

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371581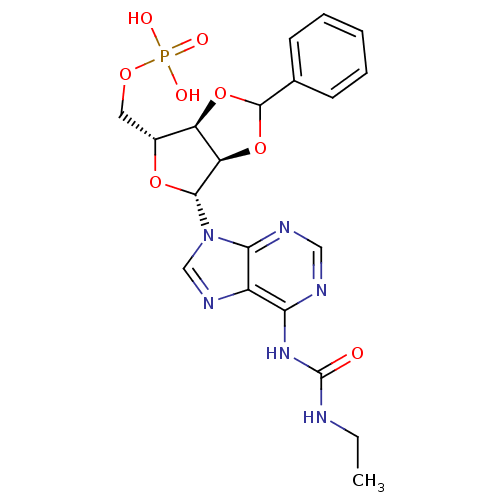 (CHEMBL1162179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50069571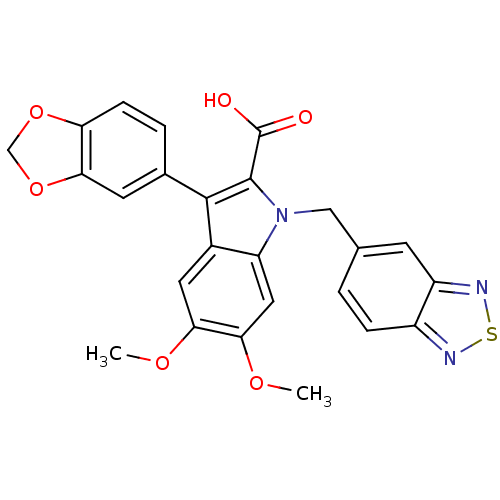 (3-Benzo[1,3]dioxol-5-yl-1-benzo[1,2,5]thiadiazol-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency of the compound at Endothelin A receptor was determined | J Med Chem 46: 3257-74 (2003) Article DOI: 10.1021/jm0300429 BindingDB Entry DOI: 10.7270/Q2W37VQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371582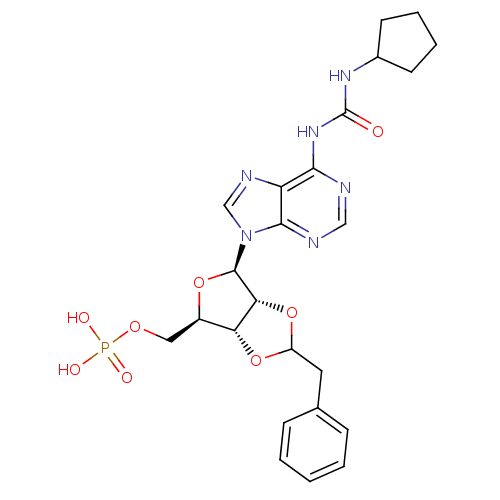 (CHEMBL1162182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371583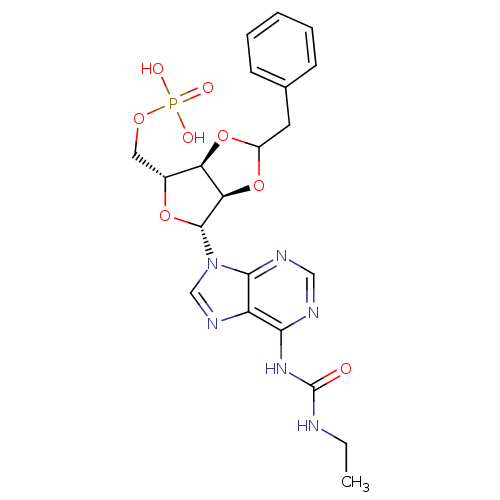 (CHEMBL1162184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371589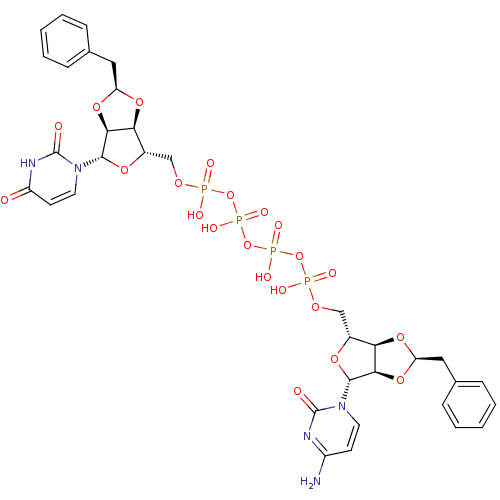 (CHEMBL1162196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371594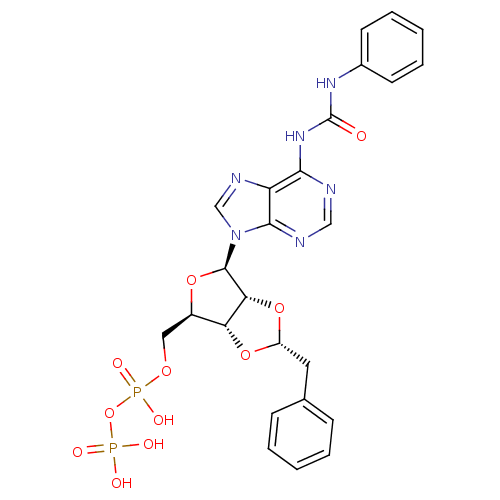 (CHEMBL1162161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371584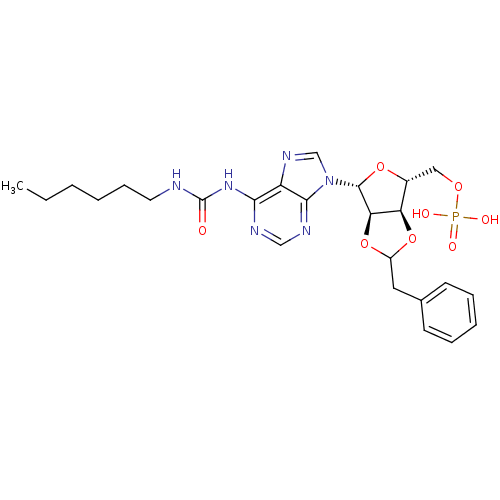 (CHEMBL1162185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50069573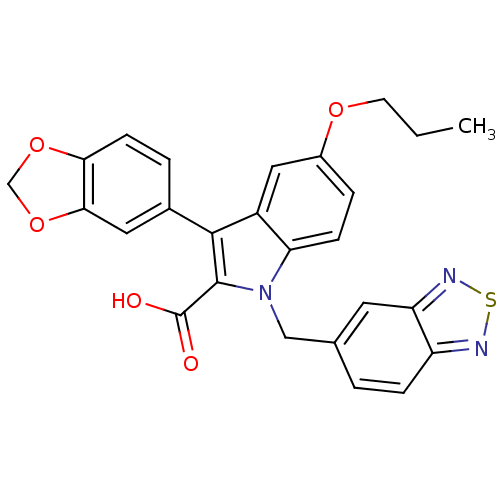 (3-Benzo[1,3]dioxol-5-yl-1-benzo[1,2,5]thiadiazol-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency of the compound at Endothelin A receptor was determined | J Med Chem 46: 3257-74 (2003) Article DOI: 10.1021/jm0300429 BindingDB Entry DOI: 10.7270/Q2W37VQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50069565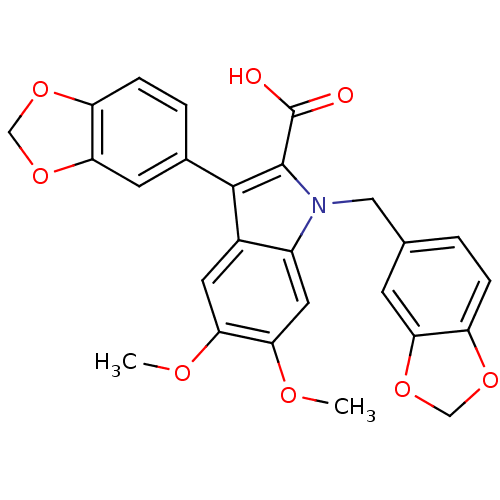 (3-Benzo[1,3]dioxol-5-yl-1-benzo[1,3]dioxol-5-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency of the compound at Endothelin A receptor was determined | J Med Chem 46: 3257-74 (2003) Article DOI: 10.1021/jm0300429 BindingDB Entry DOI: 10.7270/Q2W37VQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371585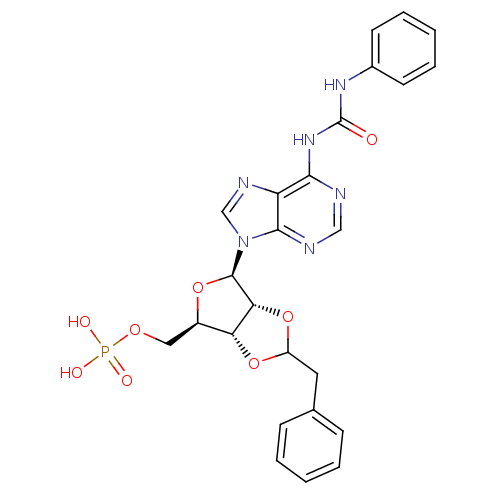 (CHEMBL1162188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562854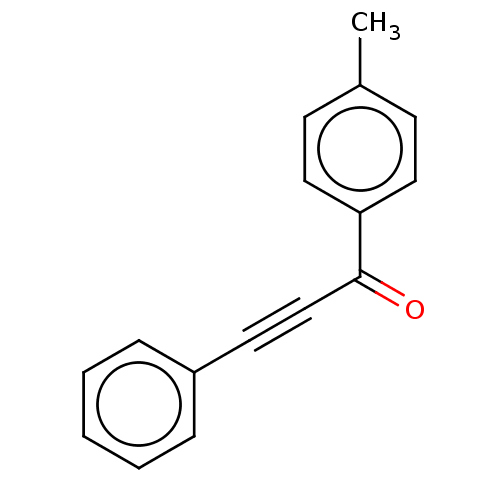 (CHEMBL4763993) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371578 (CHEMBL1162172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371577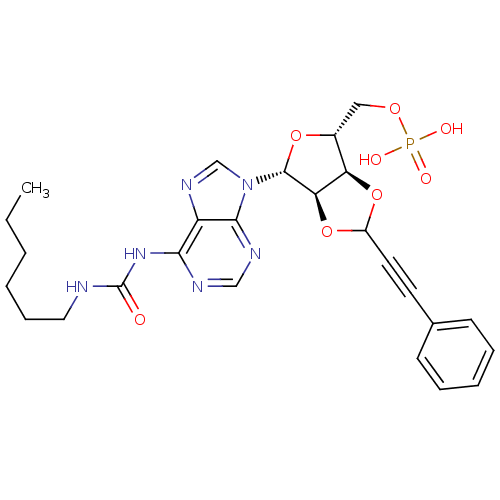 (CHEMBL1162173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371579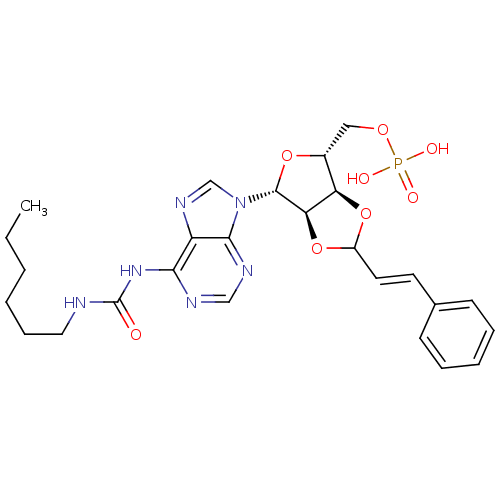 (CHEMBL1162171) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50069567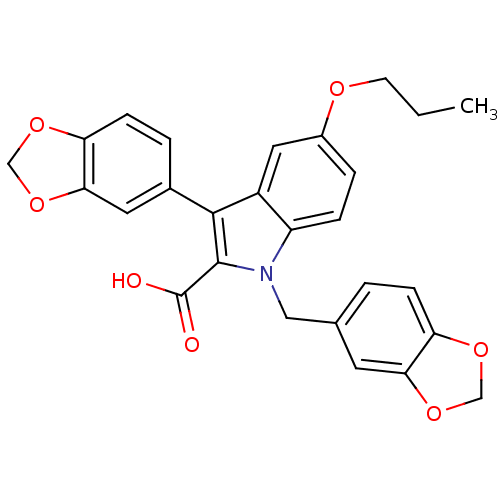 (3-Benzo[1,3]dioxol-5-yl-1-benzo[1,3]dioxol-5-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency of the compound at Endothelin A receptor was determined | J Med Chem 46: 3257-74 (2003) Article DOI: 10.1021/jm0300429 BindingDB Entry DOI: 10.7270/Q2W37VQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371576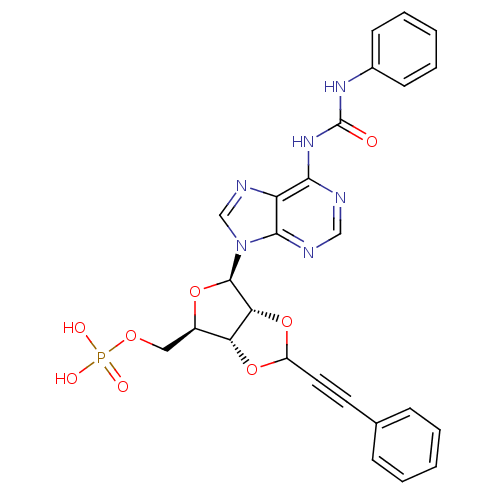 (CHEMBL1162164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371593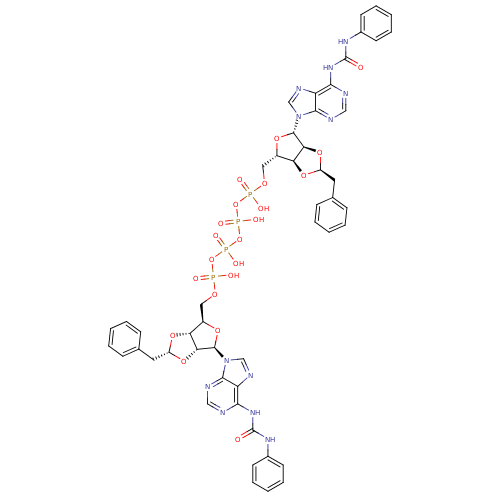 (CHEMBL1162159) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 522 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562860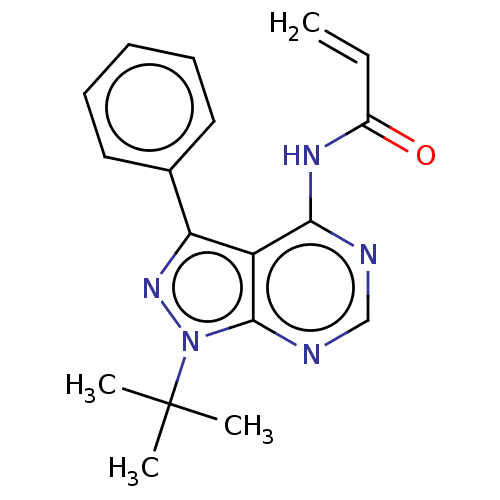 (CHEMBL4762311) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562857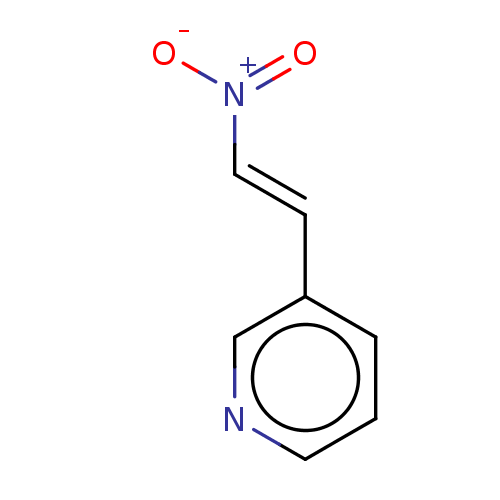 (CHEMBL4791813) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562869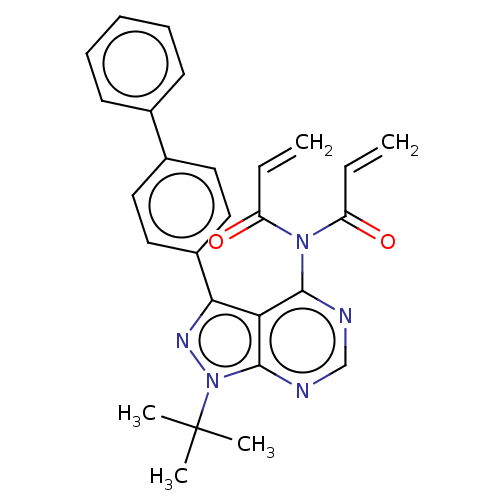 (CHEMBL4759891) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562861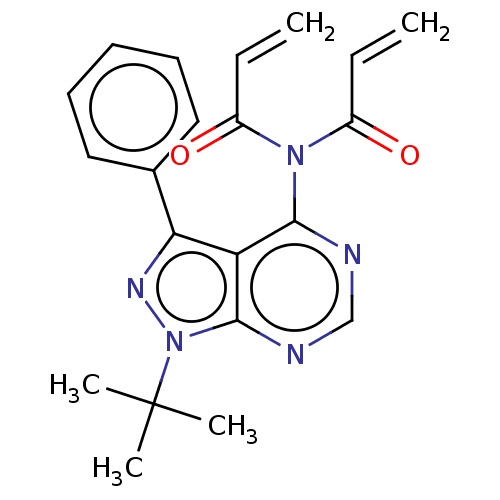 (CHEMBL4792077) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50561073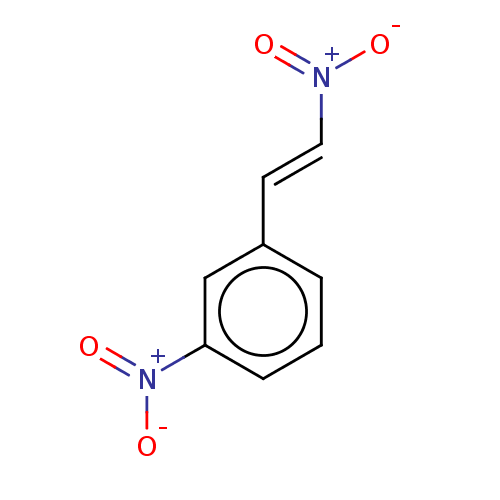 (CHEMBL4751295) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562864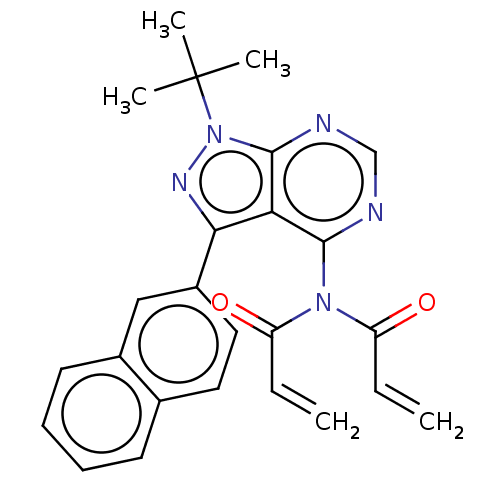 (CHEMBL4756916) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50155928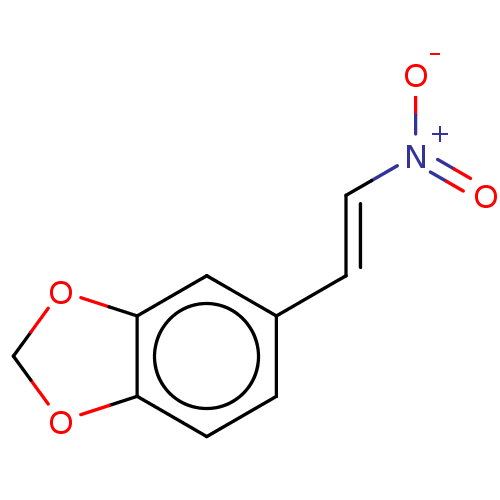 (3,4-Methylenedioxyb-Nitrostyrene | CHEMBL596380) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562860 (CHEMBL4762311) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50561073 (CHEMBL4751295) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562859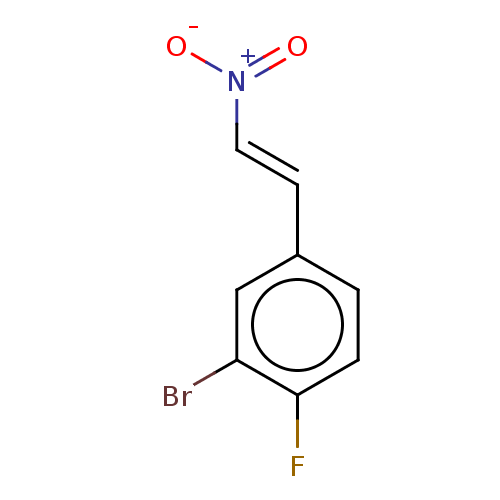 (CHEMBL4742209) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50155928 (3,4-Methylenedioxyb-Nitrostyrene | CHEMBL596380) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562859 (CHEMBL4742209) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371603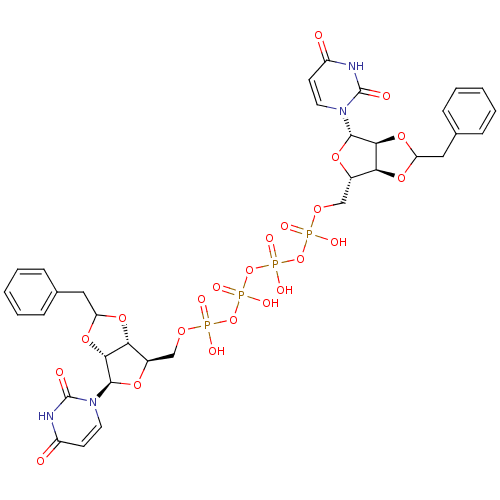 (CHEMBL1162165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50532921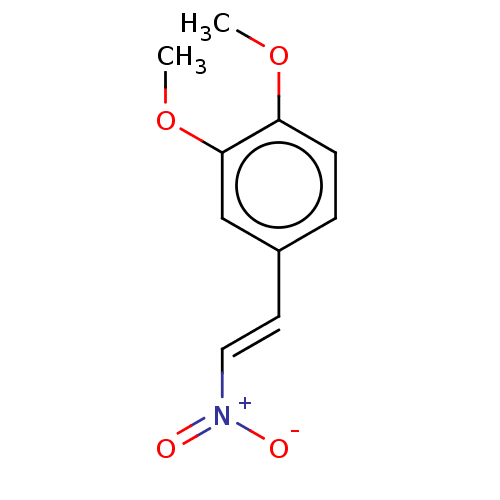 (CHEMBL594404) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562863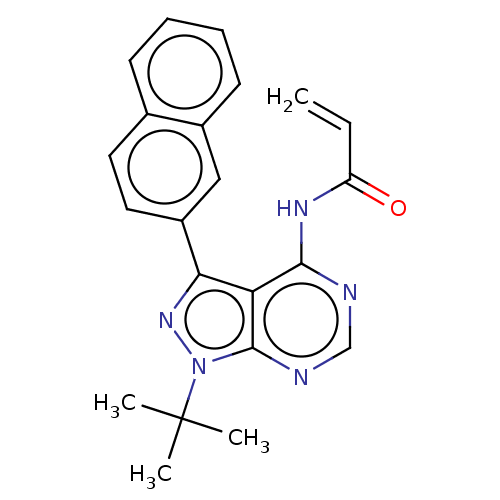 (CHEMBL4788535) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50249208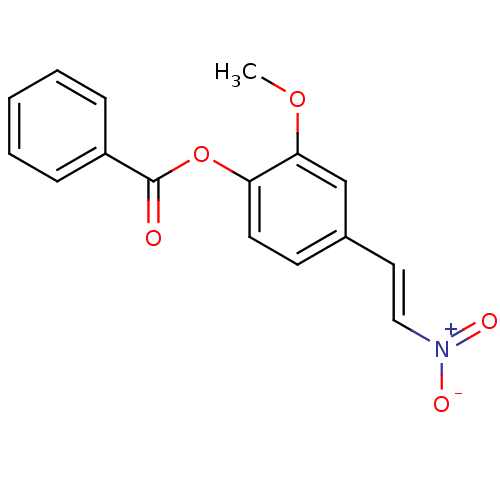 (2-methoxy-4-(2-nitrovinyl)phenyl benzoate | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562868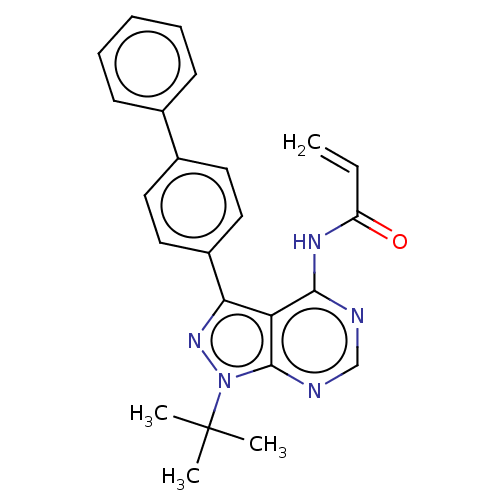 (CHEMBL4790925) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562858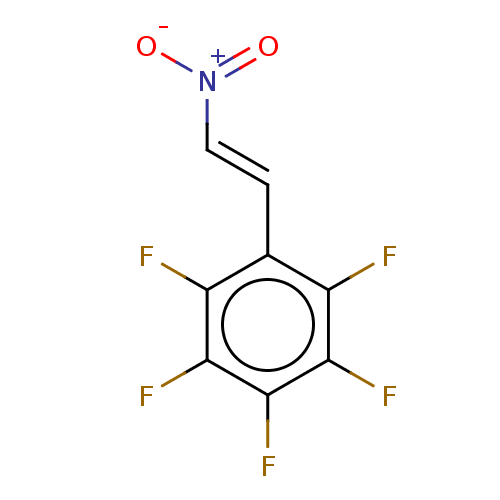 (CHEMBL2409306) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562864 (CHEMBL4756916) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50069574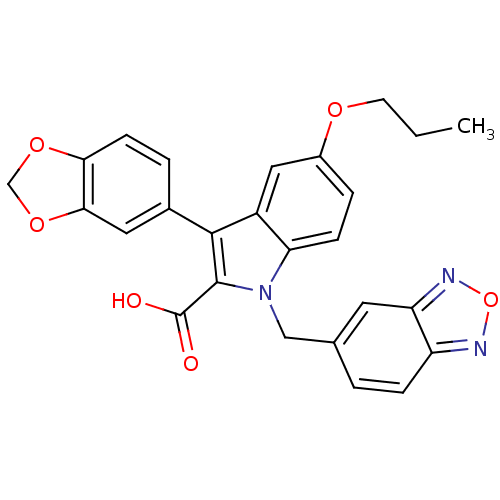 (3-Benzo[1,3]dioxol-5-yl-1-benzo[1,2,5]oxadiazol-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Potency of the compound at Endothelin A receptor was determined | J Med Chem 46: 3257-74 (2003) Article DOI: 10.1021/jm0300429 BindingDB Entry DOI: 10.7270/Q2W37VQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562858 (CHEMBL2409306) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562868 (CHEMBL4790925) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562857 (CHEMBL4791813) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562863 (CHEMBL4788535) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371589 (CHEMBL1162196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50532921 (CHEMBL594404) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371588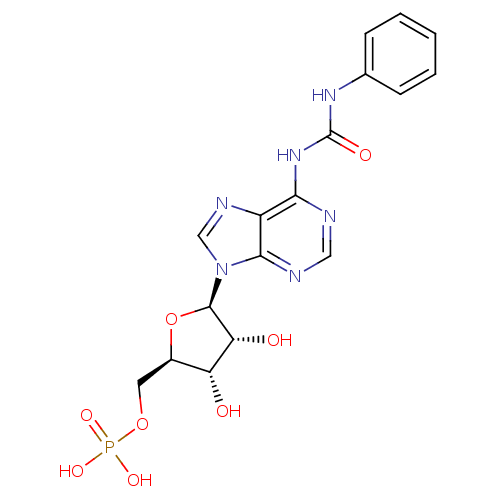 (CHEMBL1162195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371573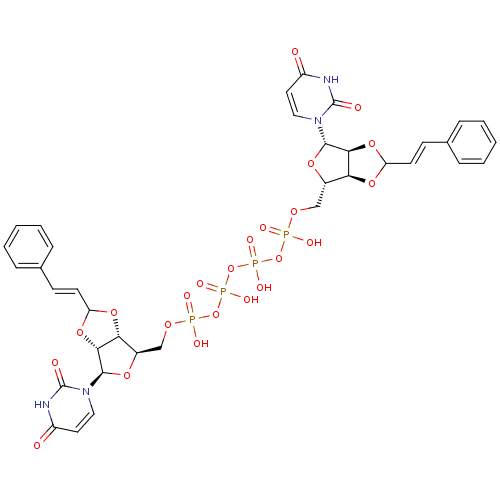 (CHEMBL1162158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371573 (CHEMBL1162158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562854 (CHEMBL4763993) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of p97 in human HeLa cells assessed as reduction in proteasomal turnover of p97-dependent reporter substrate UbG76V-GFP incubated for 120 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transitional endoplasmic reticulum ATPase (Homo sapiens (Human)) | BDBM50562856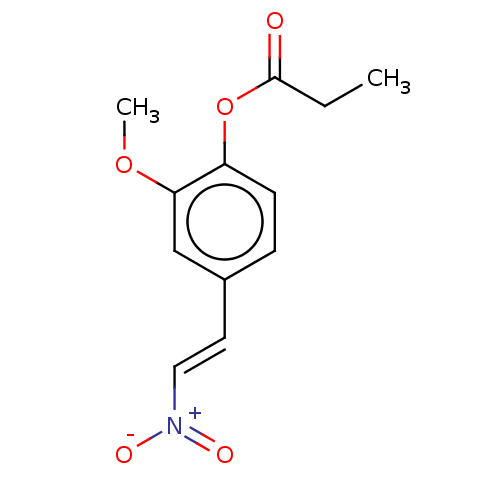 (CHEMBL4763795) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6-tagged p97 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ATPase activity measured after 60 mi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113148 BindingDB Entry DOI: 10.7270/Q2HT2T24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131 total ) | Next | Last >> |