

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25041 (N'-[(1E)-{6-cyanoimidazo[1,2-a]pyridin-3-yl}methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 5837-44 (2007) Article DOI: 10.1016/j.bmc.2007.05.070 BindingDB Entry DOI: 10.7270/Q2X928M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25036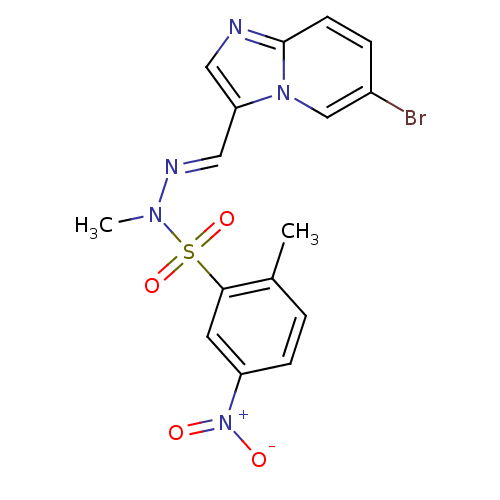 (CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 5837-44 (2007) Article DOI: 10.1016/j.bmc.2007.05.070 BindingDB Entry DOI: 10.7270/Q2X928M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Rattus norvegicus (Rat)) | BDBM130711 (US8822448, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Astellas Pharma Inc. US Patent | Assay Description The PDE9-inhibiting activity was measured by the following method. That is, to a buffer solution containing tris(hydroxymethyl)aminomethane-hydrochlo... | US Patent US8822448 (2014) BindingDB Entry DOI: 10.7270/Q2BK1B1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271230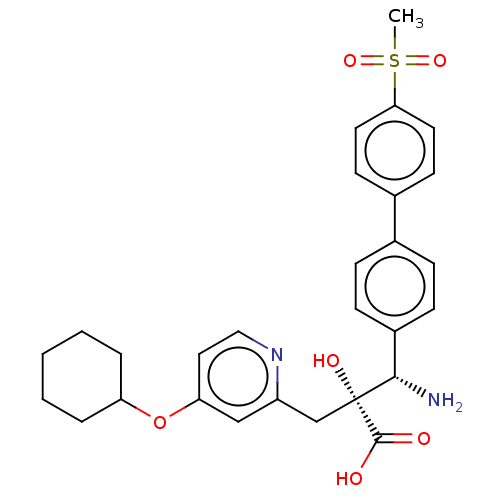 (US10059720, Example 45 | US10975091, Example 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271230 (US10059720, Example 45 | US10975091, Example 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271290 (US10059720, Example 105 | US10975091, Example 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271290 (US10059720, Example 105 | US10975091, Example 105) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271330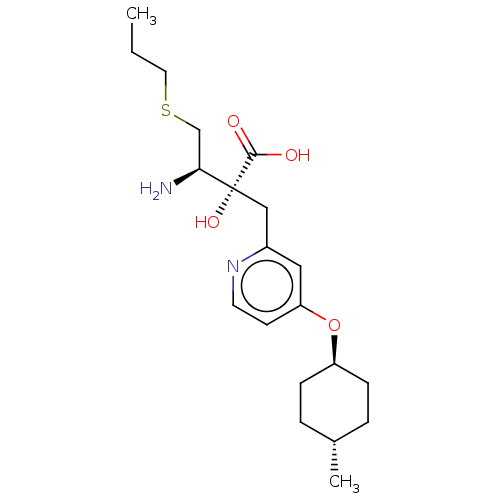 (US10059720, Example 145 | US10975091, Example 145) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM25037 (N'-[(1E)-{6-chloroimidazo[1,2-a]pyridin-3-yl}methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc. | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Bioorg Med Chem 15: 5837-44 (2007) Article DOI: 10.1016/j.bmc.2007.05.070 BindingDB Entry DOI: 10.7270/Q2X928M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271330 (US10059720, Example 145 | US10975091, Example 145) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271257 (US10059720, Example 72 | US10975091, Example 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271257 (US10059720, Example 72 | US10975091, Example 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271230 (US10059720, Example 45 | US10975091, Example 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271230 (US10059720, Example 45 | US10975091, Example 45) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271310 (US10059720, Example 125 | US10975091, Example 125) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271310 (US10059720, Example 125 | US10975091, Example 125) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271316 (US10059720, Example 131 | US10975091, Example 131) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271316 (US10059720, Example 131 | US10975091, Example 131) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271309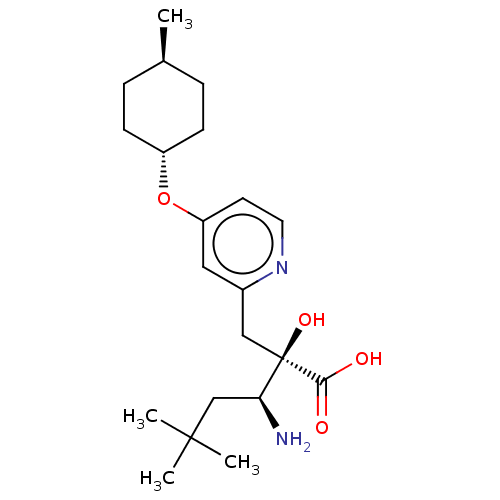 (US10059720, Example 124 | US10975091, Example 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271309 (US10059720, Example 124 | US10975091, Example 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271257 (US10059720, Example 72 | US10975091, Example 72) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271257 (US10059720, Example 72 | US10975091, Example 72) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271275 (US10059720, Example 90 | US10975091, Example 90) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271275 (US10059720, Example 90 | US10975091, Example 90) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271318 (US10059720, Example 133 | US10975091, Example 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271198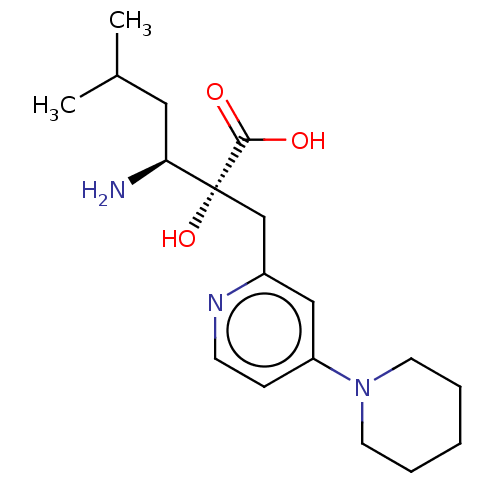 (US10059720, Example 13 | US10975091, Example 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271318 (US10059720, Example 133 | US10975091, Example 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271275 (US10059720, Example 90 | US10975091, Example 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271288 (US10059720, Example 103 | US10975091, Example 103) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271198 (US10059720, Example 13 | US10975091, Example 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271275 (US10059720, Example 90 | US10975091, Example 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271288 (US10059720, Example 103 | US10975091, Example 103) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Rattus norvegicus (Rat)) | BDBM130738 (US8822448, 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Astellas Pharma Inc. US Patent | Assay Description The PDE9-inhibiting activity was measured by the following method. That is, to a buffer solution containing tris(hydroxymethyl)aminomethane-hydrochlo... | US Patent US8822448 (2014) BindingDB Entry DOI: 10.7270/Q2BK1B1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271258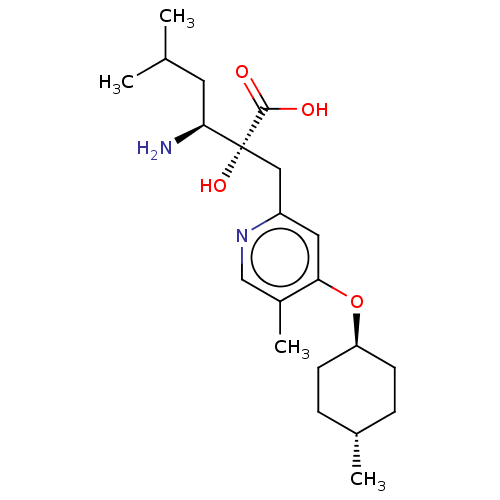 (US10059720, Example 73 | US10975091, Example 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271277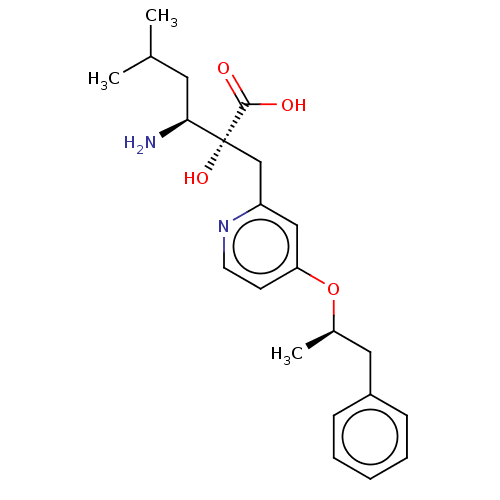 (US10059720, Example 92 | US10975091, Example 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271258 (US10059720, Example 73 | US10975091, Example 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271277 (US10059720, Example 92 | US10975091, Example 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271213 (US10059720, Example 28 | US10975091, Example 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271190 (US10059720, Example 6 | US10975091, Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (Rattus norvegicus (Rat)) | BDBM130781 (US8822448, 211) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Astellas Pharma Inc. US Patent | Assay Description The PDE9-inhibiting activity was measured by the following method. That is, to a buffer solution containing tris(hydroxymethyl)aminomethane-hydrochlo... | US Patent US8822448 (2014) BindingDB Entry DOI: 10.7270/Q2BK1B1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271260 (US10059720, Example 75 | US10975091, Example 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271277 (US10059720, Example 92 | US10975091, Example 92) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271190 (US10059720, Example 6 | US10975091, Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271213 (US10059720, Example 28 | US10975091, Example 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICAL CO., LTD. US Patent | Assay Description IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP.... | US Patent US10975091 (2021) BindingDB Entry DOI: 10.7270/Q2FF3WHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271260 (US10059720, Example 75 | US10975091, Example 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271277 (US10059720, Example 92 | US10975091, Example 92) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271243 (US10059720, Example 58 | US10975091, Example 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271330 (US10059720, Example 145 | US10975091, Example 145) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Homo sapiens (Human)) | BDBM271190 (US10059720, Example 6 | US10975091, Example 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM271198 (US10059720, Example 13 | US10975091, Example 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.; KOTOBUKI PHARMACEUTICALS CO., LTD. US Patent | Assay Description Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m... | US Patent US10059720 (2018) BindingDB Entry DOI: 10.7270/Q2XD13Q5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 760 total ) | Next | Last >> |