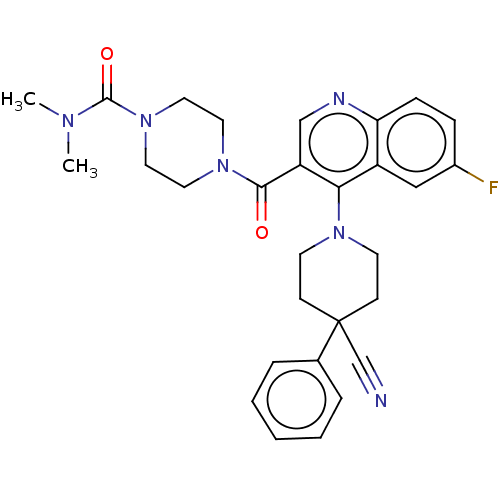
(CHEMBL4207514)Show SMILES CN(C)C(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C29H31FN6O2/c1-33(2)28(38)36-16-14-35(15-17-36)27(37)24-19-32-25-9-8-22(30)18-23(25)26(24)34-12-10-29(20-31,11-13-34)21-6-4-3-5-7-21/h3-9,18-19H,10-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456249

(CHEMBL4212671)Show SMILES CN(C)S(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C26H26FN5O3S/c1-30(2)36(34,35)32-13-11-31(12-14-32)25(33)22-16-29-23-8-7-20(27)15-21(23)24(22)18-3-5-19(6-4-18)26(17-28)9-10-26/h3-8,15-16H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223

(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 30 uM after... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456235
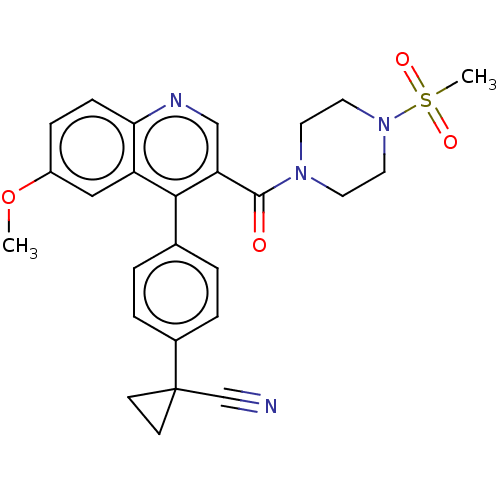
(CHEMBL4209722)Show SMILES COc1ccc2ncc(C(=O)N3CCN(CC3)S(C)(=O)=O)c(-c3ccc(cc3)C3(CC3)C#N)c2c1 Show InChI InChI=1S/C26H26N4O4S/c1-34-20-7-8-23-21(15-20)24(18-3-5-19(6-4-18)26(17-27)9-10-26)22(16-28-23)25(31)29-11-13-30(14-12-29)35(2,32)33/h3-8,15-16H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456216
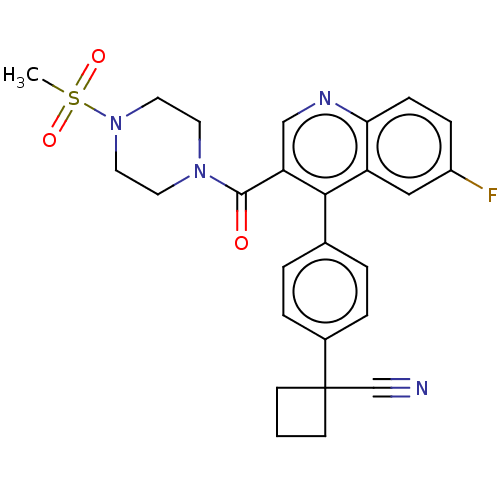
(CHEMBL4206606)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CCC1)C#N Show InChI InChI=1S/C26H25FN4O3S/c1-35(33,34)31-13-11-30(12-14-31)25(32)22-16-29-23-8-7-20(27)15-21(23)24(22)18-3-5-19(6-4-18)26(17-28)9-2-10-26/h3-8,15-16H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456254
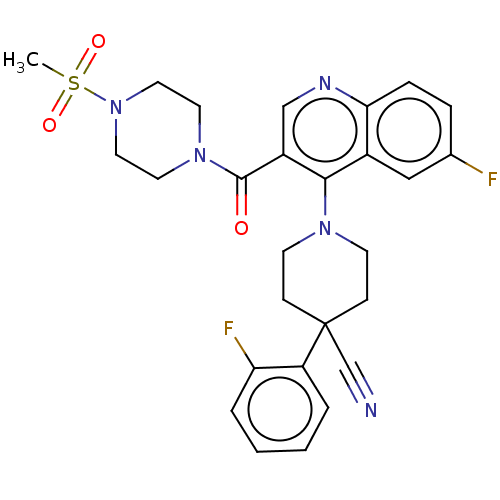
(CHEMBL4202680)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1F Show InChI InChI=1S/C27H27F2N5O3S/c1-38(36,37)34-14-12-33(13-15-34)26(35)21-17-31-24-7-6-19(28)16-20(24)25(21)32-10-8-27(18-30,9-11-32)22-4-2-3-5-23(22)29/h2-7,16-17H,8-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223

(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456232

(CHEMBL4207617)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(Cl)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23ClN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456222

(CHEMBL4206272)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H23FN4O3S/c1-34(32,33)30-12-10-29(11-13-30)24(31)21-15-28-22-7-6-19(26)14-20(22)23(21)17-2-4-18(5-3-17)25(16-27)8-9-25/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50165356

((2R,4S)-4-carbamimidamido-2-[(1R)-1-({[12-({[(1R)-...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-1-[#6@@H](-[#8]-[#6](=[#6]-[#6@@H]-1\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O)-[#6@H](-[#8]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#8]-[#6@H](-[#6](-[#8])-[#6]-[#8])-[#6@@H]-1-[#8]-[#6](=[#6]-[#6@H](\[#7]=[#6](\[#7])-[#7])-[#6]-1-[#7]-[#6](-[#6])=O)-[#6](-[#8])=O)-[#6](-[#8])-[#6]-[#8] |c:7,46| Show InChI InChI=1S/C38H64N10O16/c1-19(51)45-27-21(47-35(39)40)15-25(33(55)56)61-31(27)29(23(53)17-49)63-37(59)43-13-11-9-7-5-3-4-6-8-10-12-14-44-38(60)64-30(24(54)18-50)32-28(46-20(2)52)22(48-36(41)42)16-26(62-32)34(57)58/h15-16,21-24,27-32,49-50,53-54H,3-14,17-18H2,1-2H3,(H,43,59)(H,44,60)(H,45,51)(H,46,52)(H,55,56)(H,57,58)(H4,39,40,47)(H4,41,42,48)/t21-,22-,23?,24?,27?,28?,29+,30+,31+,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.86 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibitory concentration against neuraminidase of influenza A/Mem/Bel/71 (H3N1) virus starin; (n=5) |
J Med Chem 48: 2964-71 (2005)
Article DOI: 10.1021/jm040891b
BindingDB Entry DOI: 10.7270/Q2CJ8D10 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456267
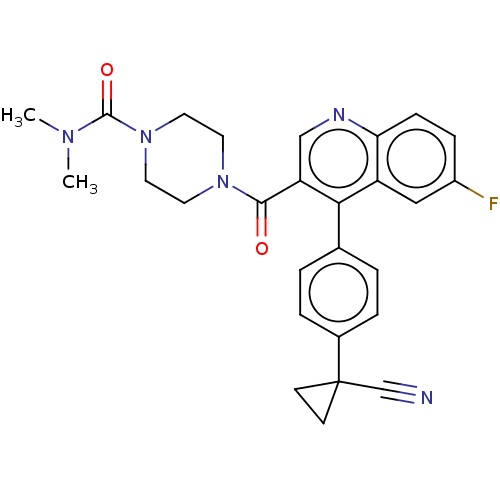
(CHEMBL4218688)Show SMILES CN(C)C(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C27H26FN5O2/c1-31(2)26(35)33-13-11-32(12-14-33)25(34)22-16-30-23-8-7-20(28)15-21(23)24(22)18-3-5-19(6-4-18)27(17-29)9-10-27/h3-8,15-16H,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456247

(CHEMBL4213848)Show SMILES Fc1ccc2ncc(C(=O)N3CCN(CC3)C(=O)C3CC3)c(N3CCC(CC3)(C#N)c3ccccc3)c2c1 Show InChI InChI=1S/C30H30FN5O2/c31-23-8-9-26-24(18-23)27(34-12-10-30(20-32,11-13-34)22-4-2-1-3-5-22)25(19-33-26)29(38)36-16-14-35(15-17-36)28(37)21-6-7-21/h1-5,8-9,18-19,21H,6-7,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456265

(CHEMBL4212891)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2cc(F)c(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H22F2N4O3S/c1-35(33,34)31-10-8-30(9-11-31)24(32)19-14-29-22-13-21(27)20(26)12-18(22)23(19)16-2-4-17(5-3-16)25(15-28)6-7-25/h2-5,12-14H,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM1710
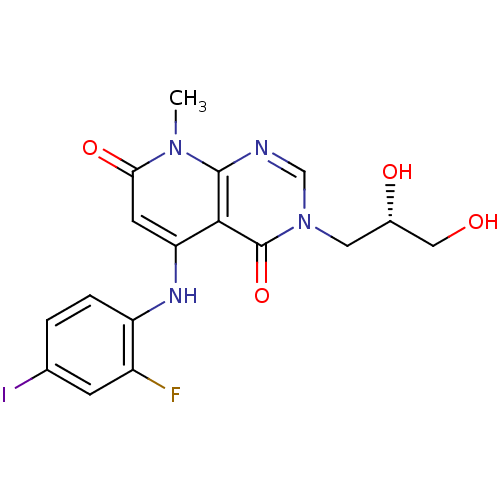
(CHEMBL1684064 | US8470837, 5)Show SMILES Cn1c2ncn(C[C@H](O)CO)c(=O)c2c(Nc2ccc(I)cc2F)cc1=O |r| Show InChI InChI=1S/C17H16FIN4O4/c1-22-14(26)5-13(21-12-3-2-9(19)4-11(12)18)15-16(22)20-8-23(17(15)27)6-10(25)7-24/h2-5,8,10,21,24-25H,6-7H2,1H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
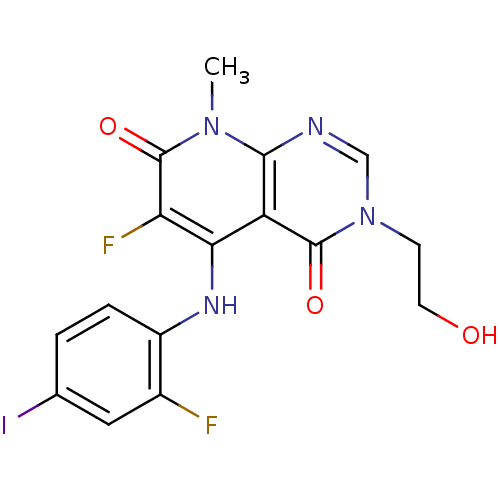
(Homo sapiens (Human)) | BDBM1716
(CHEMBL1684072 | US8470837, 17)Show SMILES Cn1c2ncn(CCO)c(=O)c2c(Nc2ccc(I)cc2F)c(F)c1=O Show InChI InChI=1S/C16H13F2IN4O3/c1-22-14-11(15(25)23(4-5-24)7-20-14)13(12(18)16(22)26)21-10-3-2-8(19)6-9(10)17/h2-3,6-7,21,24H,4-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda San Diego
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 by IMAP assay |
Bioorg Med Chem Lett 21: 1315-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.071
BindingDB Entry DOI: 10.7270/Q2833SB8 |
More data for this
Ligand-Target Pair | |
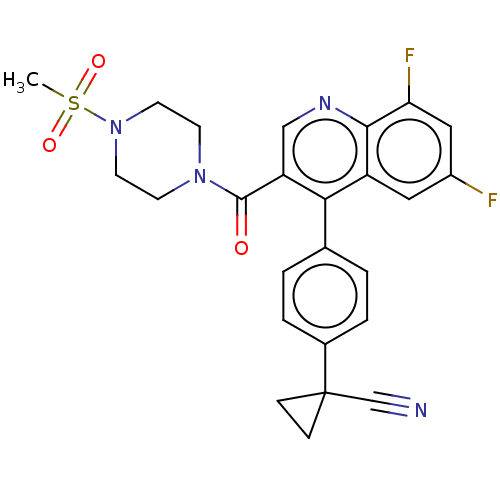
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456244
(CHEMBL4213604)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2c(F)cc(F)cc2c1-c1ccc(cc1)C1(CC1)C#N Show InChI InChI=1S/C25H22F2N4O3S/c1-35(33,34)31-10-8-30(9-11-31)24(32)20-14-29-23-19(12-18(26)13-21(23)27)22(20)16-2-4-17(5-3-16)25(15-28)6-7-25/h2-5,12-14H,6-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456312

(CHEMBL4217115)Show SMILES CC(C)(C#N)c1ccc(c(F)c1)-c1c(cnc2ccc(F)cc12)C(=O)N1CCN(CC1)S(C)(=O)=O |(35.43,.37,;34.1,-.41,;34.1,1.13,;32.77,.34,;31.42,1.11,;34.11,-1.96,;32.77,-2.73,;32.77,-4.28,;34.1,-5.03,;35.44,-4.27,;36.77,-5.04,;35.44,-2.73,;34.11,-6.57,;32.78,-7.34,;32.78,-8.89,;34.11,-9.66,;35.44,-8.88,;36.78,-9.65,;38.11,-8.87,;38.11,-7.32,;39.43,-6.54,;36.77,-6.56,;35.44,-7.34,;31.45,-6.57,;31.45,-5.03,;30.11,-7.34,;28.77,-6.56,;27.45,-7.32,;27.44,-8.86,;28.77,-9.64,;30.11,-8.87,;26.1,-9.63,;24.76,-8.85,;26.86,-10.96,;25.33,-10.95,)| Show InChI InChI=1S/C25H24F2N4O3S/c1-25(2,15-28)16-4-6-18(21(27)12-16)23-19-13-17(26)5-7-22(19)29-14-20(23)24(32)30-8-10-31(11-9-30)35(3,33)34/h4-7,12-14H,8-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
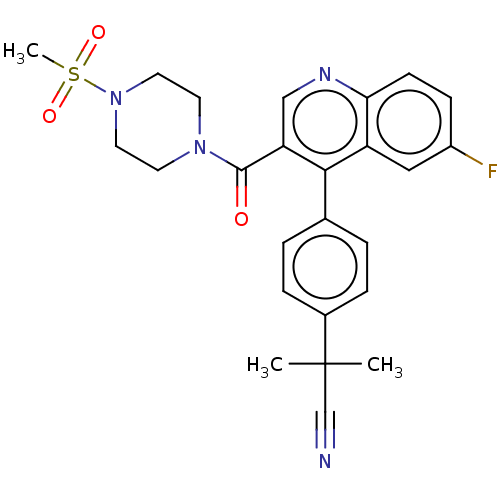
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456217
(CHEMBL4214724)Show SMILES CC(C)(C#N)c1ccc(cc1)-c1c(cnc2ccc(F)cc12)C(=O)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C25H25FN4O3S/c1-25(2,16-27)18-6-4-17(5-7-18)23-20-14-19(26)8-9-22(20)28-15-21(23)24(31)29-10-12-30(13-11-29)34(3,32)33/h4-9,14-15H,10-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human ALDH1A1 using NAD+/propionaldehyde as substrate/cofactor preincubated for 15 mins followed by substrate/cofactor addition measure... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase 1A1
(Homo sapiens (Human)) | BDBM50456223

(CHEMBL4206892)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)c1cnc2ccc(F)cc2c1N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C27H28FN5O3S/c1-37(35,36)33-15-13-32(14-16-33)26(34)23-18-30-24-8-7-21(28)17-22(24)25(23)31-11-9-27(19-29,10-12-31)20-5-3-2-4-6-20/h2-8,17-18H,9-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 20 uM after... |
J Med Chem 61: 4883-4903 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00270
BindingDB Entry DOI: 10.7270/Q2SB48BH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data