

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069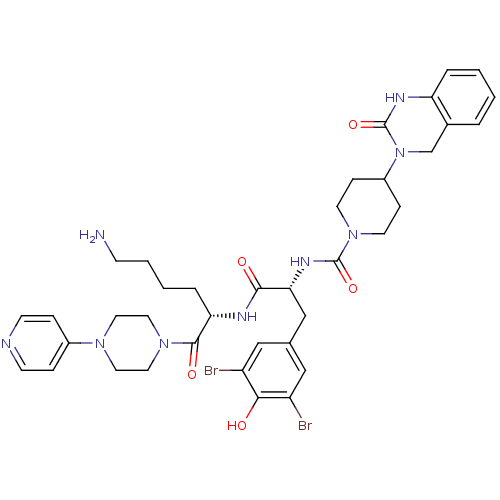 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447327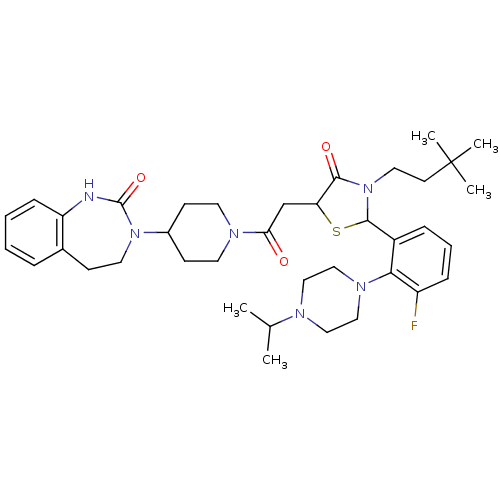 (CHEMBL3114495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447325 (CHEMBL3114676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447328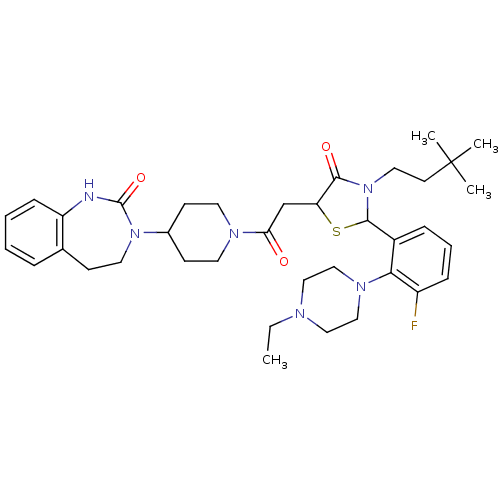 (CHEMBL3114494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447326 (CHEMBL3114496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103072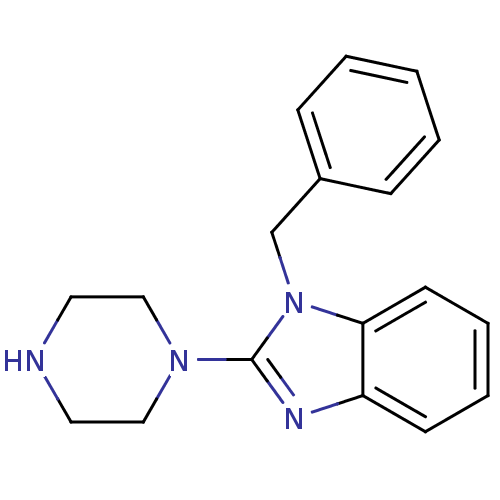 (1-Benzyl-2-piperazin-1-yl-1H-benzoimidazole | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103070 (1-(4-Methoxy-benzyl)-2-piperazin-1-yl-1H-benzoimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447332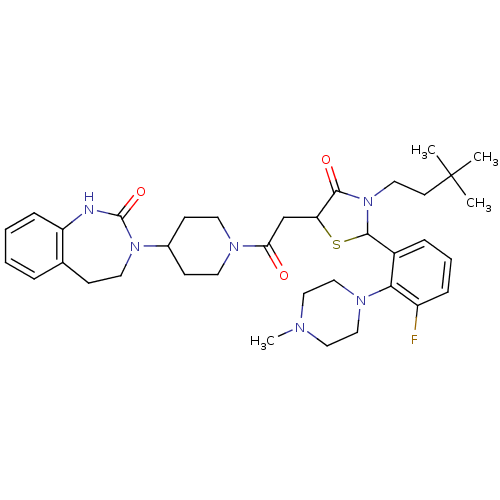 (CHEMBL3114490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447335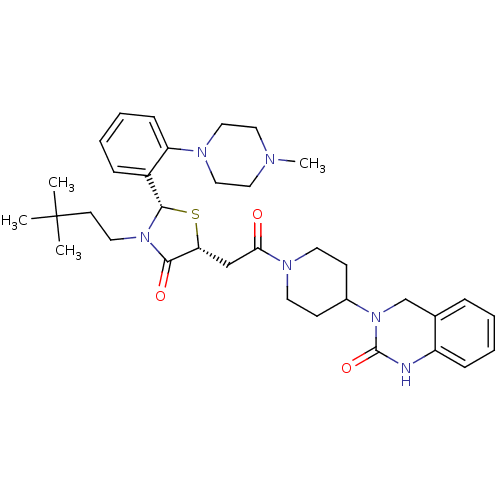 (CHEMBL3114486) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447329 (CHEMBL3114493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447324 (CHEMBL3114677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447333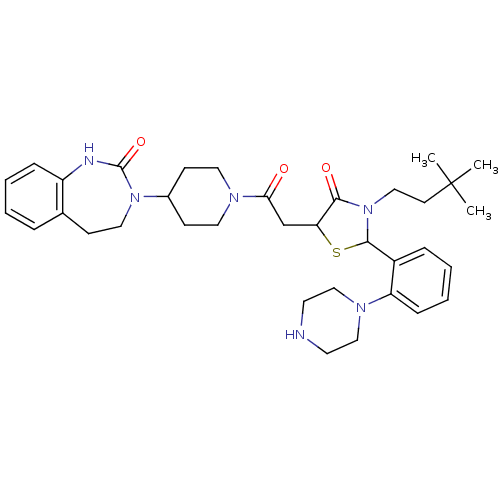 (CHEMBL3114489) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103076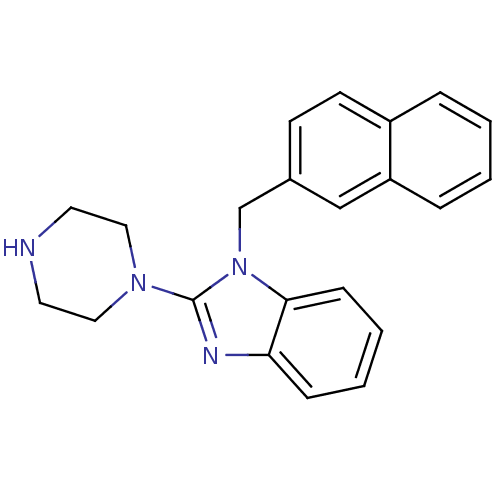 (1-Naphthalen-2-ylmethyl-2-piperazin-1-yl-1H-benzoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103075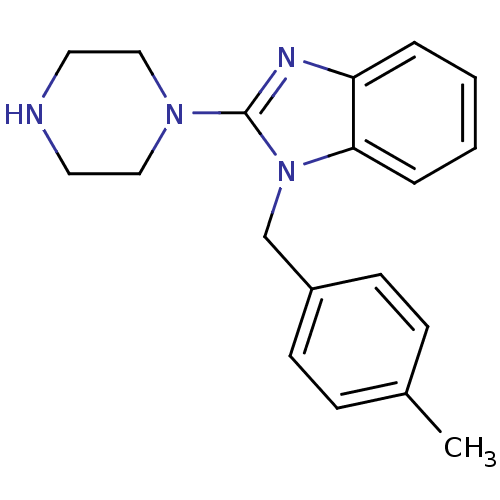 (1-(4-Methyl-benzyl)-2-piperazin-1-yl-1H-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447323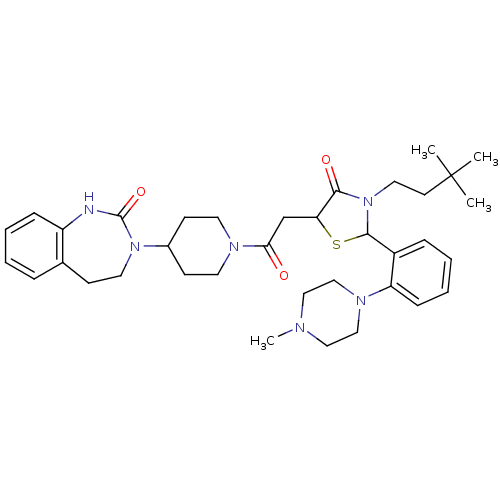 (CHEMBL3114488) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447337 (CHEMBL3114484) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447345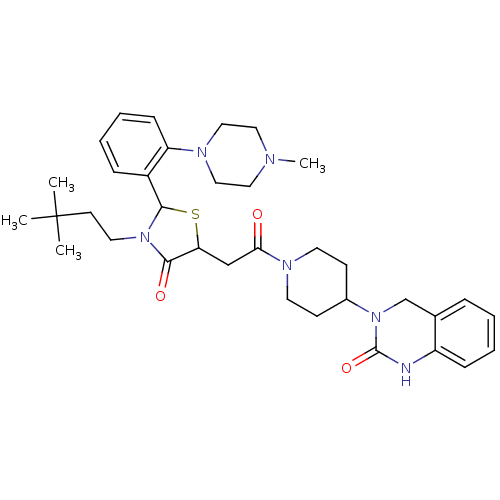 (CHEMBL3114476) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447347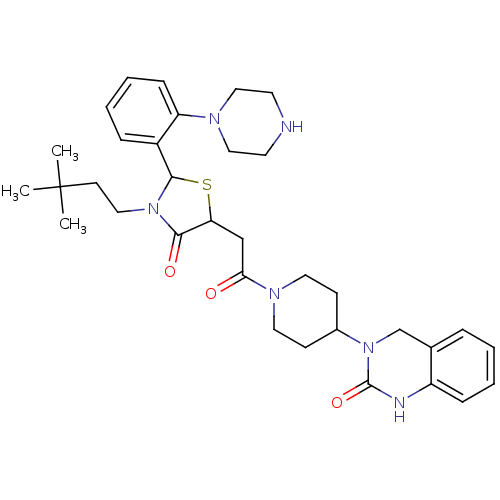 (CHEMBL3114474) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447339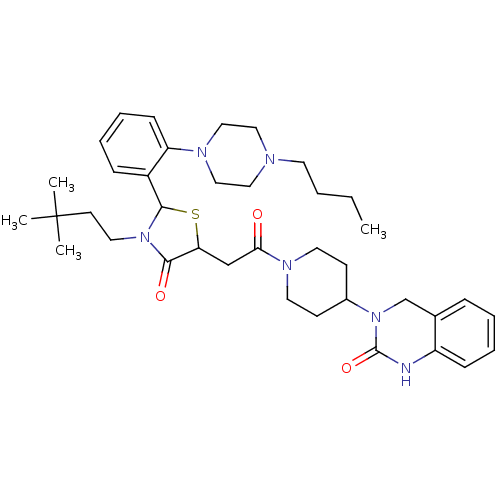 (CHEMBL3114482) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323404 (US9630956, Compound I-141) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323405 (US9630956, Compound I-142) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323406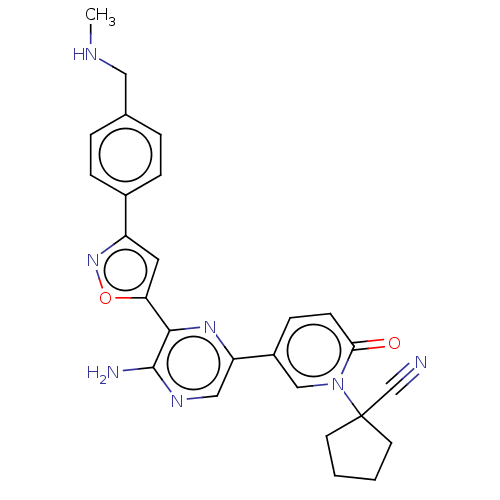 (US9630956, Compound I-143) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323407 (US9630956, Compound I-144) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323408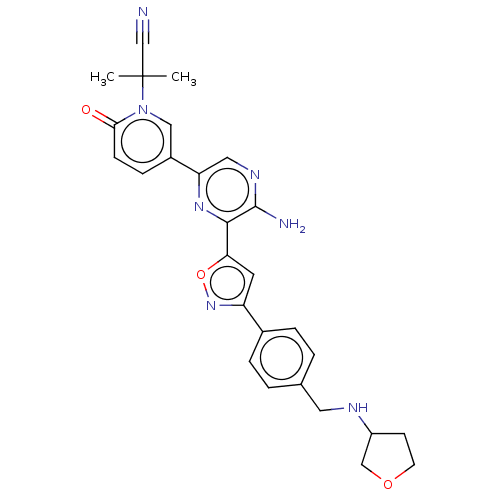 (US9630956, Compound I-145) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323409 (US9630956, Compound I-146) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323410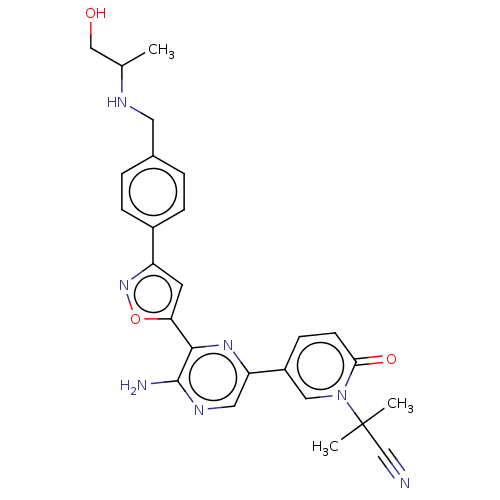 (US9630956, Compound I-147) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323411 (US9630956, Compound I-148) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323277 (US9630956, Compound I-15) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323317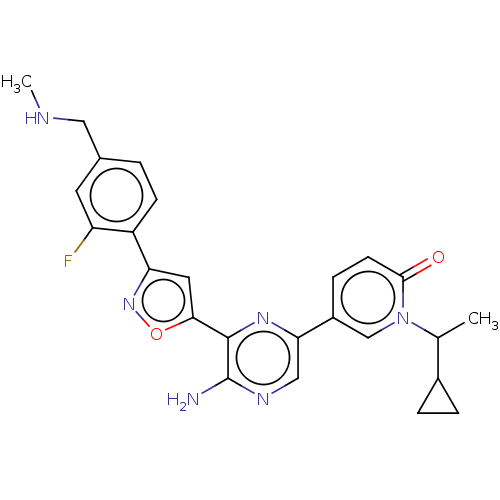 (US9630956, Compound I-54) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323325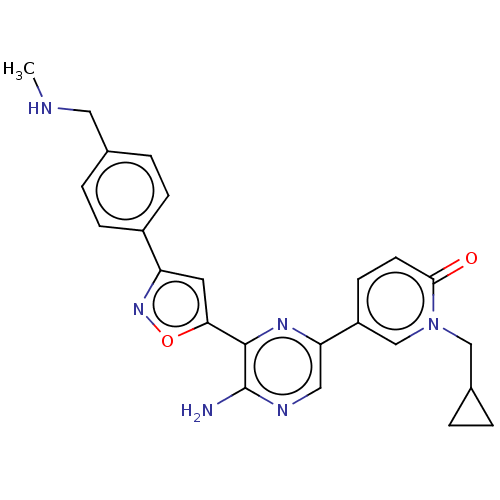 (US9630956, Compound I-62) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323298 (US9630956, Compound I-35) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323341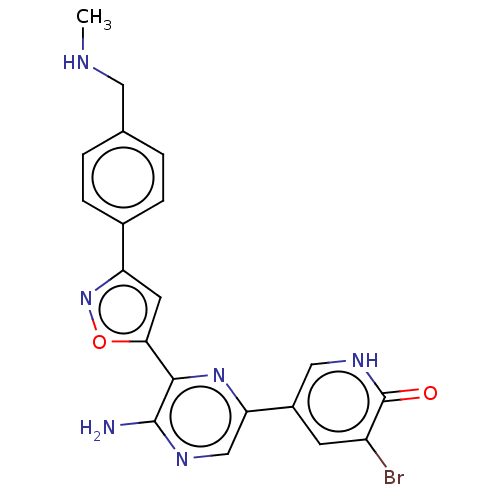 (US9630956, Compound I-78) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323349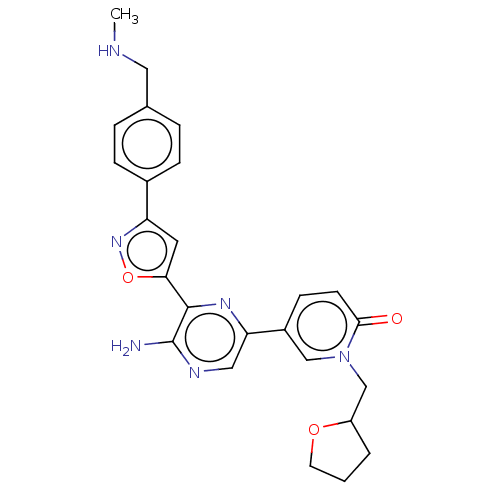 (US9630956, Compound I-86) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323331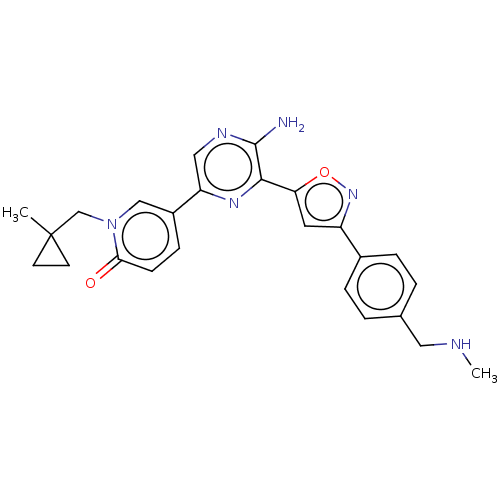 (US9630956, Compound I-68) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323332 (US9630956, Compound I-69) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323333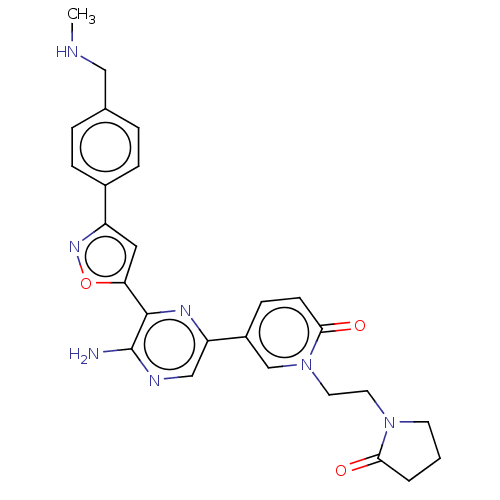 (US9630956, Compound I-70) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323335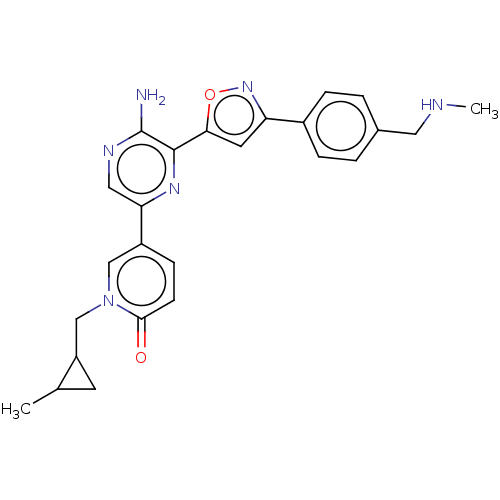 (US9630956, Compound I-72) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323336 (US9630956, Compound I-73) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323337 (US9630956, Compound I-74) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323340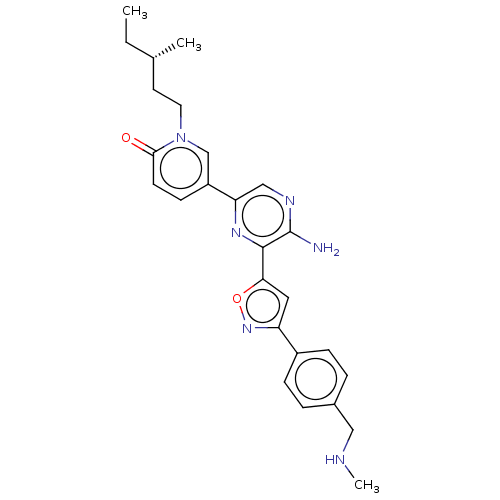 (US9630956, Compound I-77) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323342 (US9630956, Compound I-79) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323343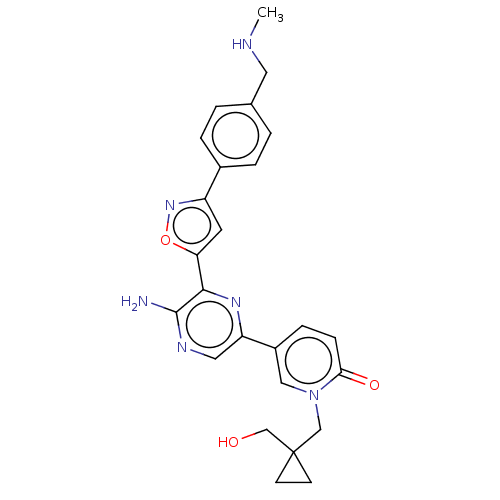 (US9630956, Compound I-80) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323344 (US9630956, Compound I-81) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323345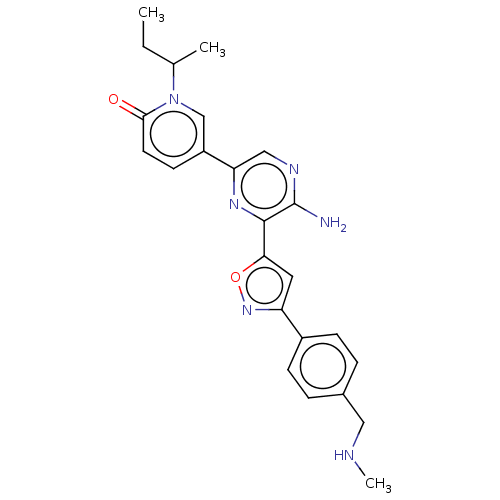 (US9630956, Compound I-82) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323346 (US9630956, Compound I-83) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323347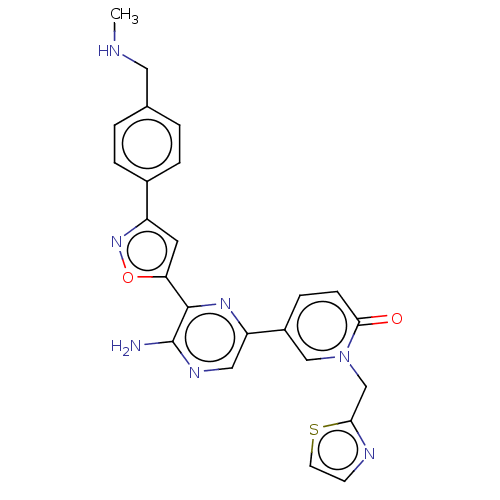 (US9630956, Compound I-84) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323348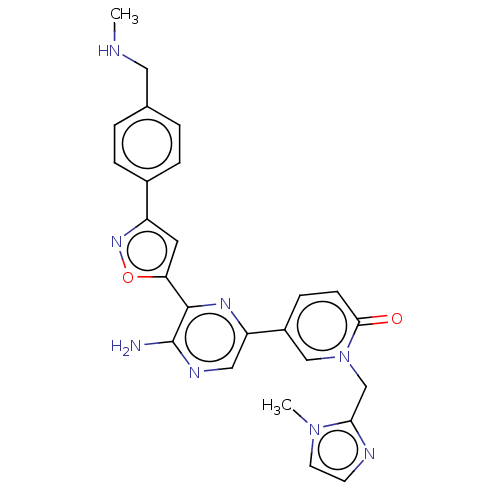 (US9630956, Compound I-85) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323350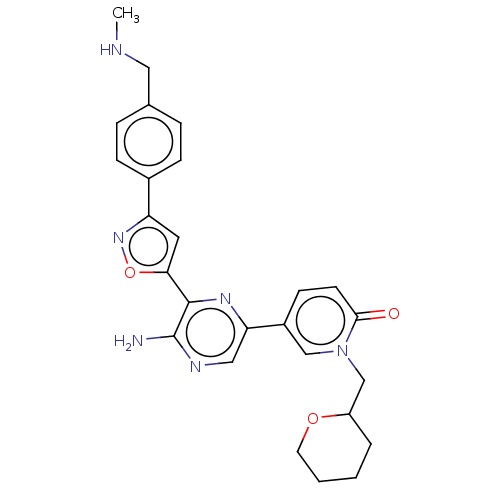 (US9630956, Compound I-87) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323351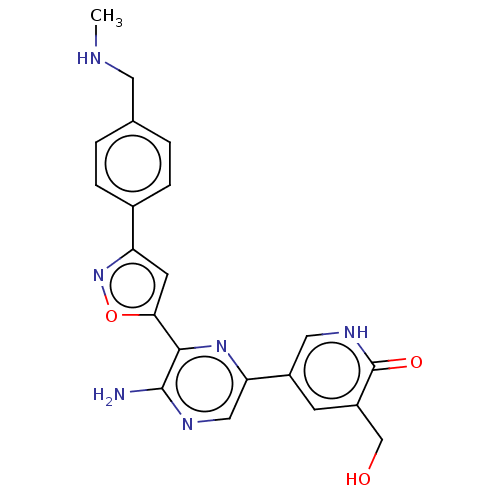 (US9630956, Compound I-88) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM323352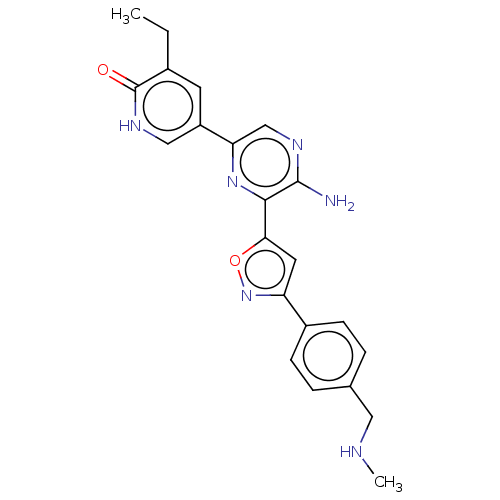 (US9630956, Compound I-89) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US9630956 (2017) BindingDB Entry DOI: 10.7270/Q2X63Q2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1254 total ) | Next | Last >> |