 Found 632 hits with Last Name = 'kang' and Initial = 'b'
Found 632 hits with Last Name = 'kang' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20323
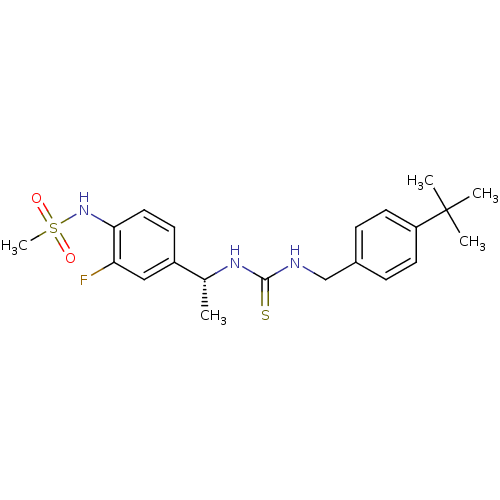
(3-[(4-tert-butylphenyl)methyl]-1-[(1R)-1-(3-fluoro...)Show SMILES C[C@@H](NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H28FN3O2S2/c1-14(16-8-11-19(18(22)12-16)25-29(5,26)27)24-20(28)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,25H,13H2,1-5H3,(H2,23,24,28)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.2 | -44.0 | n/a | n/a | 37 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20317

(3-[(4-tert-butylphenyl)methyl]-1-[(1R)-1-(4-methan...)Show SMILES C[C@@H](NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C21H29N3O2S2/c1-15(17-8-12-19(13-9-17)24-28(5,25)26)23-20(27)22-14-16-6-10-18(11-7-16)21(2,3)4/h6-13,15,24H,14H2,1-5H3,(H2,22,23,27)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | -43.9 | n/a | n/a | 4.5 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330
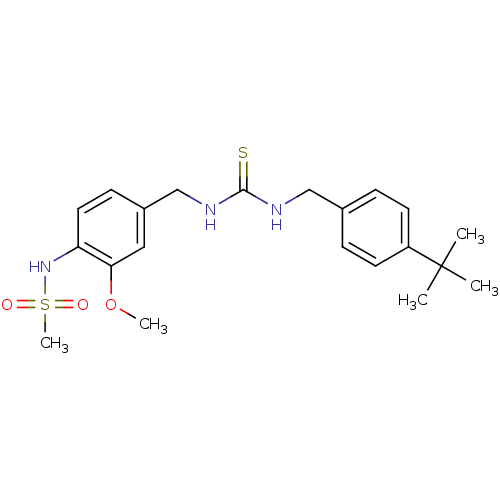
(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20321
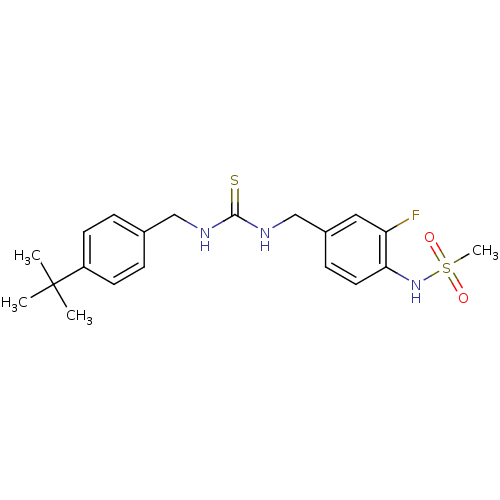
(3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NCc2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C20H26FN3O2S2/c1-20(2,3)16-8-5-14(6-9-16)12-22-19(27)23-13-15-7-10-18(17(21)11-15)24-28(4,25)26/h5-11,24H,12-13H2,1-4H3,(H2,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53.5 | -43.2 | n/a | n/a | 9.20 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20322

(3-[(4-tert-butylphenyl)methyl]-1-[1-(3-fluoro-4-me...)Show SMILES CC(NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H28FN3O2S2/c1-14(16-8-11-19(18(22)12-16)25-29(5,26)27)24-20(28)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,25H,13H2,1-5H3,(H2,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | -43.2 | n/a | n/a | 9.20 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20316
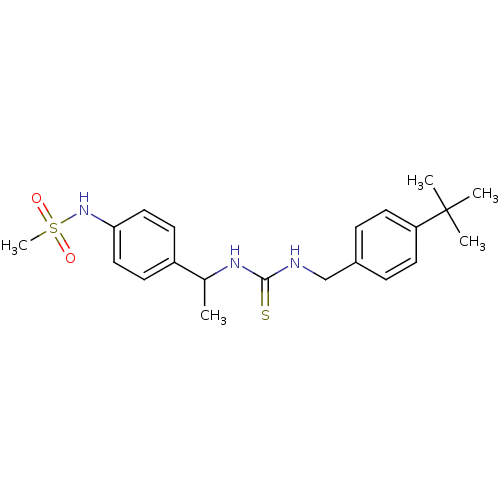
(3-[(4-tert-butylphenyl)methyl]-1-[1-(4-methanesulf...)Show SMILES CC(NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C21H29N3O2S2/c1-15(17-8-12-19(13-9-17)24-28(5,25)26)23-20(27)22-14-16-6-10-18(11-7-16)21(2,3)4/h6-13,15,24H,14H2,1-5H3,(H2,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59.3 | -42.9 | n/a | n/a | 14.7 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20315
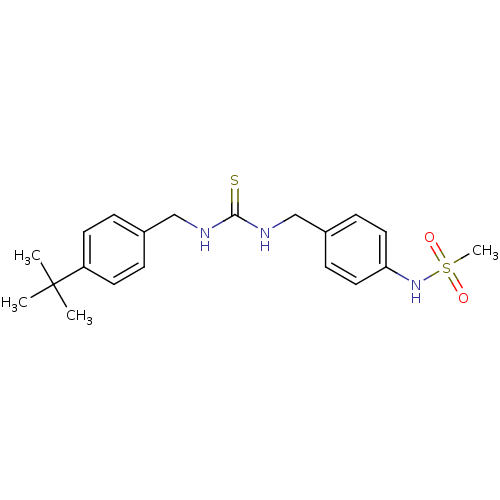
(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NCc2ccc(NS(C)(=O)=O)cc2)cc1 Show InChI InChI=1S/C20H27N3O2S2/c1-20(2,3)17-9-5-15(6-10-17)13-21-19(26)22-14-16-7-11-18(12-8-16)23-27(4,24)25/h5-12,23H,13-14H2,1-4H3,(H2,21,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | -42.8 | n/a | n/a | 54 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20331
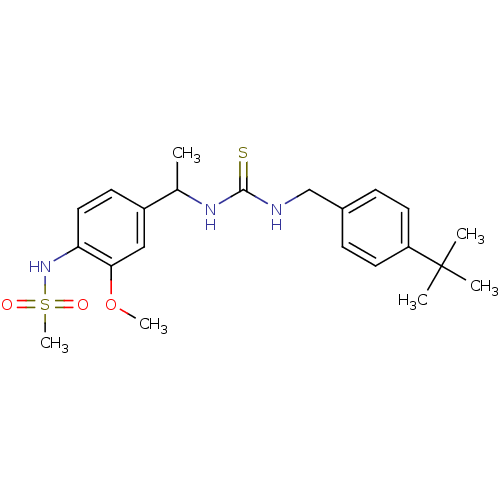
(3-[(4-tert-butylphenyl)methyl]-1-[1-(4-methanesulf...)Show SMILES COc1cc(ccc1NS(C)(=O)=O)C(C)NC(=S)NCc1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C22H31N3O3S2/c1-15(17-9-12-19(20(13-17)28-5)25-30(6,26)27)24-21(29)23-14-16-7-10-18(11-8-16)22(2,3)4/h7-13,15,25H,14H2,1-6H3,(H2,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | -42.6 | n/a | n/a | 28.6 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20328
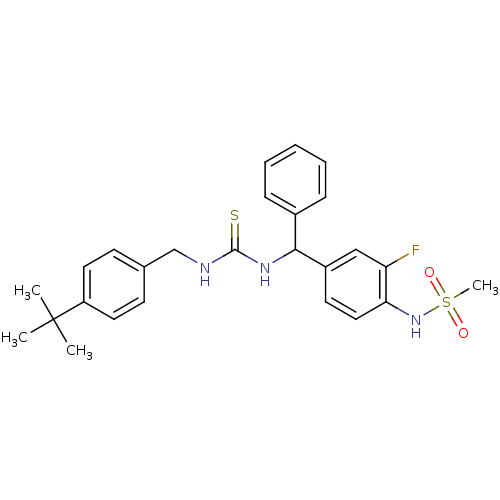
(3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NC(c2ccccc2)c2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C26H30FN3O2S2/c1-26(2,3)21-13-10-18(11-14-21)17-28-25(33)29-24(19-8-6-5-7-9-19)20-12-15-23(22(27)16-20)30-34(4,31)32/h5-16,24,30H,17H2,1-4H3,(H2,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -41.6 | n/a | n/a | 860 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20320

(1-[(4-tert-butylphenyl)methyl]-3-[1-(4-methanesulf...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NC2(CC2)c2ccc(NS(C)(=O)=O)cc2)cc1 Show InChI InChI=1S/C22H29N3O2S2/c1-21(2,3)17-7-5-16(6-8-17)15-23-20(28)24-22(13-14-22)18-9-11-19(12-10-18)25-29(4,26)27/h5-12,25H,13-15H2,1-4H3,(H2,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | -40.2 | n/a | n/a | 60 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20325
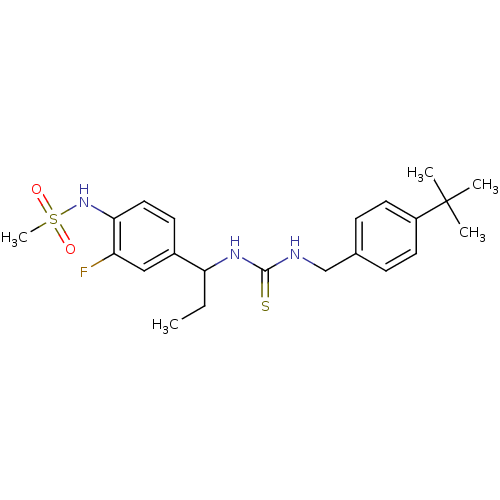
(3-[(4-tert-butylphenyl)methyl]-1-[1-(3-fluoro-4-me...)Show SMILES CCC(NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C22H30FN3O2S2/c1-6-19(16-9-12-20(18(23)13-16)26-30(5,27)28)25-21(29)24-14-15-7-10-17(11-8-15)22(2,3)4/h7-13,19,26H,6,14H2,1-5H3,(H2,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | -39.4 | n/a | n/a | 54 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20326
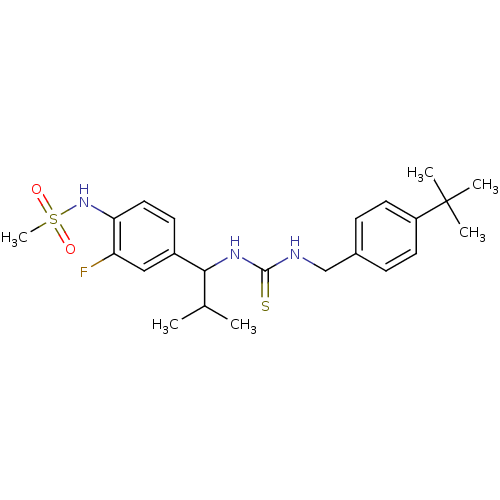
(3-[(4-tert-butylphenyl)methyl]-1-[1-(3-fluoro-4-me...)Show SMILES CC(C)C(NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C23H32FN3O2S2/c1-15(2)21(17-9-12-20(19(24)13-17)27-31(6,28)29)26-22(30)25-14-16-7-10-18(11-8-16)23(3,4)5/h7-13,15,21,27H,14H2,1-6H3,(H2,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 339 | -38.4 | n/a | n/a | 223 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20333
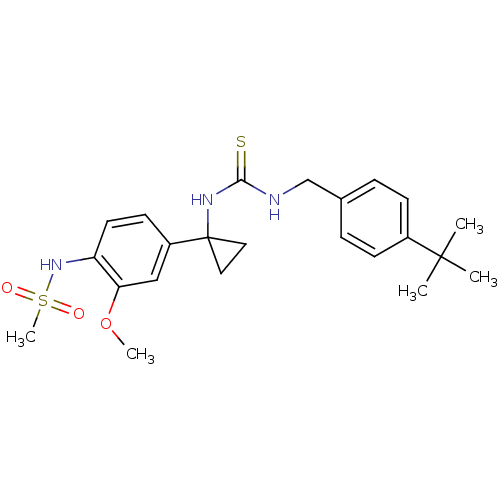
(1-[(4-tert-butylphenyl)methyl]-3-[1-(4-methanesulf...)Show SMILES COc1cc(ccc1NS(C)(=O)=O)C1(CC1)NC(=S)NCc1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C23H31N3O3S2/c1-22(2,3)17-8-6-16(7-9-17)15-24-21(30)25-23(12-13-23)18-10-11-19(20(14-18)29-4)26-31(5,27)28/h6-11,14,26H,12-13,15H2,1-5H3,(H2,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | -38.2 | n/a | n/a | 243 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20284
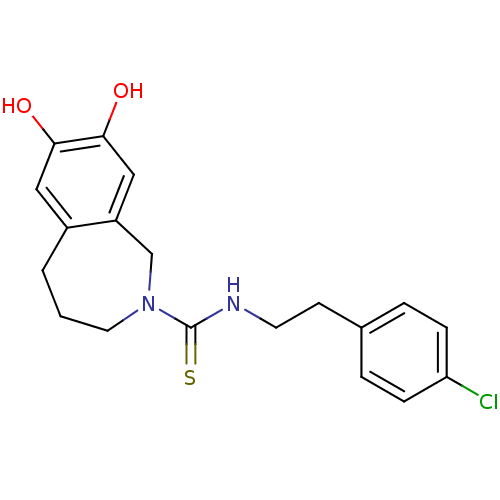
(CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...)Show InChI InChI=1S/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.30E+3 | -34.9 | n/a | n/a | 520 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20327
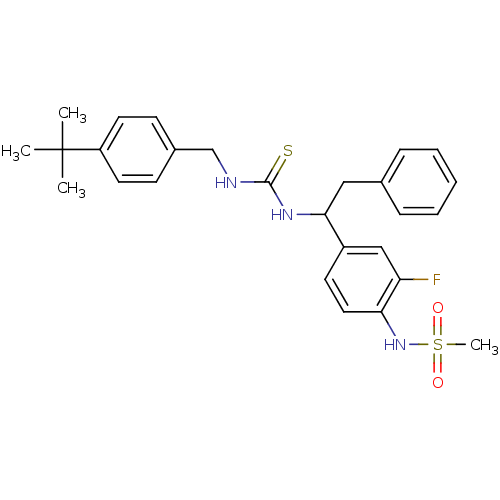
(3-[(4-tert-butylphenyl)methyl]-1-[1-(3-fluoro-4-me...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NC(Cc2ccccc2)c2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C27H32FN3O2S2/c1-27(2,3)22-13-10-20(11-14-22)18-29-26(34)30-25(16-19-8-6-5-7-9-19)21-12-15-24(23(28)17-21)31-35(4,32)33/h5-15,17,25,31H,16,18H2,1-4H3,(H2,29,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.74E+3 | -34.2 | n/a | n/a | 700 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20324
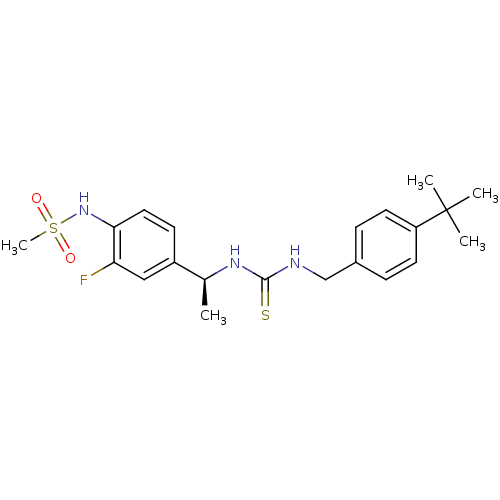
(3-[(4-tert-butylphenyl)methyl]-1-[(1S)-1-(3-fluoro...)Show SMILES C[C@H](NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C21H28FN3O2S2/c1-14(16-8-11-19(18(22)12-16)25-29(5,26)27)24-20(28)23-13-15-6-9-17(10-7-15)21(2,3)4/h6-12,14,25H,13H2,1-5H3,(H2,23,24,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.14E+3 | -33.7 | n/a | n/a | 2.67E+3 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20332
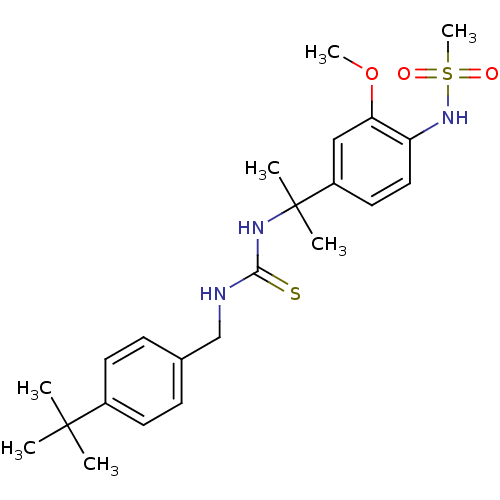
(3-[(4-tert-butylphenyl)methyl]-1-[2-(4-methanesulf...)Show SMILES COc1cc(ccc1NS(C)(=O)=O)C(C)(C)NC(=S)NCc1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C23H33N3O3S2/c1-22(2,3)17-10-8-16(9-11-17)15-24-21(30)25-23(4,5)18-12-13-19(20(14-18)29-6)26-31(7,27)28/h8-14,26H,15H2,1-7H3,(H2,24,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.89E+3 | -32.9 | n/a | n/a | 663 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20318
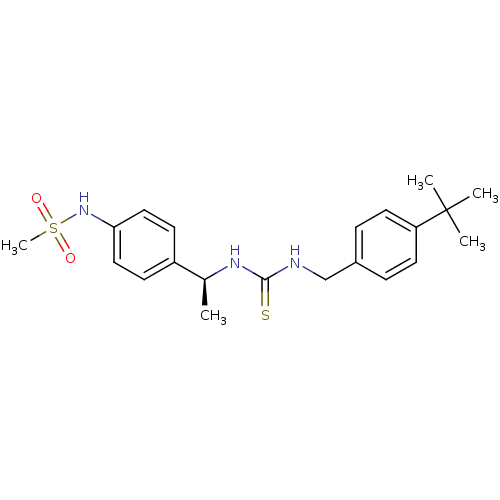
(3-[(4-tert-butylphenyl)methyl]-1-[(1S)-1-(4-methan...)Show SMILES C[C@H](NC(=S)NCc1ccc(cc1)C(C)(C)C)c1ccc(NS(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C21H29N3O2S2/c1-15(17-8-12-19(13-9-17)24-28(5,25)26)23-20(27)22-14-16-6-10-18(11-7-16)21(2,3)4/h6-13,15,24H,14H2,1-5H3,(H2,22,23,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.95E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20329
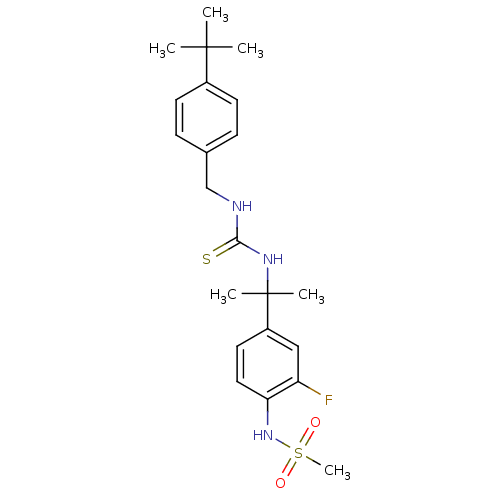
(3-[(4-tert-butylphenyl)methyl]-1-[2-(3-fluoro-4-me...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NC(C)(C)c2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C22H30FN3O2S2/c1-21(2,3)16-9-7-15(8-10-16)14-24-20(29)25-22(4,5)17-11-12-19(18(23)13-17)26-30(6,27)28/h7-13,26H,14H2,1-6H3,(H2,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70E+3 | -30.4 | n/a | n/a | 980 | n/a | n/a | 7.4 | 37 |
Seoul National University
| Assay Description
Binding assay mixtures were set up and contained [3H] RTX, various concentrations of competing ligands, and rVR1-transfected CHO cells. Nonspecific b... |
Bioorg Med Chem 15: 6043-53 (2007)
Article DOI: 10.1016/j.bmc.2007.06.041
BindingDB Entry DOI: 10.7270/Q2X928KS |
More data for this
Ligand-Target Pair | |
GTP:AMP phosphotransferase AK3, mitochondrial
(Rattus norvegicus) | BDBM50027422
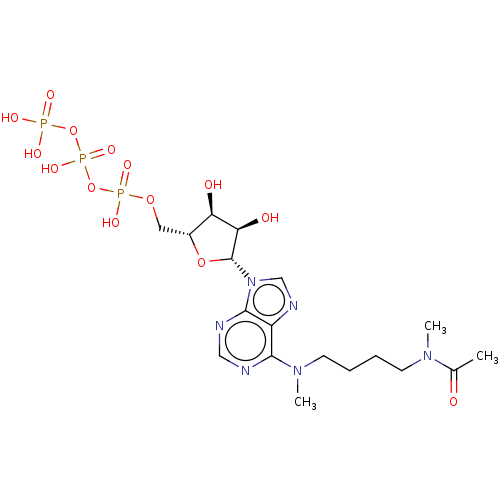
(1N-{4-[9-(3,4-dihydroxy-5-hydroxymethyltetrahydro-...)Show SMILES CN(CCCCN(C)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C(C)=O Show InChI InChI=1S/C18H31N6O14P3/c1-11(25)22(2)6-4-5-7-23(3)16-13-17(20-9-19-16)24(10-21-13)18-15(27)14(26)12(36-18)8-35-40(31,32)38-41(33,34)37-39(28,29)30/h9-10,12,14-15,18,26-27H,4-8H2,1-3H3,(H,31,32)(H,33,34)(H2,28,29,30)/t12-,14-,15-,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of adenylate kinase II in rat liver with respect to ATP |
J Med Chem 25: 373-81 (1982)
BindingDB Entry DOI: 10.7270/Q28P5ZH6 |
More data for this
Ligand-Target Pair | |
Adenylate kinase 2, mitochondrial
(Rattus norvegicus) | BDBM50027422

(1N-{4-[9-(3,4-dihydroxy-5-hydroxymethyltetrahydro-...)Show SMILES CN(CCCCN(C)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]1O)C(C)=O Show InChI InChI=1S/C18H31N6O14P3/c1-11(25)22(2)6-4-5-7-23(3)16-13-17(20-9-19-16)24(10-21-13)18-15(27)14(26)12(36-18)8-35-40(31,32)38-41(33,34)37-39(28,29)30/h9-10,12,14-15,18,26-27H,4-8H2,1-3H3,(H,31,32)(H,33,34)(H2,28,29,30)/t12-,14-,15-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of adenylate kinase III in rat liver with respect to ATP |
J Med Chem 25: 373-81 (1982)
BindingDB Entry DOI: 10.7270/Q28P5ZH6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255355
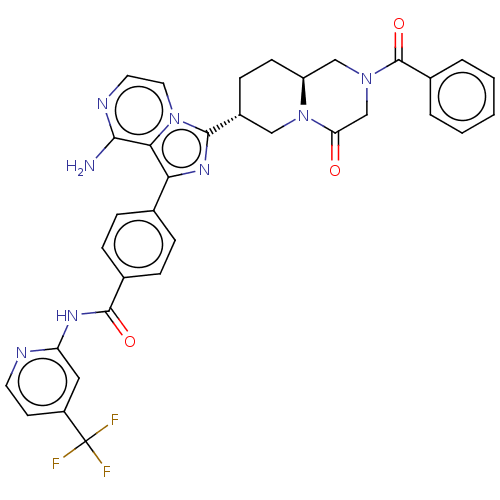
(US9481682, 105)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CN(CC(=O)N2C1)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H29F3N8O3/c35-34(36,37)24-12-13-39-26(16-24)41-32(47)21-8-6-20(7-9-21)28-29-30(38)40-14-15-44(29)31(42-28)23-10-11-25-18-43(19-27(46)45(25)17-23)33(48)22-4-2-1-3-5-22/h1-9,12-16,23,25H,10-11,17-19H2,(H2,38,40)(H,39,41,47)/t23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255335

(US9481682, 85)Show SMILES COc1cc(ccc1-c1nc([C@@H]2CC[C@H]3CN(CC(=O)N3C2)C(=O)c2ccncc2)n2ccnc(N)c12)C(=O)Nc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C34H30F3N9O4/c1-50-25-14-20(32(48)42-26-15-22(8-11-40-26)34(35,36)37)3-5-24(25)28-29-30(38)41-12-13-45(29)31(43-28)21-2-4-23-17-44(18-27(47)46(23)16-21)33(49)19-6-9-39-10-7-19/h3,5-15,21,23H,2,4,16-18H2,1H3,(H2,38,41)(H,40,42,48)/t21-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594234

(CHEMBL5200336)Show SMILES Cc1cc(C(N)=O)c2[nH]c3cc(ccc3c2c1-c1cccc(c1O)-n1c(=O)[nH]c2c(F)cccc2c1=O)C(C)(C)O |(3.12,1.54,;1.79,2.31,;1.79,3.85,;.47,4.61,;.47,6.15,;1.8,6.92,;-.87,6.92,;-.86,3.85,;-2.33,4.32,;-3.23,3.08,;-4.77,2.91,;-5.39,1.5,;-4.49,.27,;-2.95,.43,;-2.33,1.83,;-.86,2.31,;.47,1.55,;.47,.01,;-.87,-.77,;-.86,-2.31,;.47,-3.07,;1.8,-2.3,;1.8,-.76,;3.14,.01,;3.13,-3.07,;3.13,-4.61,;1.8,-5.38,;4.47,-5.38,;5.8,-4.61,;7.14,-5.38,;7.14,-6.92,;8.47,-4.6,;8.47,-3.07,;7.13,-2.3,;5.8,-3.07,;4.47,-2.3,;4.47,-.76,;-6.93,1.5,;-7.7,.17,;-7.7,2.84,;-8.47,1.5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621073
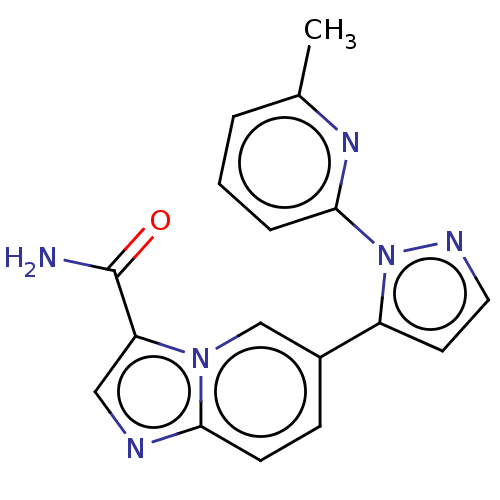
(Synthesis of 5-(3-(4-fluorobenzylamino)-1-(6-methy...) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594232

(CHEMBL5171706)Show SMILES OC[C@H]1CCC[C@H](Nc2ncnc3[nH]cc(C(=O)c4ccc(Oc5ccccc5)cc4Cl)c23)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1/2
(Homo sapiens (Human)) | BDBM50531540
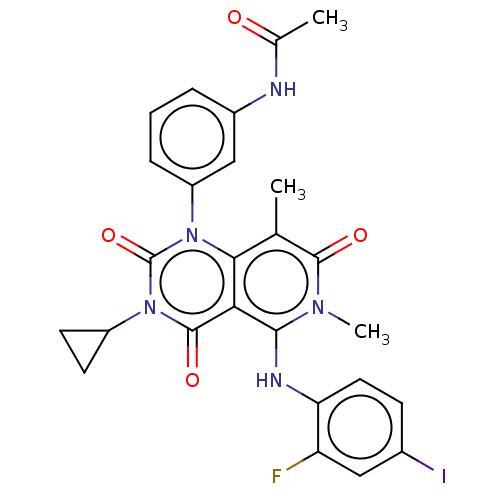
(CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...)Show SMILES CC(=O)Nc1cccc(c1)-n1c2c(C)c(=O)n(C)c(Nc3ccc(I)cc3F)c2c(=O)n(C2CC2)c1=O Show InChI InChI=1S/C26H23FIN5O4/c1-13-22-21(23(31(3)24(13)35)30-20-10-7-15(28)11-19(20)27)25(36)33(17-8-9-17)26(37)32(22)18-6-4-5-16(12-18)29-14(2)34/h4-7,10-12,17,30H,8-9H2,1-3H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01170
BindingDB Entry DOI: 10.7270/Q2CC14BB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594233
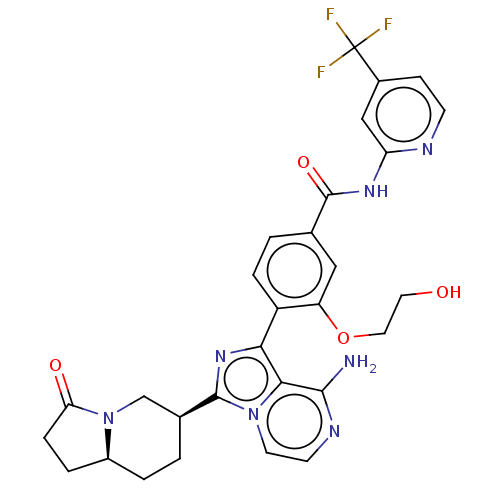
(CHEMBL5175002)Show SMILES [H][C@]12CCC(=O)N1C[C@H](CC2)c1nc(-c2ccc(cc2OCCO)C(=O)Nc2cc(ccn2)C(F)(F)F)c2c(N)nccn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM255370

(US9481682, 121)Show SMILES Nc1nccn2c(nc(-c3ccc(cc3OC3CC3)C(=O)Nc3cc(ccn3)C(F)(F)F)c12)[C@@H]1CC[C@H]2CCC(=O)N2C1 |r| Show InChI InChI=1S/C30H28F3N7O3/c31-30(32,33)18-9-10-35-23(14-18)37-29(42)16-2-7-21(22(13-16)43-20-5-6-20)25-26-27(34)36-11-12-39(26)28(38-25)17-1-3-19-4-8-24(41)40(19)15-17/h2,7,9-14,17,19-20H,1,3-6,8,15H2,(H2,34,36)(H,35,37,42)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621068
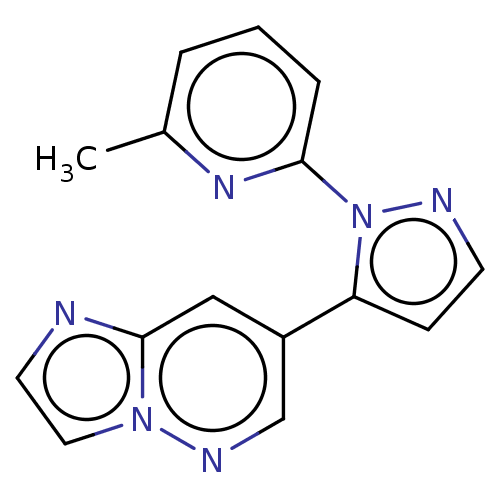
(US20230303562, Example 6) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594227

(CHEMBL5169992)Show SMILES CN1CCn2nc(Nc3cc(nn4ccnc34)-c3ccnc(N4CCn5c6CCCCc6cc5C4=O)c3CO)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594229

(CHEMBL5177238)Show SMILES Cc1cc(Nc2cc(cn(C)c2=O)-c2ccnc(c2CO)-n2ncc3cc(cc(F)c3c2=O)C(C)(C)C)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594229

(CHEMBL5177238)Show SMILES Cc1cc(Nc2cc(cn(C)c2=O)-c2ccnc(c2CO)-n2ncc3cc(cc(F)c3c2=O)C(C)(C)C)nn1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594227

(CHEMBL5169992)Show SMILES CN1CCn2nc(Nc3cc(nn4ccnc34)-c3ccnc(N4CCn5c6CCCCc6cc5C4=O)c3CO)cc2C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613898

(CHEMBL5283753) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613915
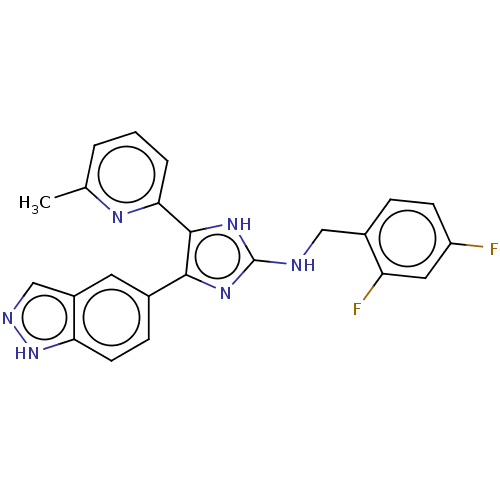
(CHEMBL5268868) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613920

(CHEMBL5273412) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613917

(CHEMBL5271258) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594228

(CHEMBL5197089)Show SMILES CCC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Cc3ccccc3)cc2)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621072
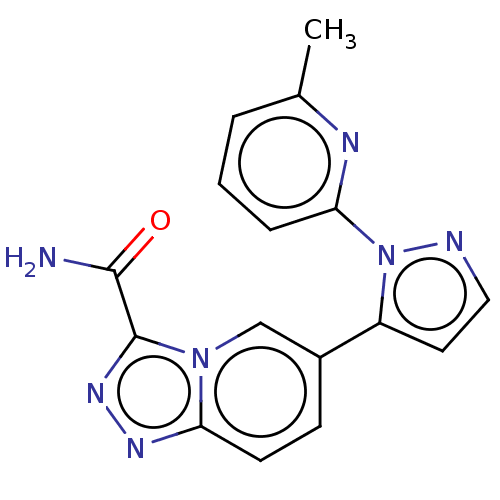
(US20230303562, Example 10) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594228

(CHEMBL5197089)Show SMILES CCC(=O)N1CCC[C@H](C1)n1nc(-c2ccc(Cc3ccccc3)cc2)c2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613909

(CHEMBL5279947) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613898

(CHEMBL5283753) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613902
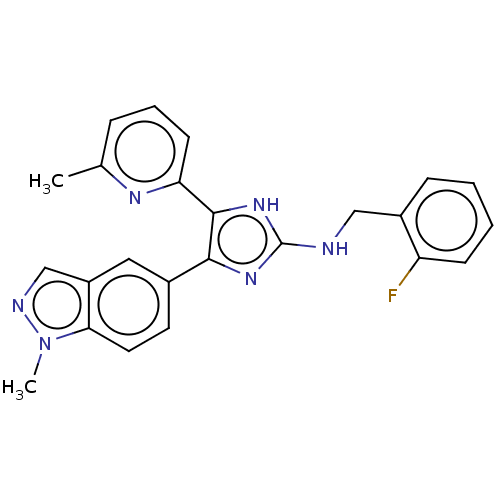
(CHEMBL5282597) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613914

(CHEMBL5274746) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50594230

(CHEMBL5187230)Show SMILES Cc1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(c2CO)-n2ncc3cc(cc(F)c3c2=O)C(C)(C)C)nc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00030
BindingDB Entry DOI: 10.7270/Q2FX7FHQ |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50613905

(CHEMBL5289293) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data