

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029326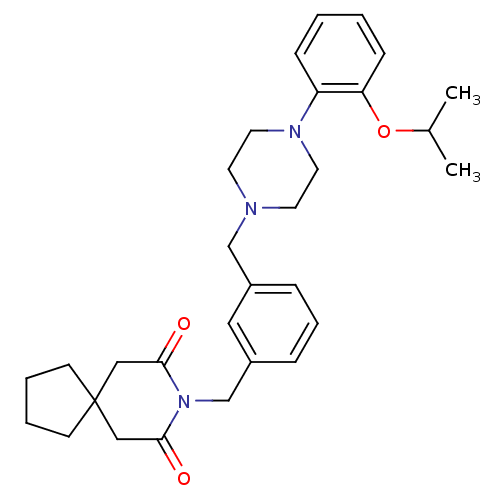 (8-{3-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064563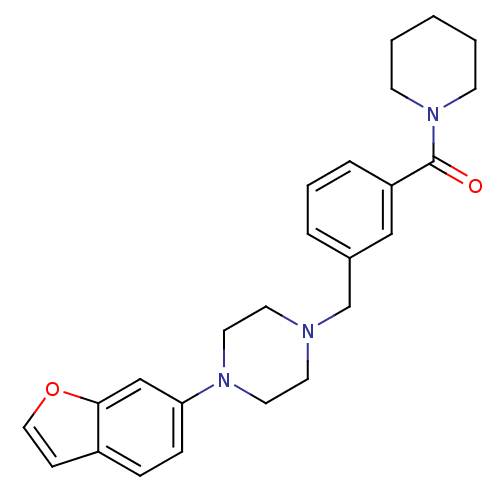 (CHEMBL61816 | [3-(4-Benzofuran-6-yl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064537 (CHEMBL293658 | {3-[4-(2,3-Dihydro-benzo[1,4]dioxin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity with Dopamine receptor D2 using membranes prepared from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064529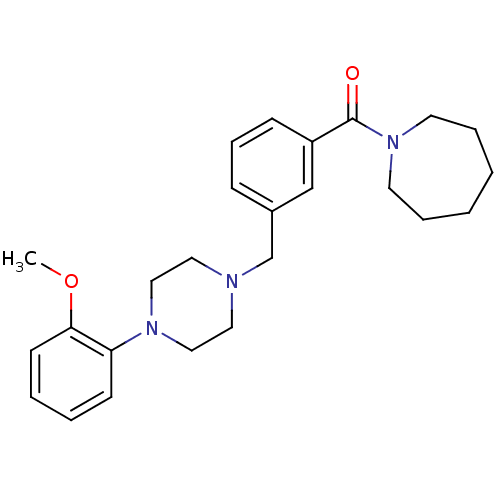 (Azepan-1-yl-{3-[4-(2-methoxy-phenyl)-piperazin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064536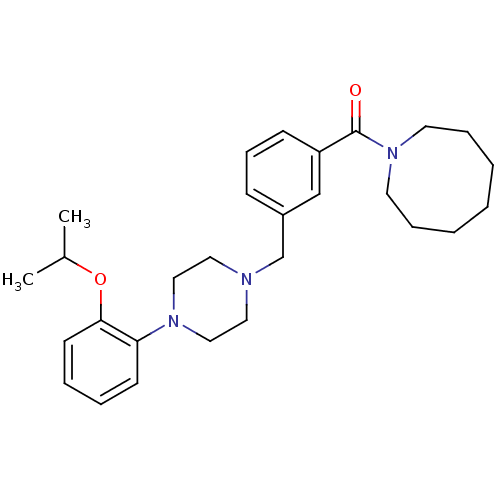 (Azocan-1-yl-{3-[4-(2-isopropoxy-phenyl)-piperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064548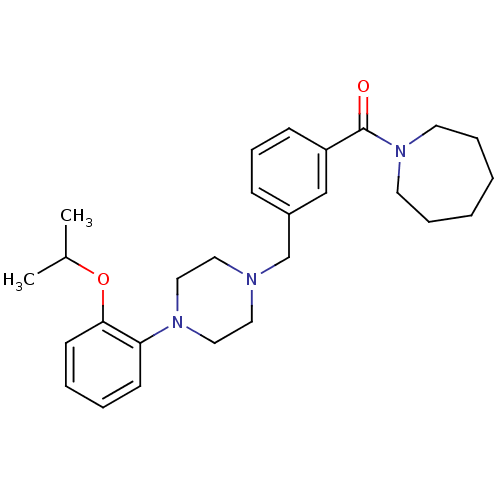 (Azepan-1-yl-{3-[4-(2-isopropoxy-phenyl)-piperazin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254012 (3-(4-Amino-3-fluorobenzyl)-7-(2-furyl)-3H-[1,2,3]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064547 (Azepan-1-yl-{3-[4-(2-ethoxy-phenyl)-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064534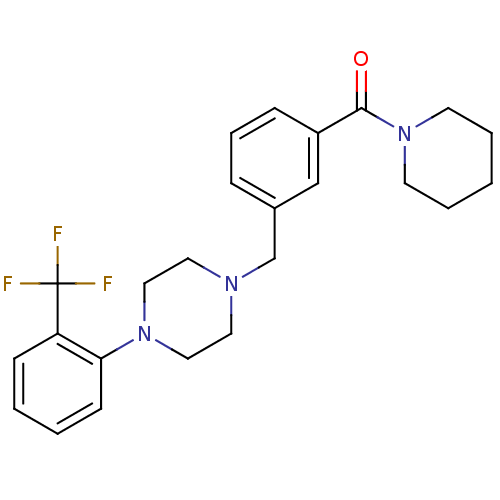 (CHEMBL413546 | Piperidin-1-yl-{3-[4-(2-trifluorome...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869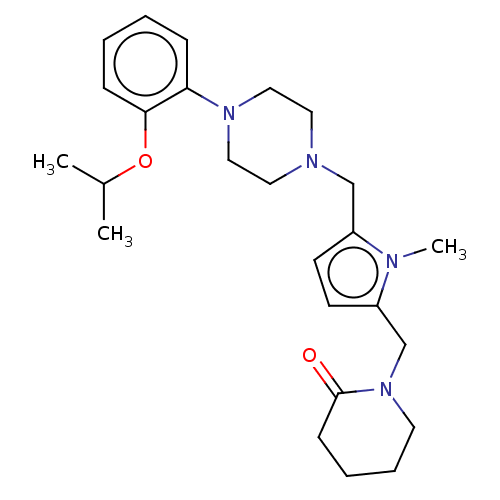 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the dopamine receptor D2 using [3H]spiperinone. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001869 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity with Dopamine receptor D2 using membranes prepared from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064565 (CHEMBL59167 | N,N-Dibutyl-3-[4-(2-isopropoxy-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50064564 (CHEMBL64528 | {3-[4-(2-Isopropoxy-phenyl)-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064564 (CHEMBL64528 | {3-[4-(2-Isopropoxy-phenyl)-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377538 (CHEMBL257757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50239036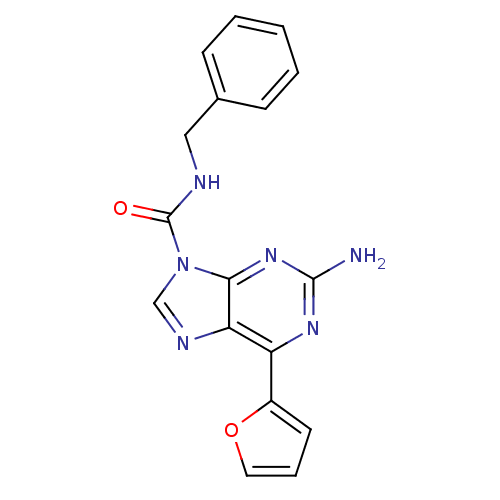 (2-amino-N-benzyl-6-(furan-2-yl)-9H-purine-9-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM35836 (pyrimidine-4-carboxamide, 118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd | Assay Description Binding assays were performed in a total volume of 250 uL, containing radioligand, membranes and test compounds. Following 60 (A2B, A3) or 90 (A1, A2... | Bioorg Med Chem 17: 6590-605 (2009) Article DOI: 10.1016/j.bmc.2009.07.078 BindingDB Entry DOI: 10.7270/Q27M069H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377558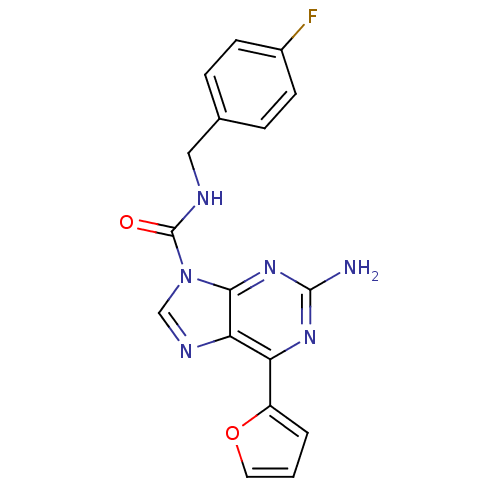 (CHEMBL429144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377543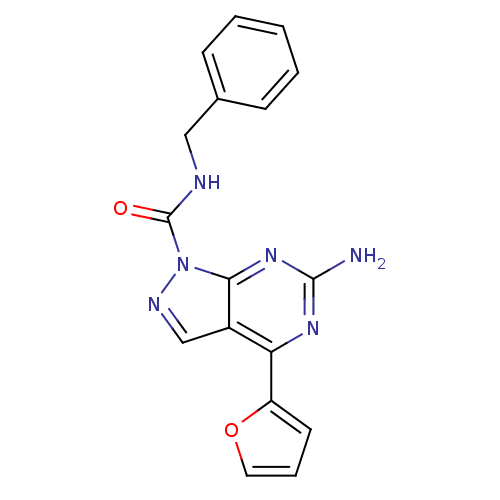 (CHEMBL260146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377556 (CHEMBL411034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254353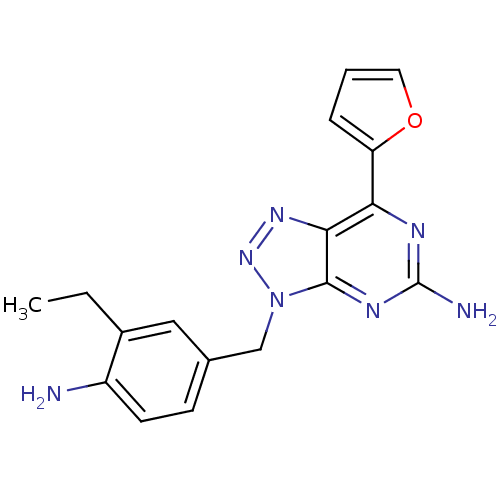 (3-(4-Amino-3-ethylbenzyl)-7-(2-furyl)-3H-[1,2,3]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377567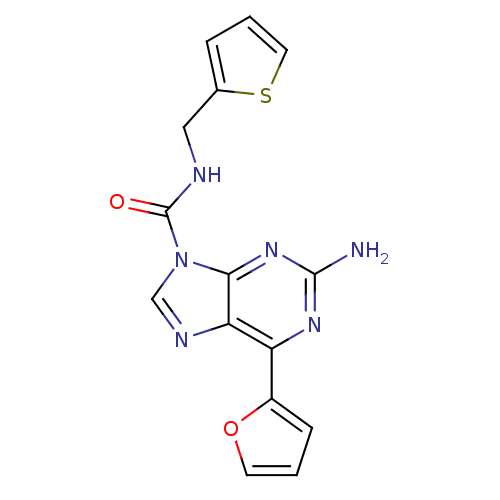 (CHEMBL409915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377566 (CHEMBL259049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377557 (CHEMBL264432) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254398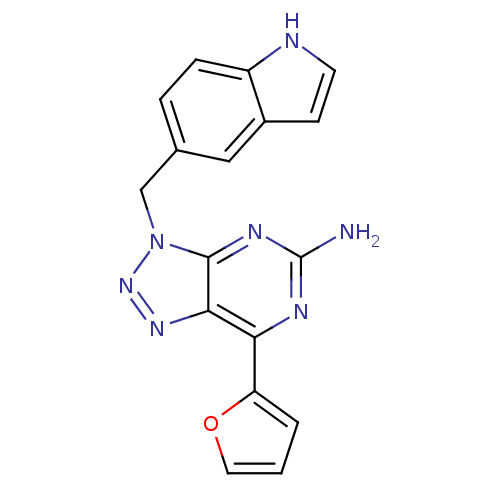 (7-(2-Furyl)-3-(5-indolylmethyl)-3H-[1,2,3]triazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50064556 (1-(2-Isopropoxy-phenyl)-4-[3-(piperidine-1-sulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Dopamine receptor D2 from rat striatum | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064561 (CHEMBL60122 | {3-[4-(2-Isopropoxy-phenyl)-piperazi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064556 (1-(2-Isopropoxy-phenyl)-4-[3-(piperidine-1-sulfony...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064568 (CHEMBL292107 | N-Cyclohexyl-3-[4-(2-isopropoxy-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064569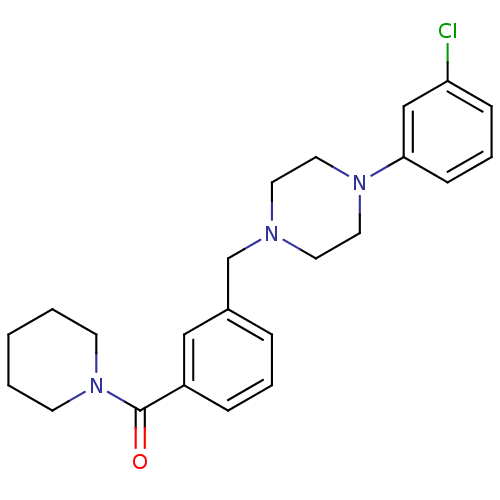 (CHEMBL61117 | {3-[4-(3-Chloro-phenyl)-piperazin-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029301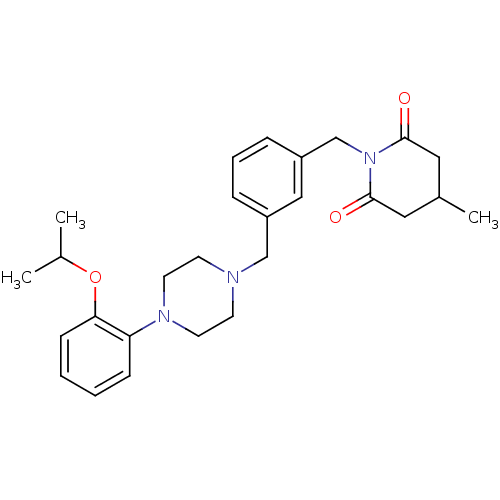 (1-{3-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377502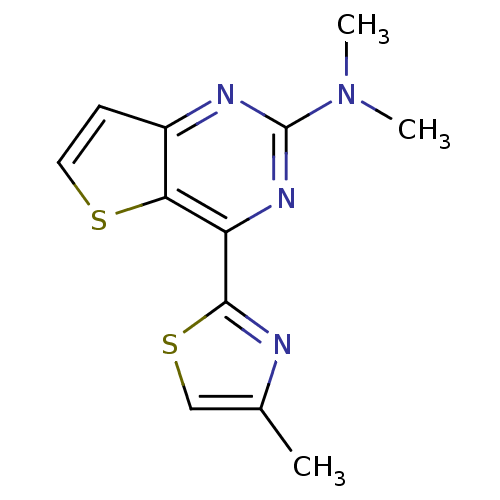 (CHEMBL406315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2920-3 (2008) Article DOI: 10.1016/j.bmcl.2008.03.076 BindingDB Entry DOI: 10.7270/Q270829P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254013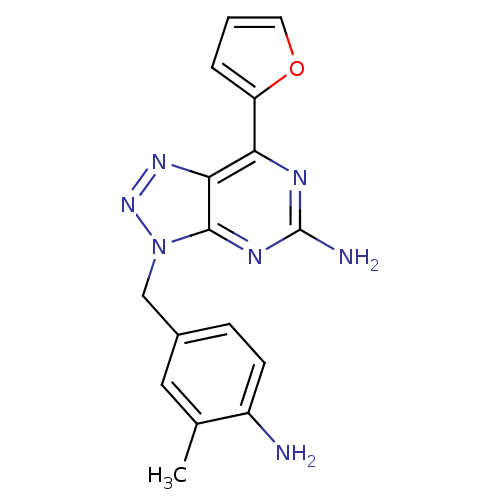 (3-(4-Amino-3-methylbenzyl)-7-(2-furyl)-3H-[1,2,3]t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50238959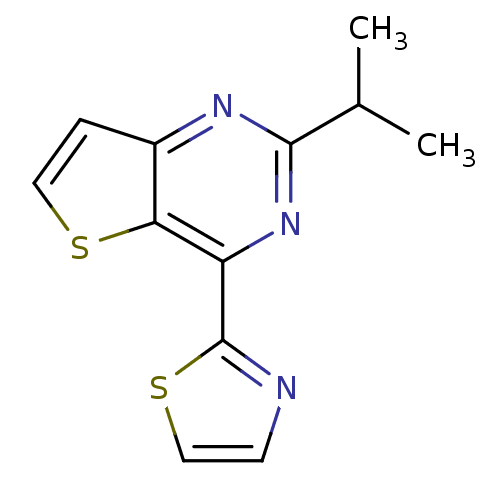 (2-isopropyl-4-(thiazol-2-yl)thieno[3,2-d]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2920-3 (2008) Article DOI: 10.1016/j.bmcl.2008.03.076 BindingDB Entry DOI: 10.7270/Q270829P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the dopamine receptor D2 using [3H]spiperinone. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254009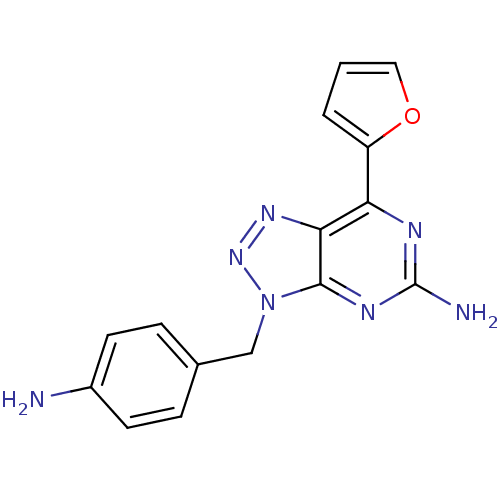 (3-(4-Aminobenzyl)-7-(2-furyl)-3H-[1,2,3]triazolo[4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254354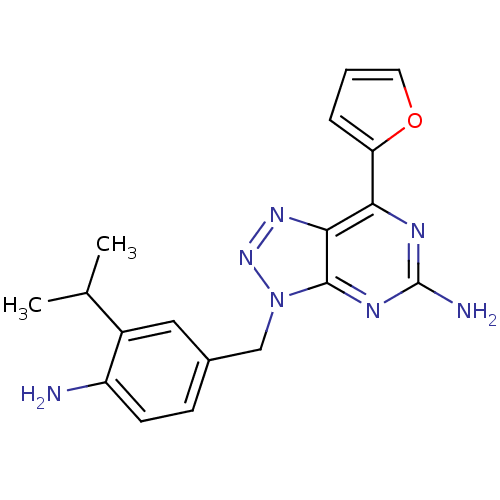 (3-(4-Amino-3-isopropylbenzyl)-7-(2-furyl)-3H-[1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029319 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377492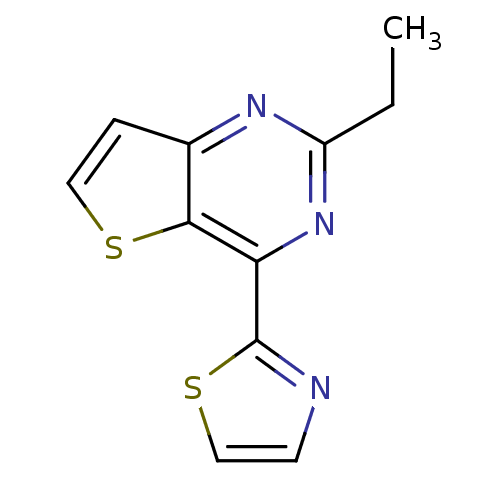 (CHEMBL407650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2920-3 (2008) Article DOI: 10.1016/j.bmcl.2008.03.076 BindingDB Entry DOI: 10.7270/Q270829P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254356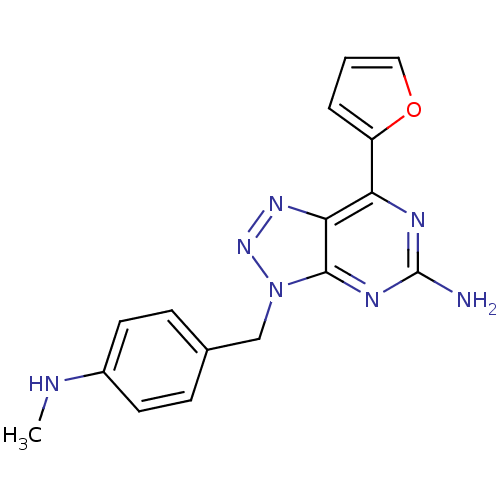 (7-(2-Furyl)-3-(4-(N-methylamino)benzyl)-3H-[1,2,3]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254355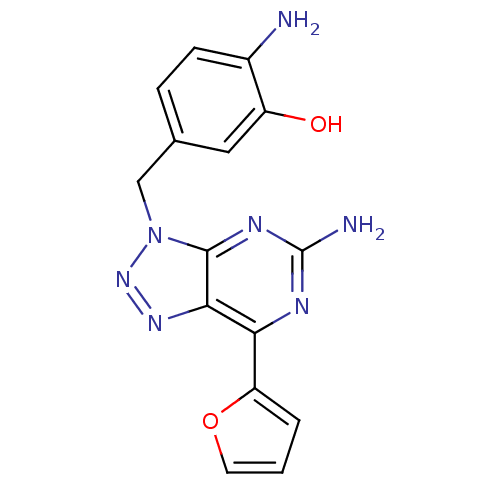 (3-(4-Amino-3-hydroxybenzyl)-7-(2-furyl)-3H-[1,2,3]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50294501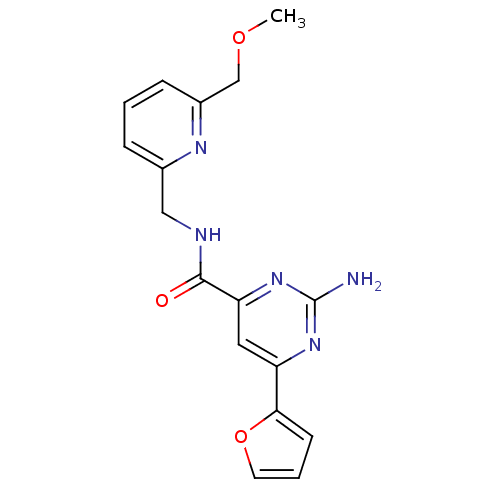 (2-amino-6-(furan-2-yl)-N-((6-(methoxymethyl)pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R+D) Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor | Bioorg Med Chem Lett 19: 2664-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.142 BindingDB Entry DOI: 10.7270/Q2RF5V1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029257 ((3-((4-(2-isopropoxyphenyl)piperazin-1-yl)methyl)p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50064558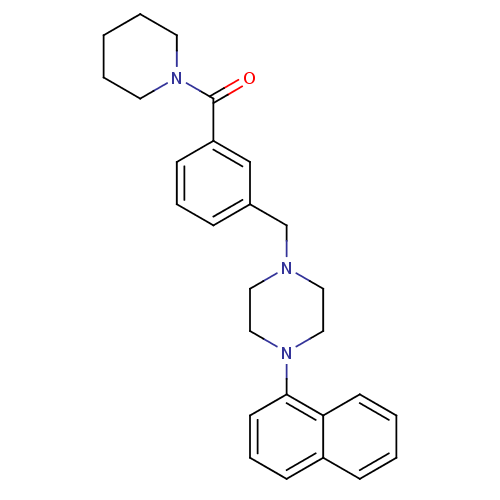 (CHEMBL59853 | [3-(4-Naphthalen-1-yl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against 5-hydroxytryptamine 1A receptor from rat cerebral cortex | J Med Chem 41: 1997-2009 (1998) Article DOI: 10.1021/jm970164z BindingDB Entry DOI: 10.7270/Q2KK9CG0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254004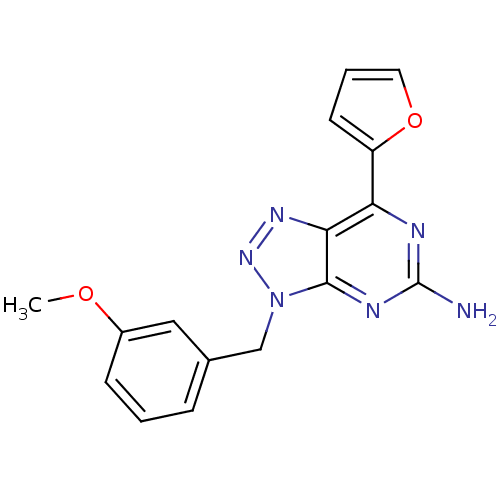 (7-(2-Furyl)-3-(3-methoxybenzyl)-3H-[1,2,3]triazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254295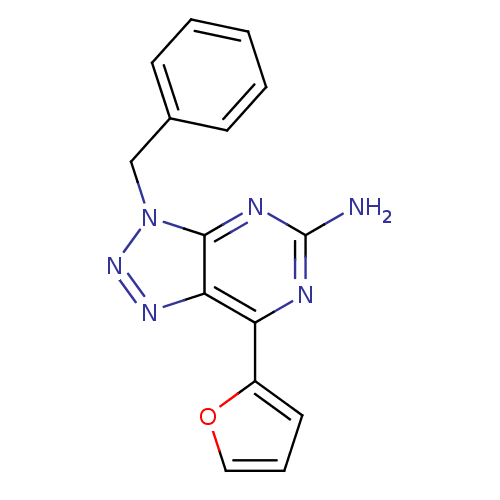 (3-Benzyl-7-(2-furyl)-3H-[1,2,3]triazolo[4,5-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50254300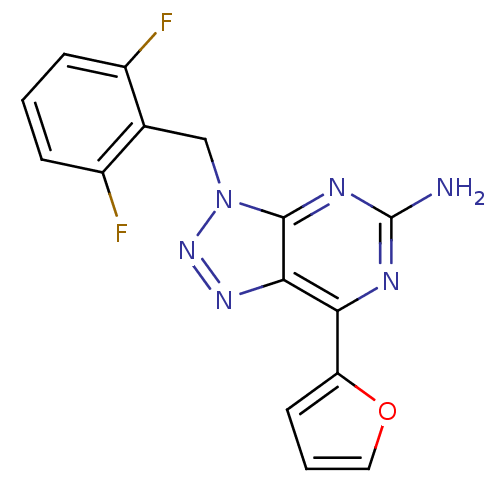 (3-(2,6-Difluorobenzyl)-7-(2-furyl)-3H-[1,2,3]triaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50253994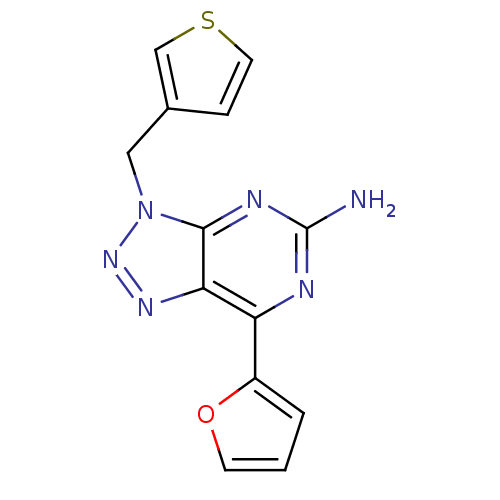 (7-(2-Furyl)-3-(3-thienylmethyl)-3H-[1,2,3]triazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis R&D Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human recombinant adenosine A2A receptor at 21 degC after 90 mins by cell-based microplate scintillation counting | J Med Chem 52: 33-47 (2009) Article DOI: 10.1021/jm800961g BindingDB Entry DOI: 10.7270/Q2XG9R0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5671 total ) | Next | Last >> |