 Found 346 hits with Last Name = 'johnson' and Initial = 'to'
Found 346 hits with Last Name = 'johnson' and Initial = 'to' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779
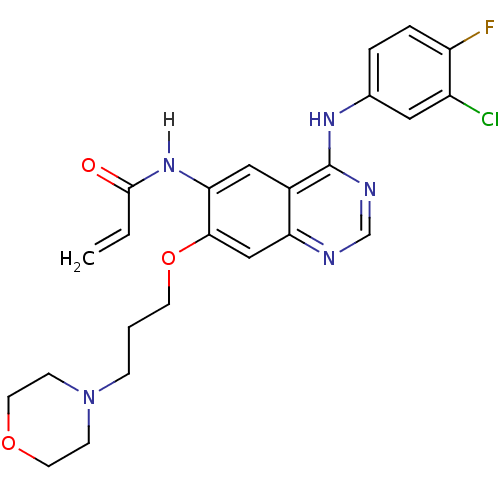
(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50428107
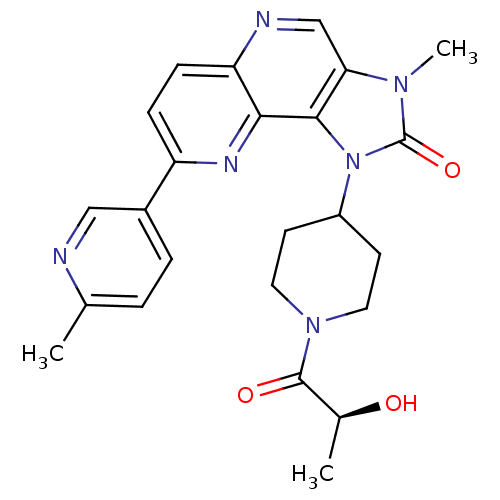
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428109
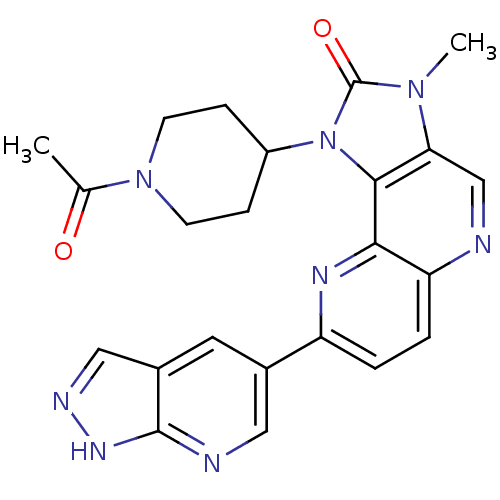
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111
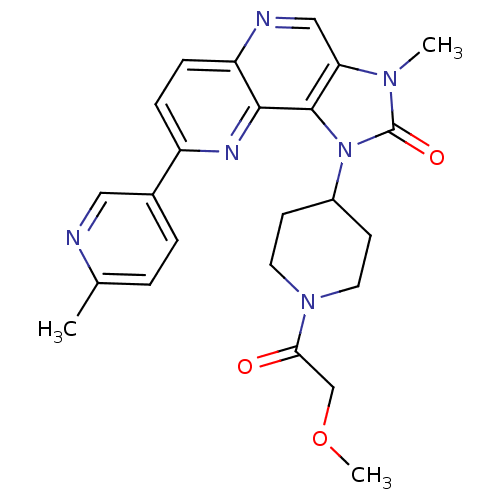
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428108
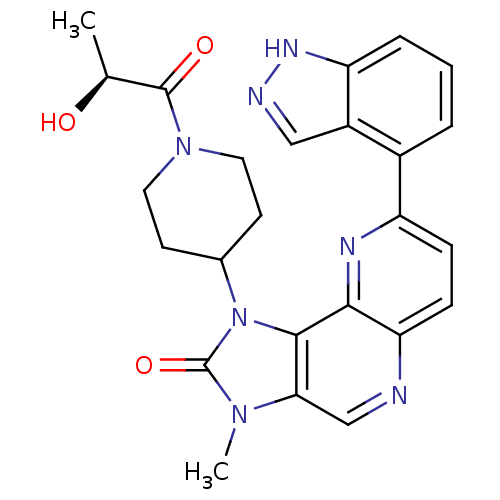
(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428113
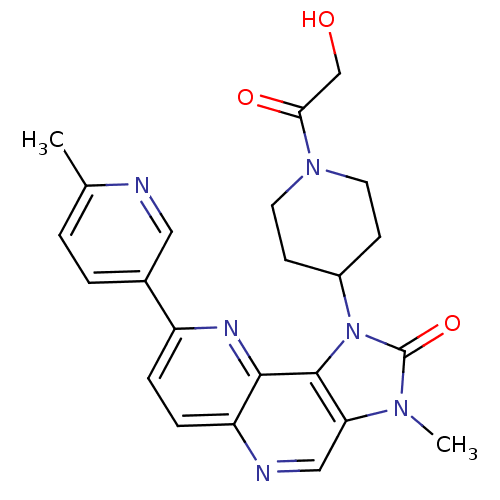
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428115
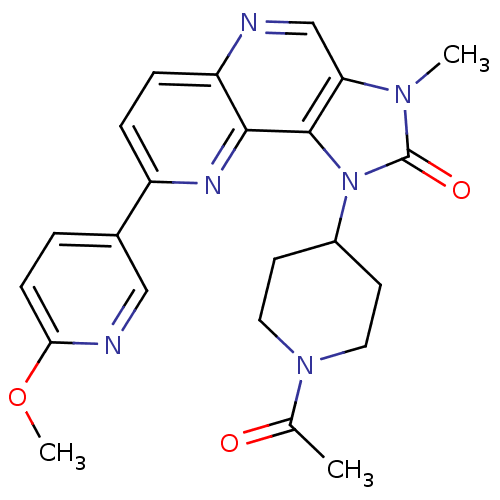
(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428110
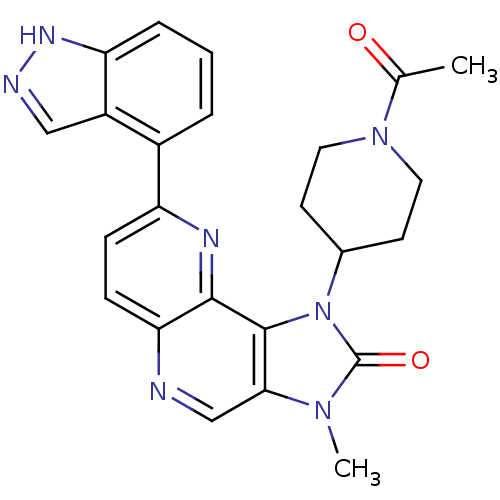
(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.584 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327904

(2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(nc12)-c1cc[nH]n1 Show InChI InChI=1S/C15H17N7O2/c1-8-11-13(20-15(16)18-8)22(9-3-6-24-7-4-9)14(23)12(19-11)10-2-5-17-21-10/h2,5,9H,3-4,6-7H2,1H3,(H,17,21)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113

(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327907
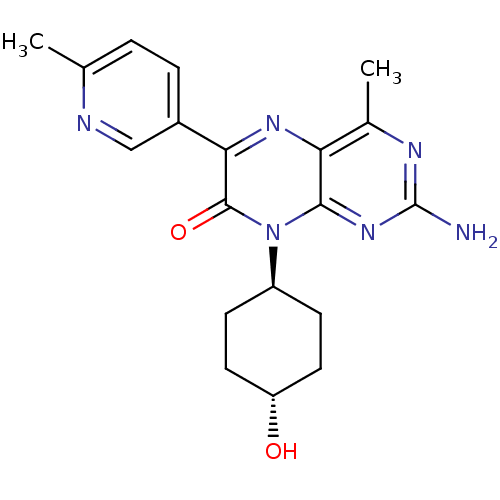
(CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.23,wD:18.19,(6.09,-29.48,;4.76,-30.26,;4.77,-31.8,;3.44,-32.58,;2.1,-31.81,;2.09,-30.28,;3.42,-29.5,;.78,-32.59,;-.55,-31.83,;-1.88,-32.6,;-3.22,-31.84,;-3.22,-30.3,;-4.54,-32.61,;-4.55,-34.15,;-5.88,-34.92,;-3.21,-34.92,;-1.88,-34.14,;-.54,-34.91,;-.54,-36.45,;-1.87,-37.22,;-1.87,-38.76,;-.54,-39.53,;-.54,-41.07,;.79,-38.76,;.8,-37.22,;.79,-34.14,;2.13,-34.9,)| Show InChI InChI=1S/C19H22N6O2/c1-10-3-4-12(9-21-10)16-18(27)25(13-5-7-14(26)8-6-13)17-15(23-16)11(2)22-19(20)24-17/h3-4,9,13-14,26H,5-8H2,1-2H3,(H2,20,22,24)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428111

(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29864
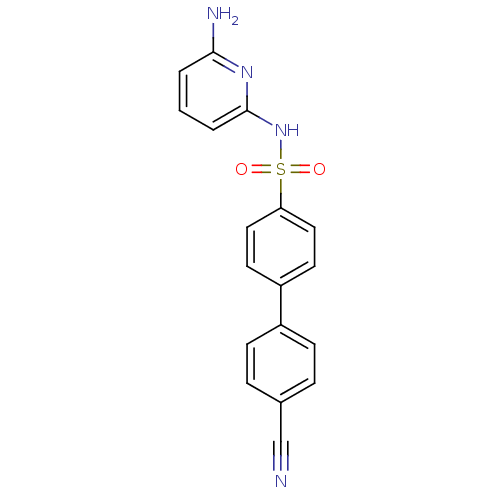
(N-(Pyridin-2-yl) arylsulfonamide, 26)Show SMILES Nc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C18H14N4O2S/c19-12-13-4-6-14(7-5-13)15-8-10-16(11-9-15)25(23,24)22-18-3-1-2-17(20)21-18/h1-11H,(H3,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428117
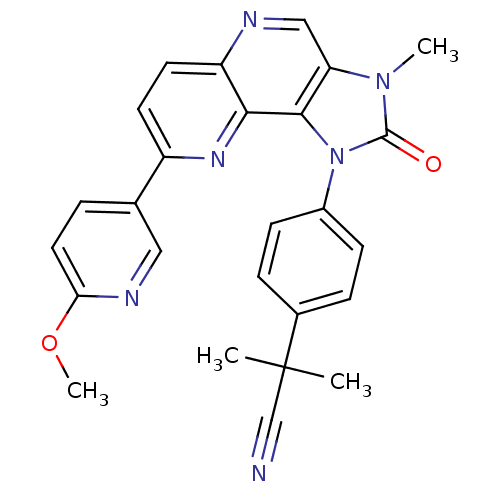
(CHEMBL2331657)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(cc4)C(C)(C)C#N)c3c2n1 Show InChI InChI=1S/C26H22N6O2/c1-26(2,15-27)17-6-8-18(9-7-17)32-24-21(31(3)25(32)33)14-28-20-11-10-19(30-23(20)24)16-5-12-22(34-4)29-13-16/h5-14H,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428110

(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29863

(N-(Pyridin-2-yl) arylsulfonamide, 25)Show SMILES CC(C)c1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C21H19N3O2S/c1-15(2)20-4-3-5-21(23-20)24-27(25,26)19-12-10-18(11-13-19)17-8-6-16(14-22)7-9-17/h3-13,15H,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | -49.6 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92906
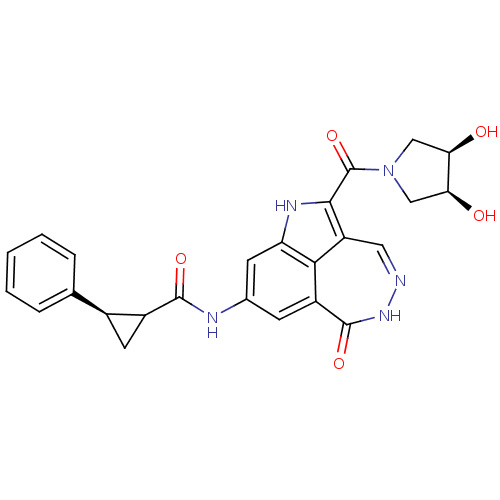
(CHK1 compound 1)Show SMILES O[C@H]1CN(C[C@H]1O)C(=O)c1[nH]c2cc(NC(=O)C3C[C@H]3c3ccccc3)cc3c2c1cn[nH]c3=O |r| Show InChI InChI=1S/C25H21N5O5/c31-19-10-30(11-20(19)32)25(35)22-17-9-26-29-24(34)16-6-13(7-18(28-22)21(16)17)27-23(33)15-8-14(15)12-4-2-1-3-5-12/h1-7,9,14-15,19-20,31-32H,8,10-11H2,(H,27,33)/t14-,15?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer
| Assay Description
The inhibitors reported in this study bind to CHK1 according to a general mechanism illustrated in Scheme 1 where E, S, and I stand for enzyme, subst... |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29862
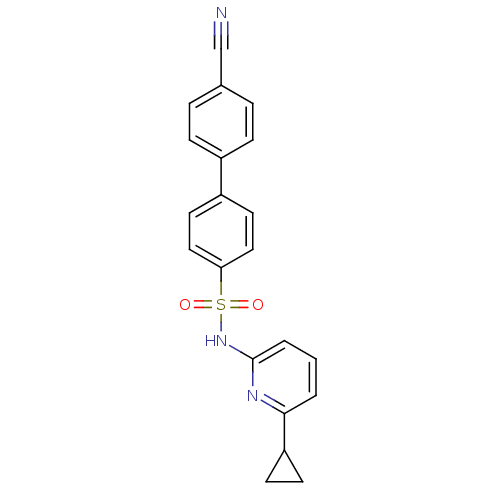
(N-(Pyridin-2-yl) arylsulfonamide, 24)Show SMILES O=S(=O)(Nc1cccc(n1)C1CC1)c1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H17N3O2S/c22-14-15-4-6-16(7-5-15)17-10-12-19(13-11-17)27(25,26)24-21-3-1-2-20(23-21)18-8-9-18/h1-7,10-13,18H,8-9H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50112173
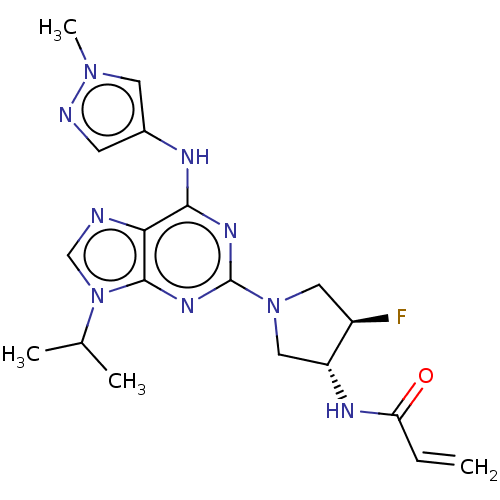
(CHEMBL3608429)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O/c1-5-15(30)24-14-9-28(8-13(14)20)19-25-17(23-12-6-22-27(4)7-12)16-18(26-19)29(10-21-16)11(2)3/h5-7,10-11,13-14H,1,8-9H2,2-4H3,(H,24,30)(H,23,25,26)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159347
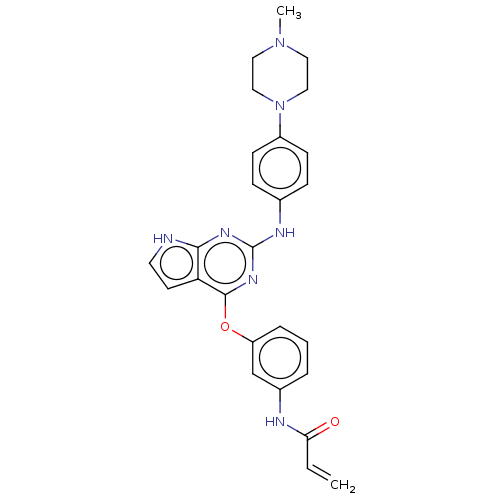
(CHEMBL3787662 | US9586965, Cpd 1)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H27N7O2/c1-3-23(34)28-19-5-4-6-21(17-19)35-25-22-11-12-27-24(22)30-26(31-25)29-18-7-9-20(10-8-18)33-15-13-32(2)14-16-33/h3-12,17H,1,13-16H2,2H3,(H,28,34)(H2,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428118
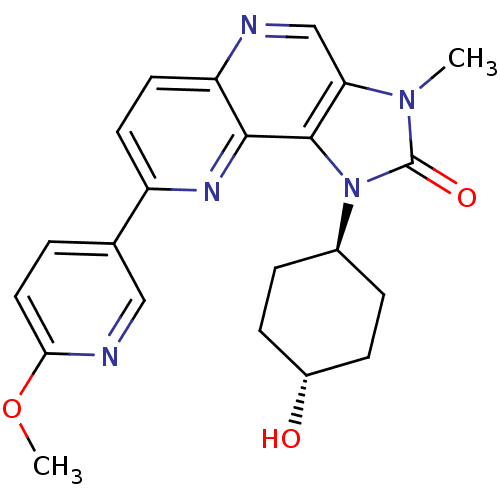
(CHEMBL2331659 | US8791131, 134)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:20.20,wD:23.24,(32.03,-7.87,;33.36,-7.1,;34.69,-7.87,;34.69,-9.41,;36.03,-10.18,;37.36,-9.41,;37.36,-7.86,;36.02,-7.1,;38.7,-10.18,;38.69,-11.72,;40.02,-12.49,;41.35,-11.71,;42.69,-12.48,;44.03,-11.7,;44.01,-10.15,;45.14,-9.12,;46.65,-9.43,;44.51,-7.72,;45.28,-6.39,;42.99,-7.89,;42.18,-6.59,;40.64,-6.65,;39.83,-5.35,;40.55,-3.98,;39.73,-2.68,;42.09,-3.93,;42.9,-5.23,;42.68,-9.39,;41.35,-10.17,;40.02,-9.41,)| Show InChI InChI=1S/C22H23N5O3/c1-26-18-12-23-17-9-8-16(13-3-10-19(30-2)24-11-13)25-20(17)21(18)27(22(26)29)14-4-6-15(28)7-5-14/h3,8-12,14-15,28H,4-7H2,1-2H3/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428114

(CHEMBL2331662 | US8791131, 173)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C23H24N6O2/c1-14-4-5-16(12-24-14)18-6-7-19-21(26-18)22-20(13-25-19)27(3)23(31)29(22)17-8-10-28(11-9-17)15(2)30/h4-7,12-13,17H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159358
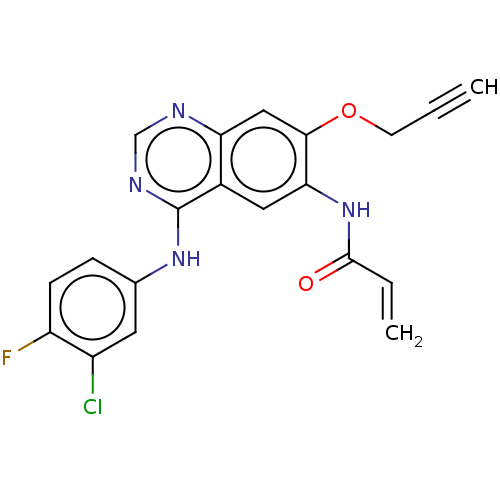
(CHEMBL3787386)Show SMILES Fc1ccc(Nc2ncnc3cc(OCC#C)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450885
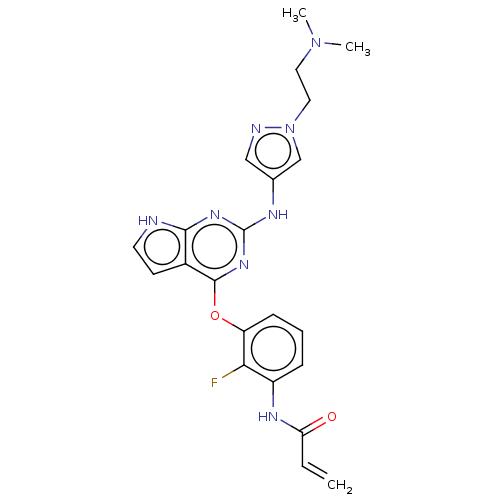
(CHEMBL4216749)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3F)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H23FN8O2/c1-4-18(32)27-16-6-5-7-17(19(16)23)33-21-15-8-9-24-20(15)28-22(29-21)26-14-12-25-31(13-14)11-10-30(2)3/h4-9,12-13H,1,10-11H2,2-3H3,(H,27,32)(H2,24,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428115

(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428114

(CHEMBL2331662 | US8791131, 173)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C23H24N6O2/c1-14-4-5-16(12-24-14)18-6-7-19-21(26-18)22-20(13-25-19)27(3)23(31)29(22)17-8-10-28(11-9-17)15(2)30/h4-7,12-13,17H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428108

(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450887
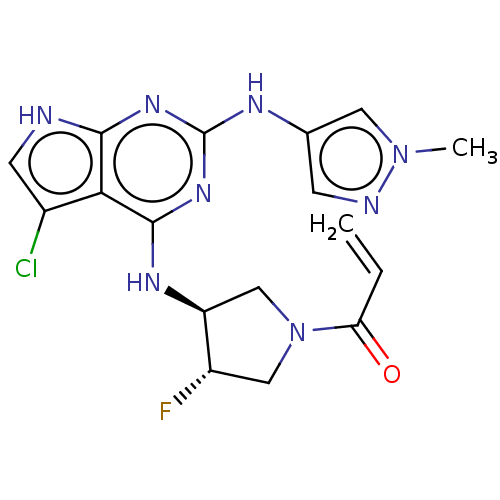
(CHEMBL4211367)Show SMILES Cn1cc(Nc2nc(N[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H18ClFN8O/c1-3-13(28)27-7-11(19)12(8-27)23-16-14-10(18)5-20-15(14)24-17(25-16)22-9-4-21-26(2)6-9/h3-6,11-12H,1,7-8H2,2H3,(H3,20,22,23,24,25)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159353

(CHEMBL3787220)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H19ClFN7O2/c1-3-14(28)27-6-10(13(20)8-27)9-29-17-15-12(19)5-21-16(15)24-18(25-17)23-11-4-22-26(2)7-11/h3-5,7,10,13H,1,6,8-9H2,2H3,(H2,21,23,24,25)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159352
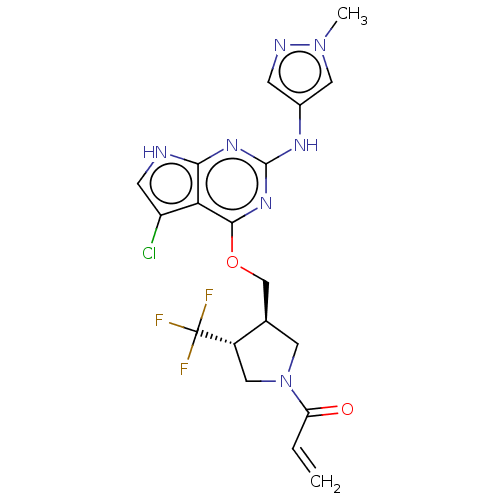
(CHEMBL3786802)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3C(F)(F)F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C19H19ClF3N7O2/c1-3-14(31)30-6-10(12(8-30)19(21,22)23)9-32-17-15-13(20)5-24-16(15)27-18(28-17)26-11-4-25-29(2)7-11/h3-5,7,10,12H,1,6,8-9H2,2H3,(H2,24,26,27,28)/t10-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159349
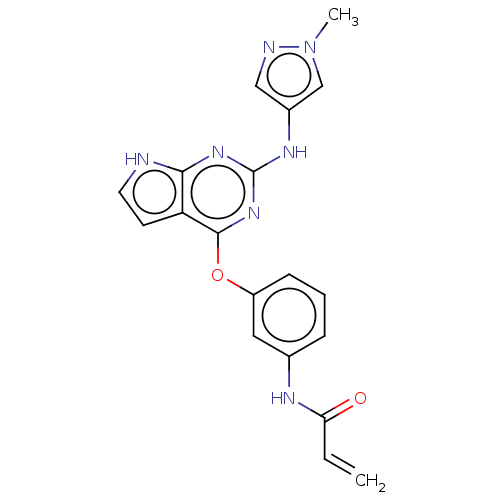
(CHEMBL3786962)Show SMILES Cn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C19H17N7O2/c1-3-16(27)22-12-5-4-6-14(9-12)28-18-15-7-8-20-17(15)24-19(25-18)23-13-10-21-26(2)11-13/h3-11H,1H2,2H3,(H,22,27)(H2,20,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450884
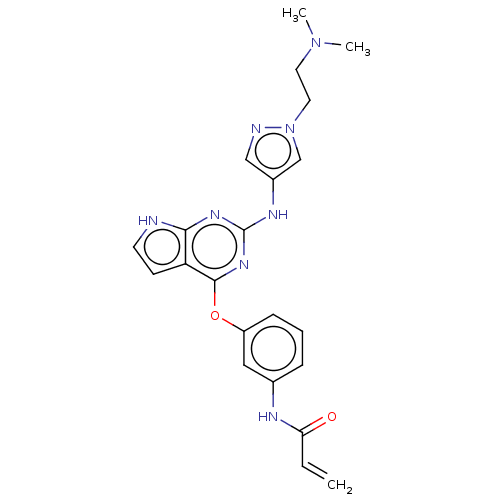
(CHEMBL4211782)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H24N8O2/c1-4-19(31)25-15-6-5-7-17(12-15)32-21-18-8-9-23-20(18)27-22(28-21)26-16-13-24-30(14-16)11-10-29(2)3/h4-9,12-14H,1,10-11H2,2-3H3,(H,25,31)(H2,23,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428112
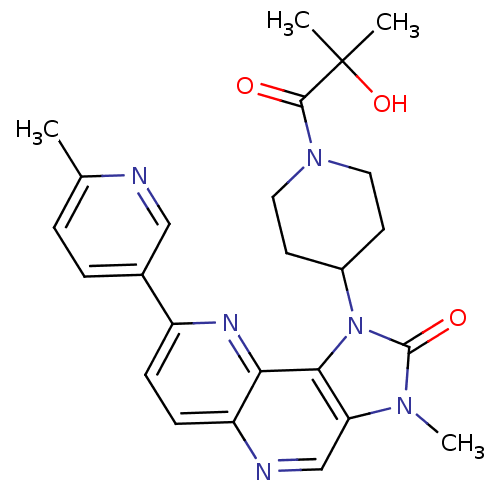
(CHEMBL2331665 | US8791131, 162)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)C(C)(C)O)c3c2n1 Show InChI InChI=1S/C25H28N6O3/c1-15-5-6-16(13-26-15)18-7-8-19-21(28-18)22-20(14-27-19)29(4)24(33)31(22)17-9-11-30(12-10-17)23(32)25(2,3)34/h5-8,13-14,17,34H,9-12H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159360
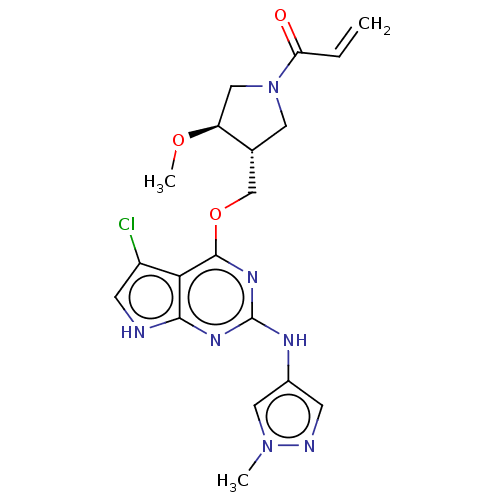
(CHEMBL3786098)Show SMILES CO[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C19H22ClN7O3/c1-4-15(28)27-7-11(14(9-27)29-3)10-30-18-16-13(20)6-21-17(16)24-19(25-18)23-12-5-22-26(2)8-12/h4-6,8,11,14H,1,7,9-10H2,2-3H3,(H2,21,23,24,25)/t11-,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159356
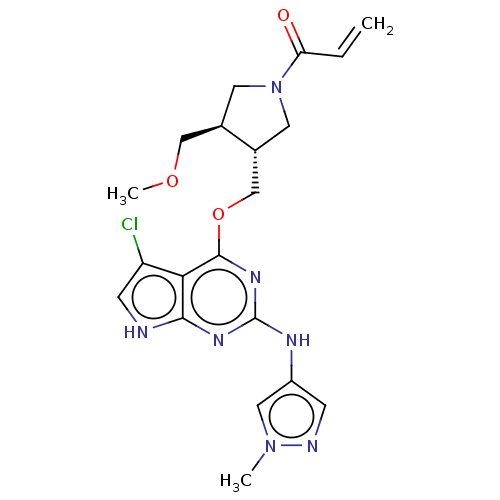
(CHEMBL3786523)Show SMILES COC[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C20H24ClN7O3/c1-4-16(29)28-7-12(10-30-3)13(8-28)11-31-19-17-15(21)6-22-18(17)25-20(26-19)24-14-5-23-27(2)9-14/h4-6,9,12-13H,1,7-8,10-11H2,2-3H3,(H2,22,24,25,26)/t12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29860
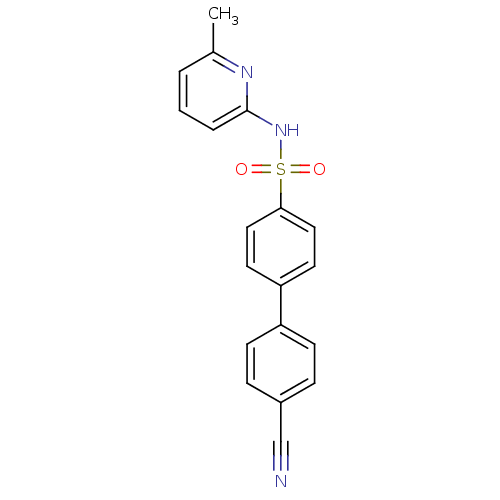
(N-(Pyridin-2-yl) arylsulfonamide, 22)Show SMILES Cc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C19H15N3O2S/c1-14-3-2-4-19(21-14)22-25(23,24)18-11-9-17(10-12-18)16-7-5-15(13-20)6-8-16/h2-12H,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450886
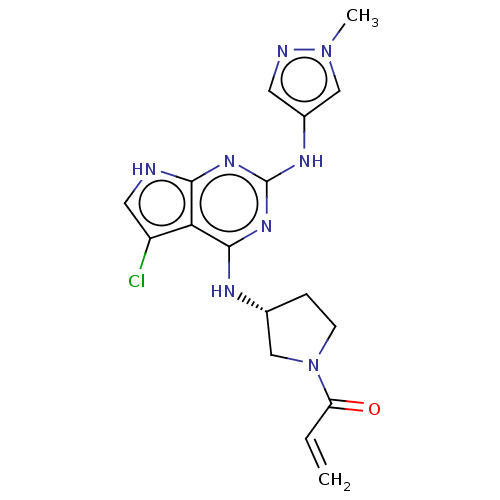
(CHEMBL4206481)Show SMILES Cn1cc(Nc2nc(N[C@@H]3CCN(C3)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H19ClN8O/c1-3-13(27)26-5-4-10(9-26)21-16-14-12(18)7-19-15(14)23-17(24-16)22-11-6-20-25(2)8-11/h3,6-8,10H,1,4-5,9H2,2H3,(H3,19,21,22,23,24)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428117

(CHEMBL2331657)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(-c4ccc(cc4)C(C)(C)C#N)c3c2n1 Show InChI InChI=1S/C26H22N6O2/c1-26(2,15-27)17-6-8-18(9-7-17)32-24-21(31(3)25(32)33)14-28-20-11-10-19(30-23(20)24)16-5-12-22(34-4)29-13-16/h5-14H,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428119
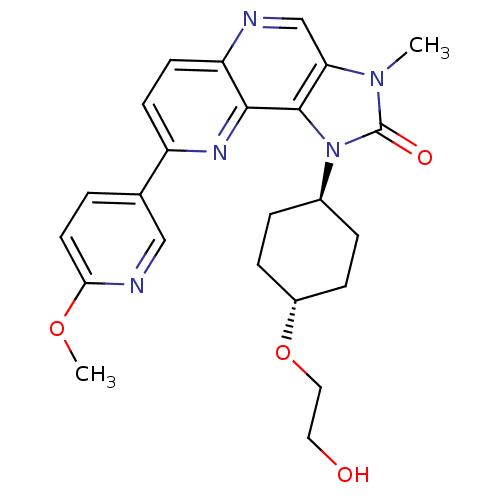
(CHEMBL2331658 | US8791131, 133)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@@H](CC4)OCCO)c3c2n1 |r,wU:20.20,wD:23.27,(17.65,-10.2,;18.98,-9.43,;20.31,-10.2,;20.31,-11.74,;21.64,-12.51,;22.98,-11.74,;22.98,-10.19,;21.64,-9.43,;24.32,-12.51,;24.31,-14.05,;25.64,-14.81,;26.97,-14.04,;28.31,-14.81,;29.65,-14.03,;29.63,-12.48,;30.76,-11.45,;32.27,-11.76,;30.13,-10.05,;30.9,-8.71,;28.61,-10.22,;27.8,-8.92,;26.26,-8.97,;25.44,-7.67,;26.17,-6.31,;27.71,-6.25,;28.52,-7.56,;25.35,-5,;26.07,-3.64,;25.25,-2.34,;25.98,-.97,;28.3,-11.72,;26.97,-12.49,;25.63,-11.73,)| Show InChI InChI=1S/C24H27N5O4/c1-28-20-14-25-19-9-8-18(15-3-10-21(32-2)26-13-15)27-22(19)23(20)29(24(28)31)16-4-6-17(7-5-16)33-12-11-30/h3,8-10,13-14,16-17,30H,4-7,11-12H2,1-2H3/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM29866

(N-(Pyridin-2-yl) arylsulfonamide, 28)Show SMILES CCNc1cccc(NS(=O)(=O)c2ccc(cc2)-c2ccc(cc2)C#N)n1 Show InChI InChI=1S/C20H18N4O2S/c1-2-22-19-4-3-5-20(23-19)24-27(25,26)18-12-10-17(11-13-18)16-8-6-15(14-21)7-9-16/h3-13H,2H2,1H3,(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | -47.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 22 |
Pfizer
| Assay Description
The enzyme assay was performed in a round-bottom 96-well plate. The enzyme was pre-incubated in the assay buffer in the presence of NADPH and inhibit... |
Bioorg Med Chem Lett 19: 3493-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.011
BindingDB Entry DOI: 10.7270/Q2SB4428 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327906
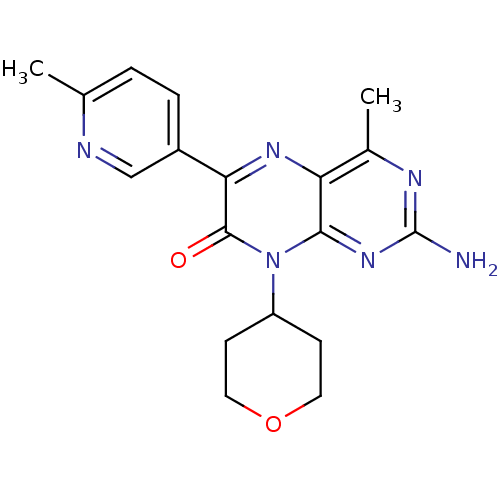
(2-amino-4-methyl-6-(6-methylpyridin-3-yl)-8-(tetra...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n(C2CCOCC2)c1=O Show InChI InChI=1S/C18H20N6O2/c1-10-3-4-12(9-20-10)15-17(25)24(13-5-7-26-8-6-13)16-14(22-15)11(2)21-18(19)23-16/h3-4,9,13H,5-8H2,1-2H3,(H2,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327894

(2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...)Show InChI InChI=1S/C17H19N5O2/c1-9(2)22-15-12(10(3)20-17(18)21-15)7-13(16(22)23)11-5-6-14(24-4)19-8-11/h5-9H,1-4H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327903
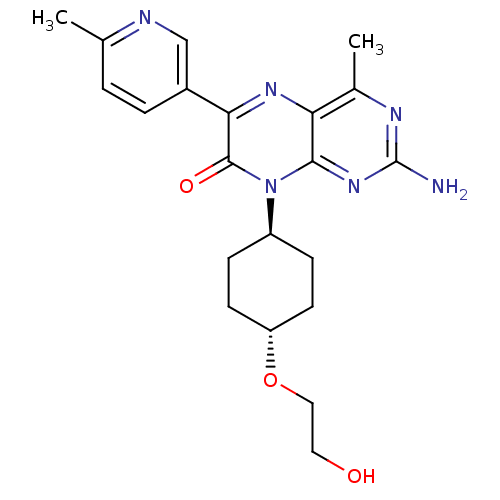
(CHEMBL1258910 | trans-2-amino-8-(4-(2-hydroxyethox...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:18.19,wD:21.26,(6.04,1.56,;4.72,.78,;4.73,-.76,;3.4,-1.53,;2.06,-.77,;2.05,.76,;3.37,1.55,;.74,-1.54,;-.6,-.78,;-1.93,-1.56,;-3.26,-.79,;-3.27,.75,;-4.59,-1.56,;-4.59,-3.11,;-5.93,-3.88,;-3.26,-3.88,;-1.93,-3.1,;-.59,-3.87,;-.59,-5.41,;.75,-6.17,;.75,-7.72,;-.59,-8.49,;-1.92,-7.71,;-1.92,-6.18,;-.59,-10.03,;-1.93,-10.8,;-1.93,-12.34,;-3.26,-13.1,;.74,-3.09,;2.08,-3.86,)| Show InChI InChI=1S/C21H26N6O3/c1-12-3-4-14(11-23-12)18-20(29)27(15-5-7-16(8-6-15)30-10-9-28)19-17(25-18)13(2)24-21(22)26-19/h3-4,11,15-16,28H,5-10H2,1-2H3,(H2,22,24,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM92906

(CHK1 compound 1)Show SMILES O[C@H]1CN(C[C@H]1O)C(=O)c1[nH]c2cc(NC(=O)C3C[C@H]3c3ccccc3)cc3c2c1cn[nH]c3=O |r| Show InChI InChI=1S/C25H21N5O5/c31-19-10-30(11-20(19)32)25(35)22-17-9-26-29-24(34)16-6-13(7-18(28-22)21(16)17)27-23(33)15-8-14(15)12-4-2-1-3-5-12/h1-7,9,14-15,19-20,31-32H,8,10-11H2,(H,27,33)/t14-,15?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.14 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 20 |
Pfizer
| Assay Description
Surface plasmon resonance (SPR) biosensor binding studies were conducted using a Biacore 3000 instrument (GE Healtchare). |
Biochemistry 48: 9823-30 (2009)
Article DOI: 10.1021/bi900258v
BindingDB Entry DOI: 10.7270/Q25M649B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428116
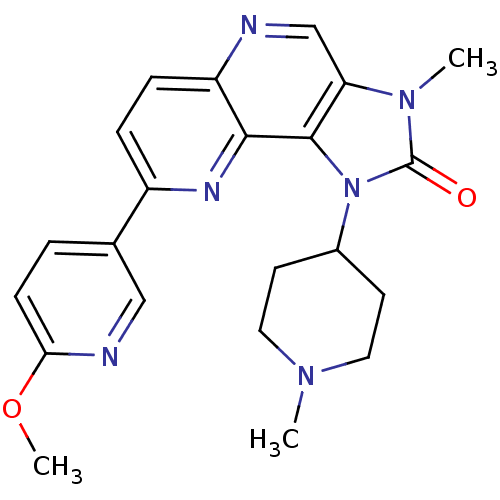
(CHEMBL2331660)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(C)CC4)c3c2n1 Show InChI InChI=1S/C22H24N6O2/c1-26-10-8-15(9-11-26)28-21-18(27(2)22(28)29)13-23-17-6-5-16(25-20(17)21)14-4-7-19(30-3)24-12-14/h4-7,12-13,15H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data