

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25256 (bisphosphonate, 9 | hydrogen [2-(dodecyldimethylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25259 (3-(decyloxy)-1-(2-hydrogen phosphonato-2-phosphono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25258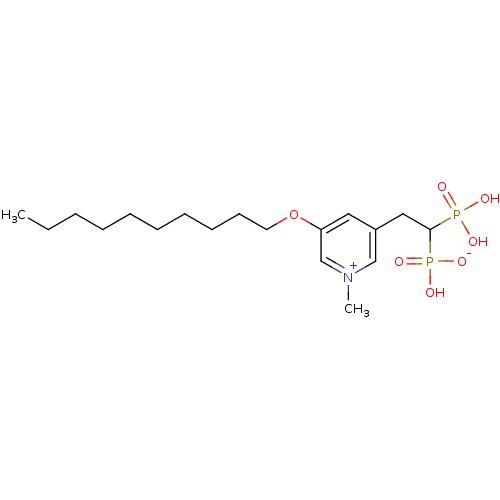 (3-(decyloxy)-5-(2-hydrogen phosphonato-2-phosphono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25260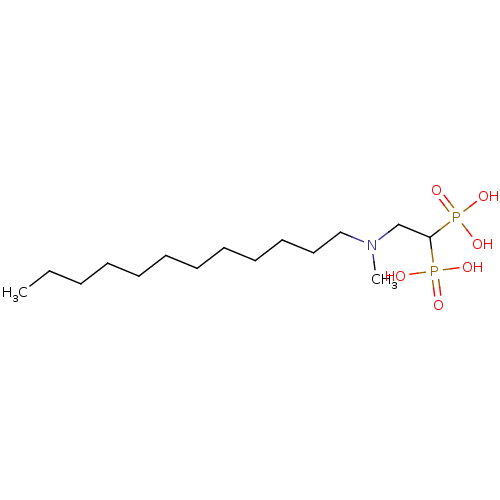 (bisphosphonate, 16 | {2-[dodecyl(methyl)amino]-1-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25261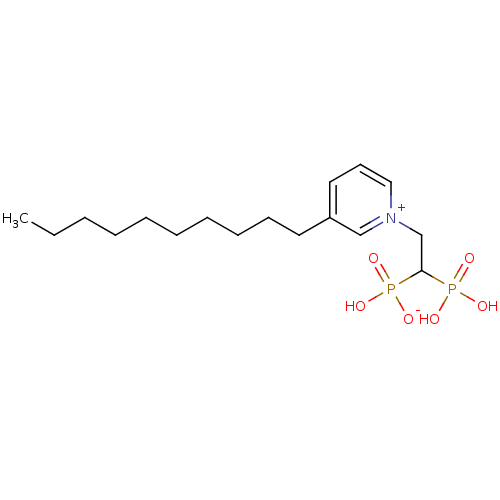 (3-decyl-1-(2-hydrogen phosphonato-2-phosphonoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25262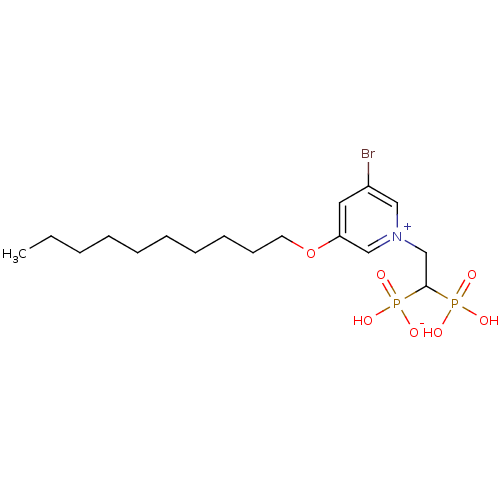 (3-bromo-5-(decyloxy)-1-(2-hydrogen phosphonato-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25263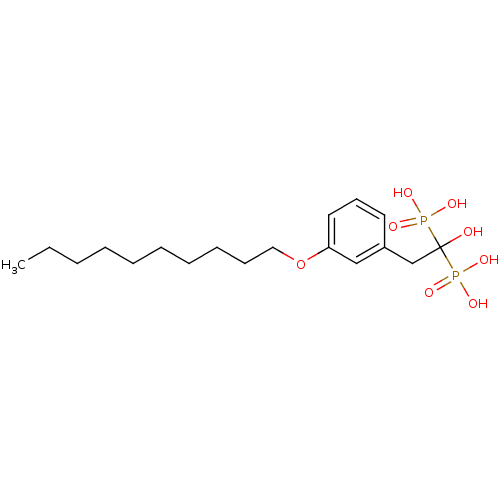 (bisphosphonate, 19 | {2-[3-(decyloxy)phenyl]-1-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25264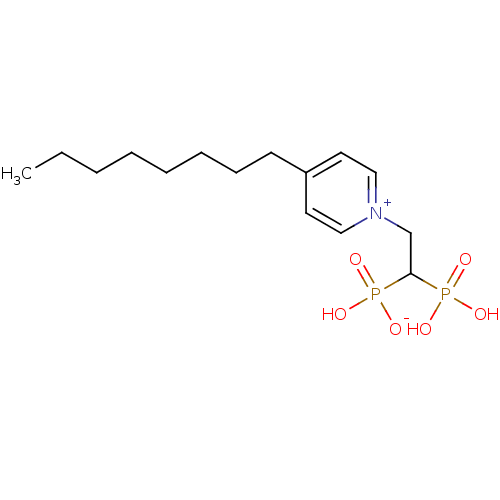 (1-(2-hydrogen phosphonato-2-phosphonoethyl)-4-octy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25265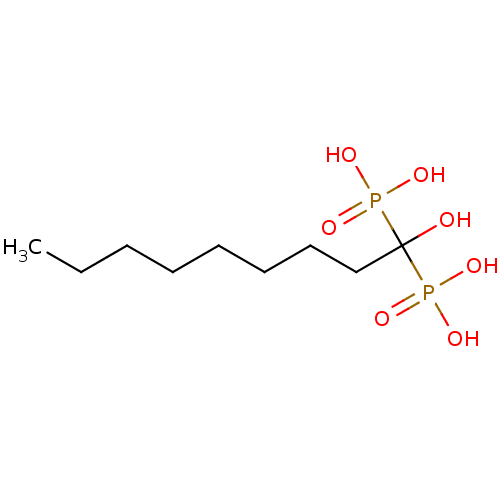 ((1-hydroxy-1-phosphonononyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25266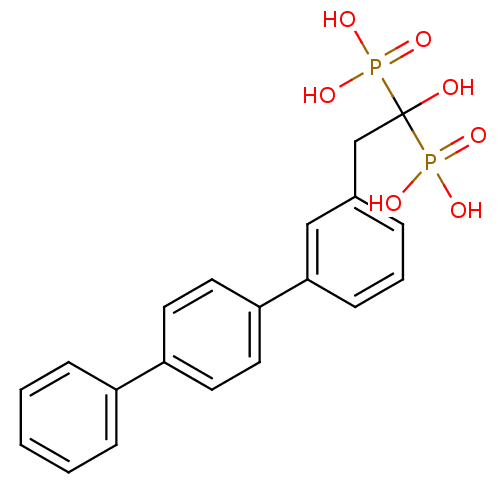 (BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25267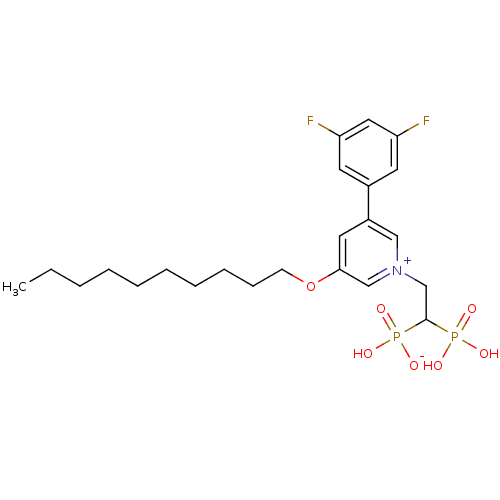 (3-(decyloxy)-5-(3,5-difluorophenyl)-1-(2-hydrogen ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25268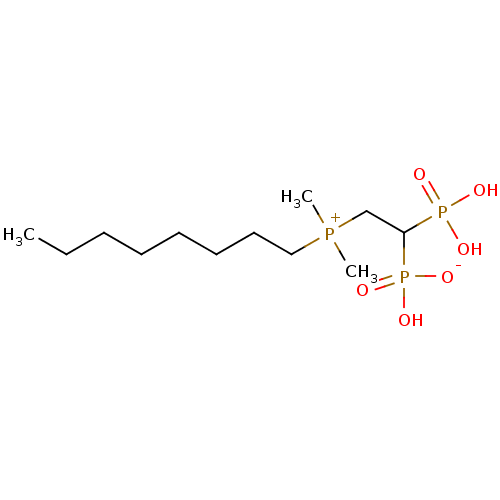 (bisphosphonate, 22 | hydrogen {2-[dimethyl(octyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25269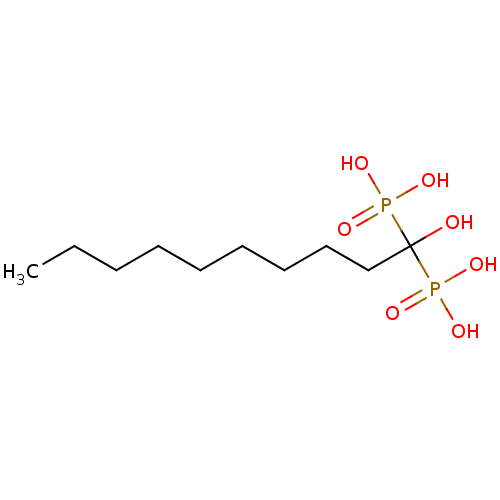 ((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25270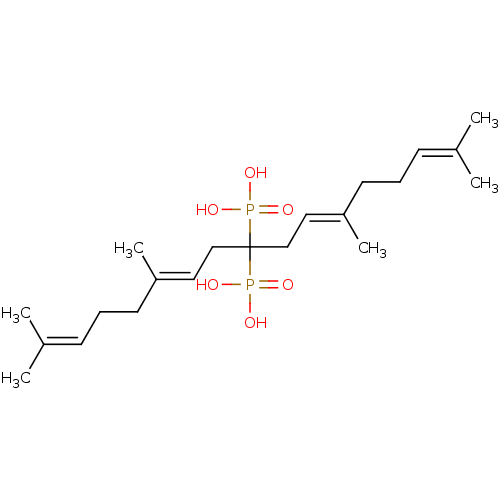 ([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25271 (3-[(3,7-dimethyloctyl)oxy]-1-(2-hydrogen phosphona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25272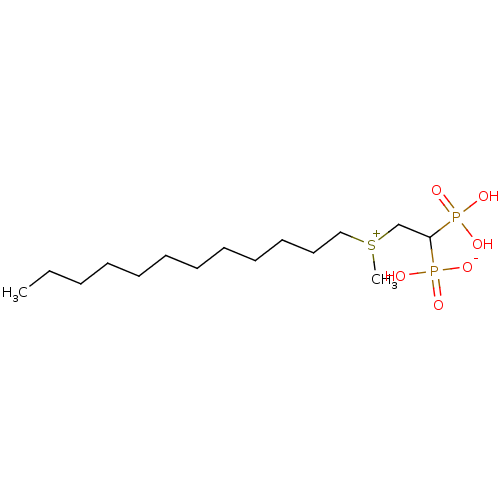 (CHEMBL238046 | bisphosphonate, 25 | hydrogen {2-[d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25273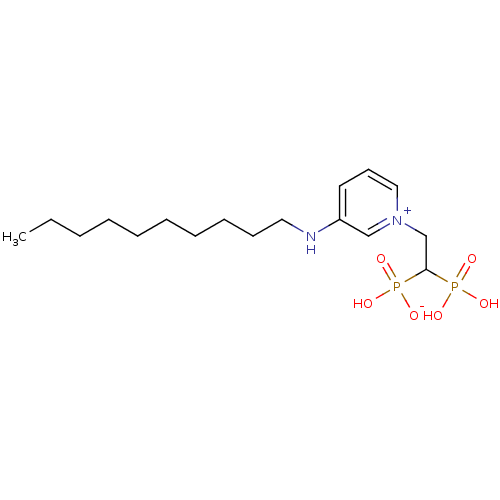 (3-(decylamino)-1-(2-hydrogen phosphonato-2-phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25274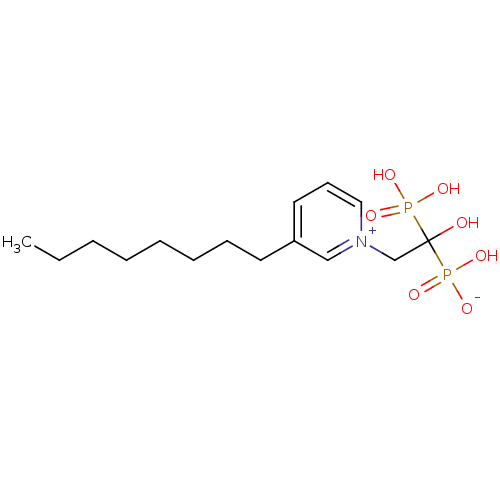 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25275 (CHEMBL237808 | bisphosphonate, 28 | hydrogen {2-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25276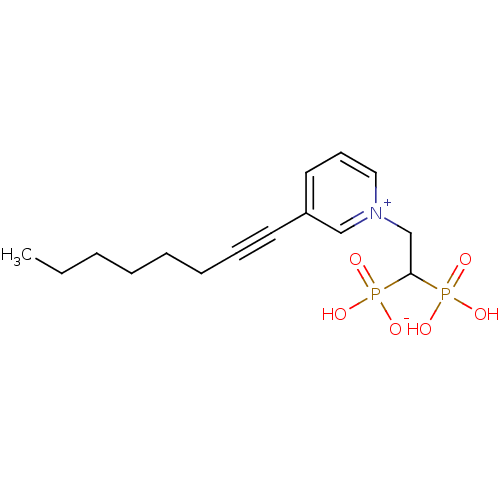 (1-(2-hydrogen phosphonato-2-phosphonoethyl)-3-(oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25277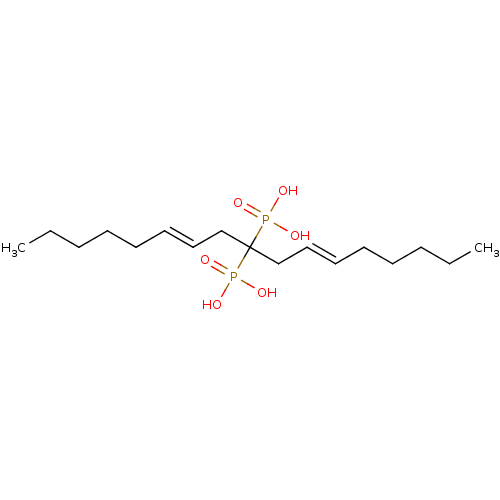 ([(6E,11E)-9-phosphonoheptadeca-6,11-dien-9-yl]phos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25279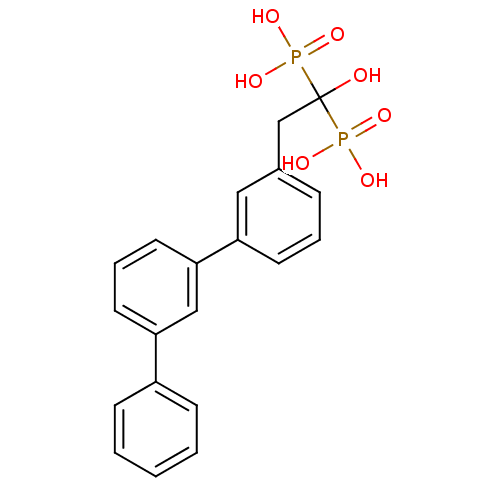 (BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25278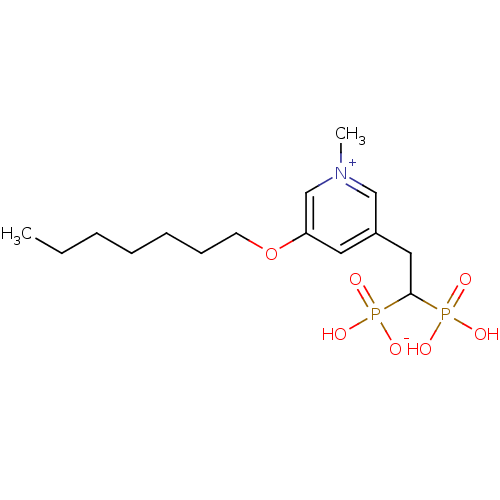 (3-(heptyloxy)-5-(2-hydrogen phosphonato-2-phosphon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25280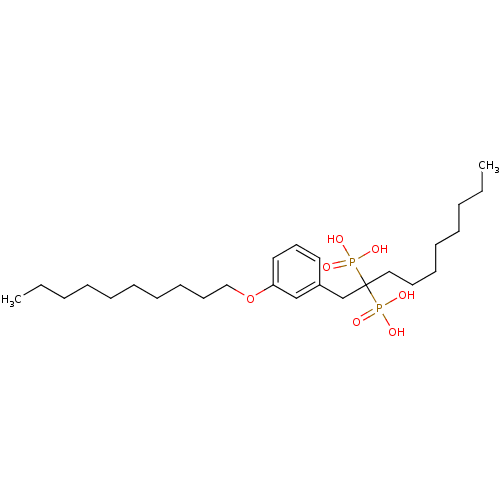 (bisphosphonate, 32 | {1-[3-(decyloxy)phenyl]-2-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25281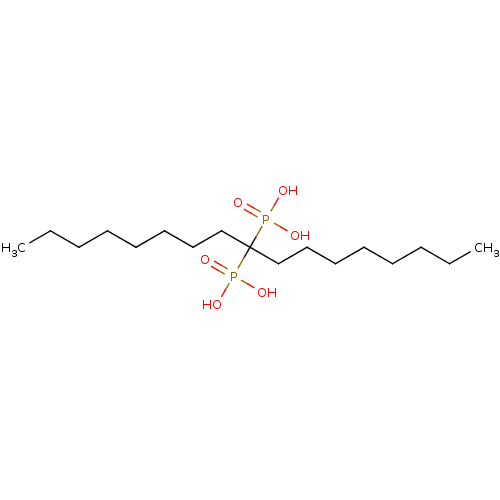 ((9-phosphonoheptadecan-9-yl)phosphonic acid | bisp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25282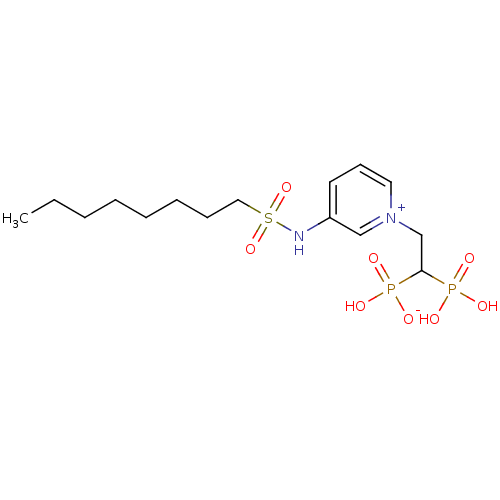 (1-(2-hydrogen phosphonato-2-phosphonoethyl)-3-(oct...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25283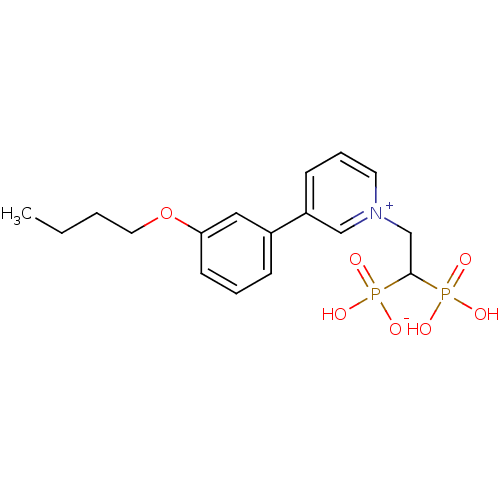 (3-(3-butoxyphenyl)-1-(2-hydrogen phosphonato-2-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25285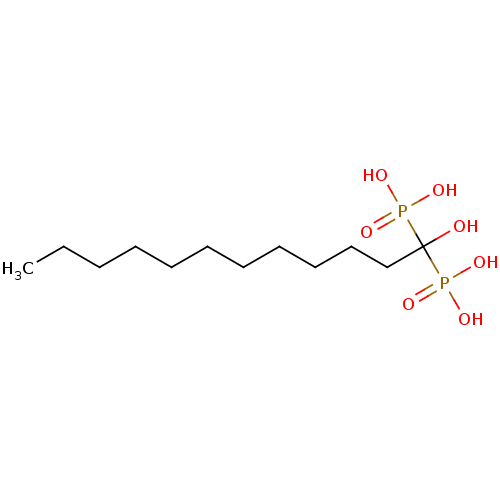 ((1-hydroxy-1-phosphonododecyl)phosphonic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25284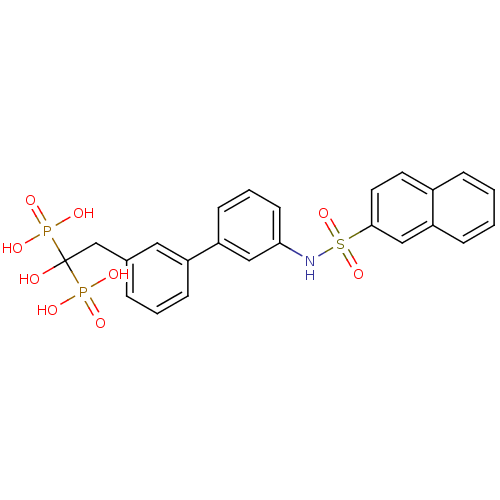 ((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25286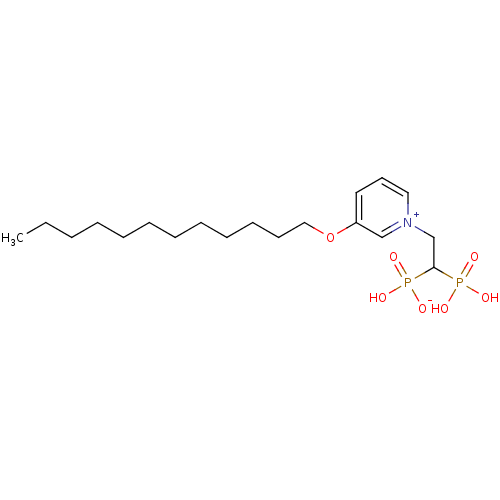 (3-(dodecyloxy)-1-(2-hydrogen phosphonato-2-phospho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25287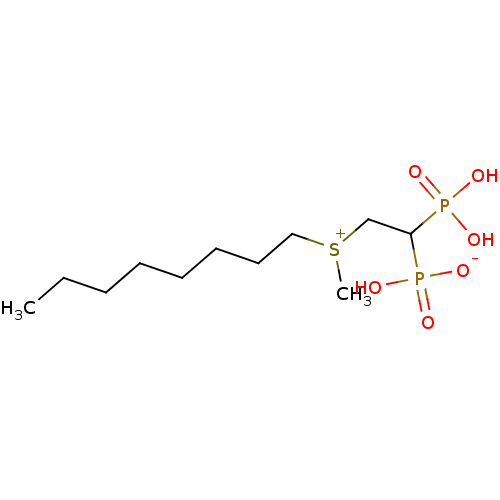 (CHEMBL235690 | bisphosphonate, 37 | hydrogen {2-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25288 (BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25289 (bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25290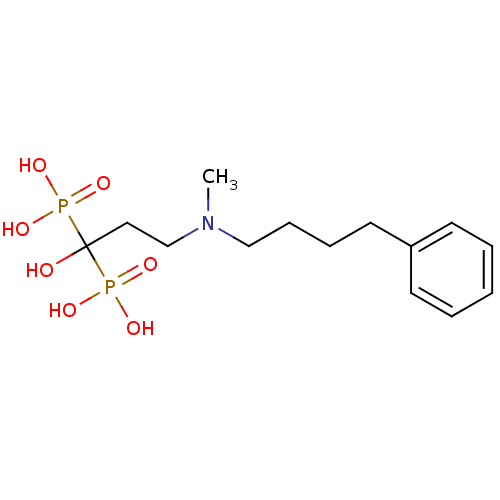 (CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25291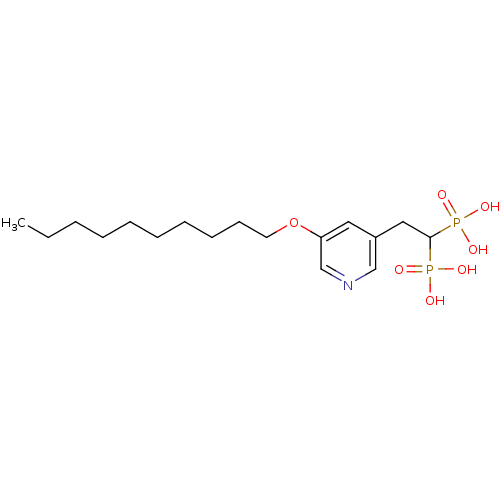 (bisphosphonate, 40 | {2-[5-(decyloxy)pyridin-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25292 (bisphosphonate, 41 | {2-[5-(dodecyloxy)pyridin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25293 (3-hexyl-1-(2-hydrogen phosphonato-2-phosphonoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25294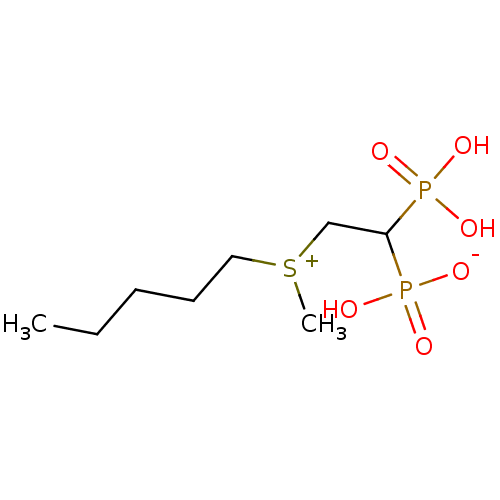 (CHEMBL392884 | bisphosphonate, 43 | hydrogen {2-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25295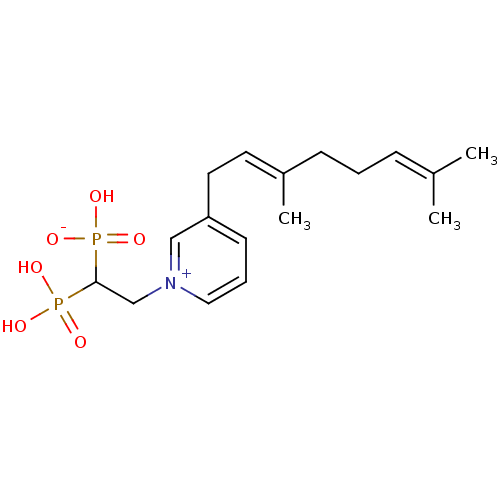 (3-[(2E)-3,7-dimethylocta-2,6-dien-1-yl]-1-(2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25296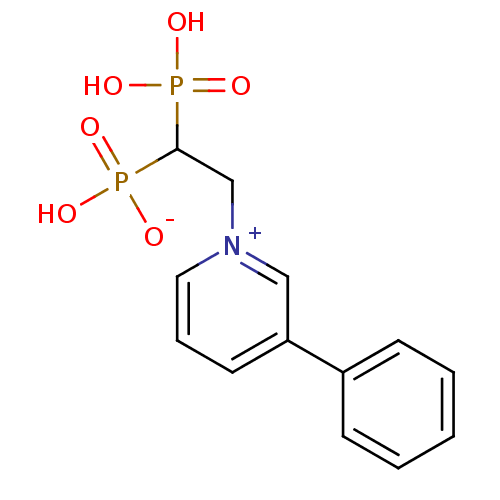 (1-(2-hydrogen phosphonato-2-phosphonoethyl)-3-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25297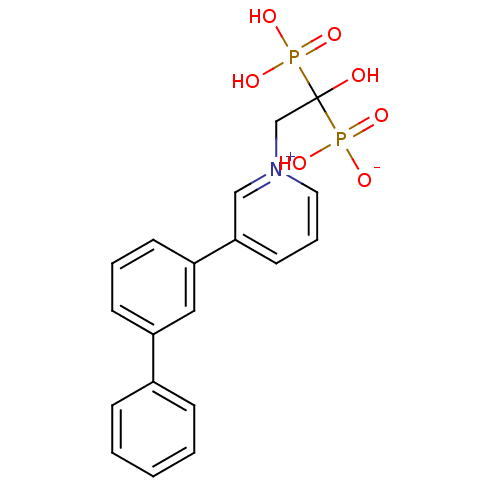 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25298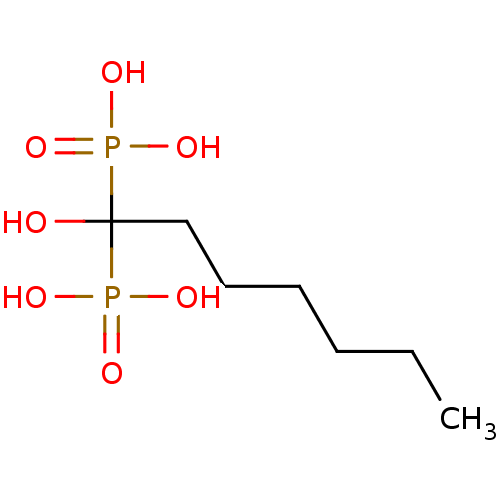 ((1-hydroxy-1-phosphonoheptyl)phosphonic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25299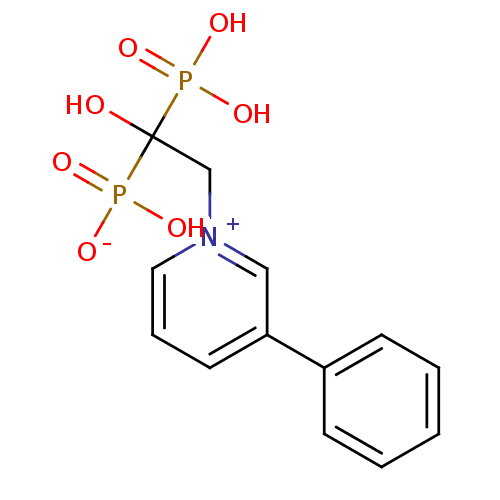 (1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25300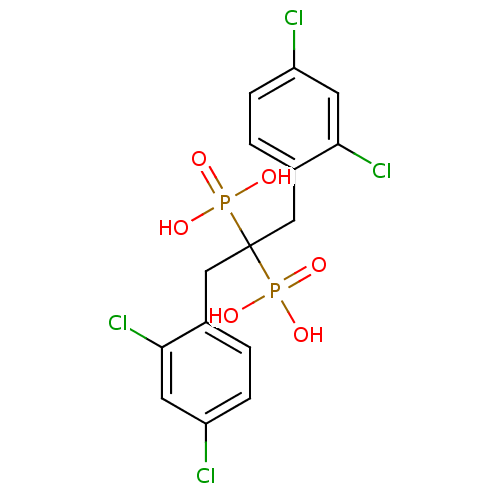 ([1,3-bis(2,4-dichlorophenyl)-2-phosphonopropan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25301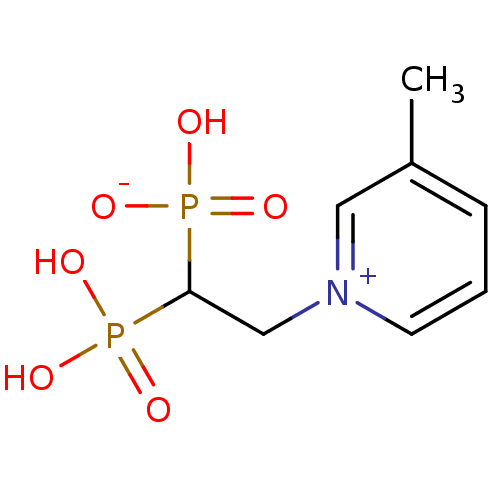 (1-(2-hydrogen phosphonato-2-phosphonoethyl)-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25302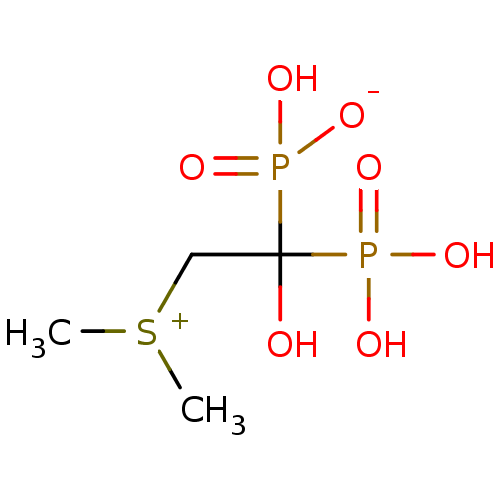 (CHEMBL277580 | bisphosphonate, 49 | hydrogen [2-(d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25303 (CHEMBL235059 | bisphosphonate, 50 | hydrogen {2-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25304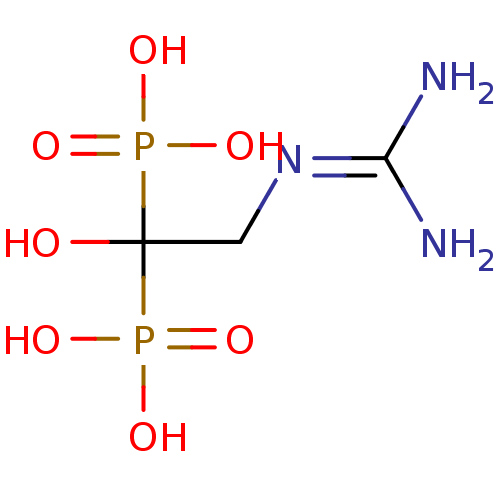 ((2-carbamimidamido-1-hydroxy-1-phosphonoethyl)phos...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25305 (bisphosphonate, 52 | hydrogen {2-[(10-carboxydecyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12577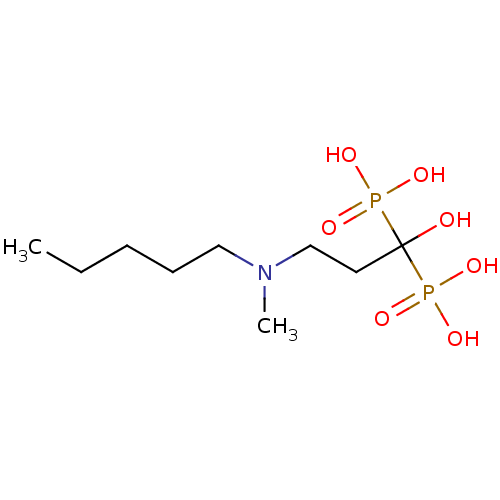 (Bisphosphonate 2 | CHEMBL997 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University | Assay Description The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... | J Med Chem 51: 5594-607 (2008) Article DOI: 10.1021/jm800325y BindingDB Entry DOI: 10.7270/Q2028PVT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |