

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50111101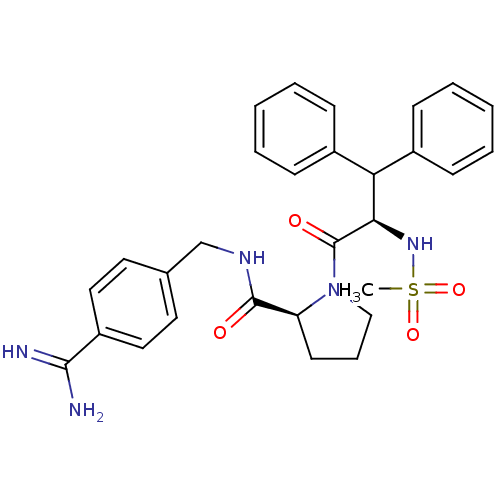 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111110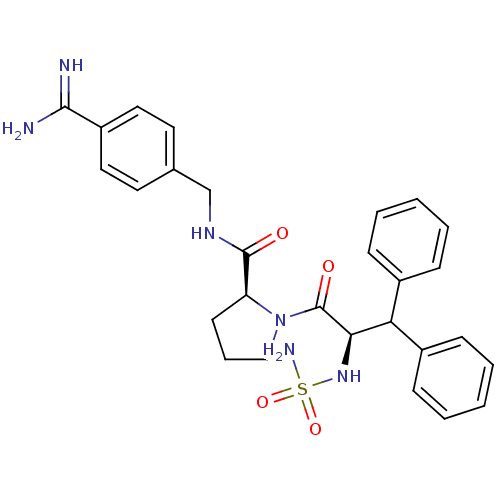 (2N-(4-Benzamidinemethyl)-1-[2-aminosulfonamido-3,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131789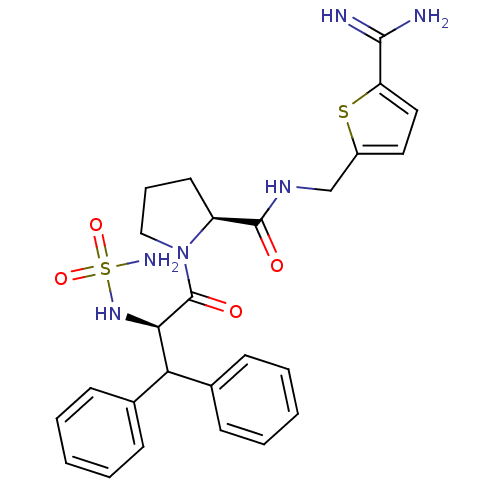 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111105 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131790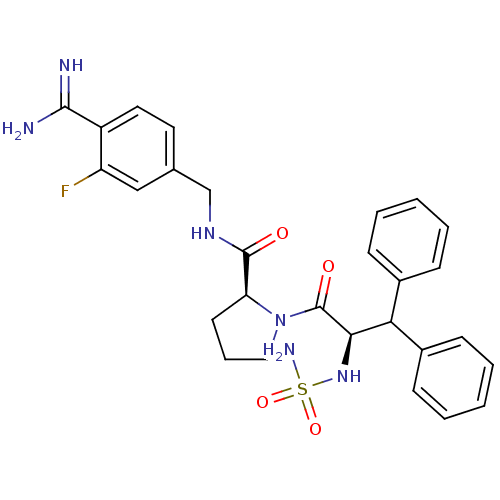 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131778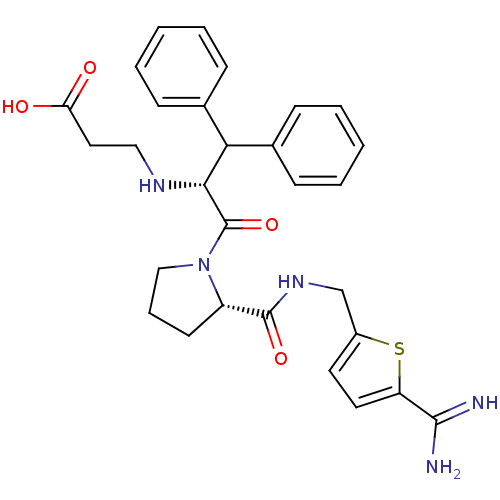 (3-(1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131795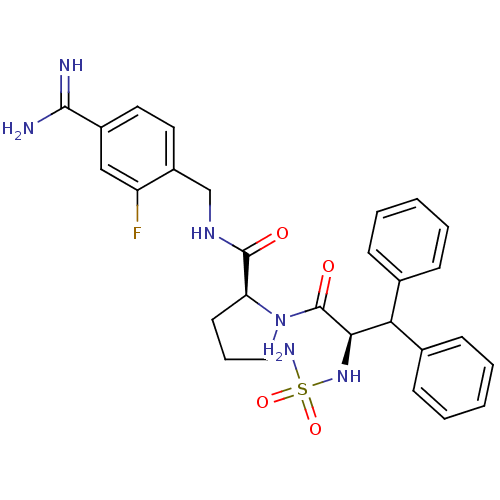 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111120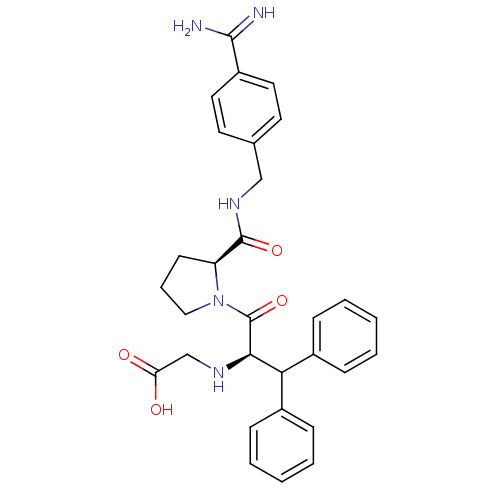 (2N-(4-Benzamidinemethyl)-1-[2-Aminoaceticacid-3,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131780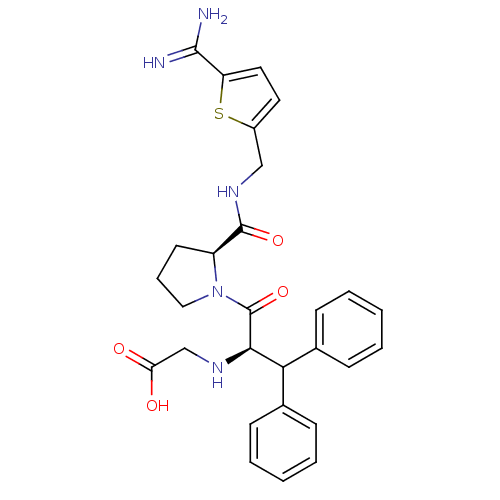 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131791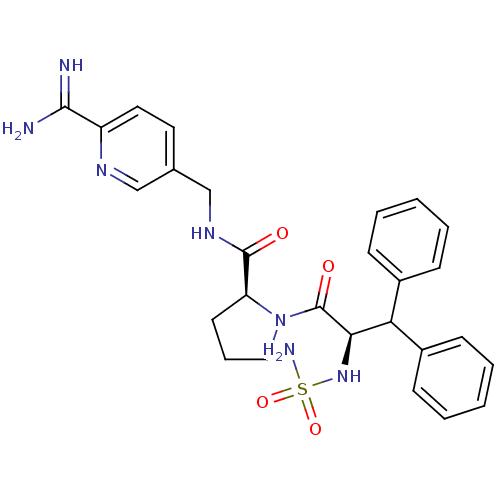 (1-(2-sulfonamideamino-3,3-diphenyl-propionyl)-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131796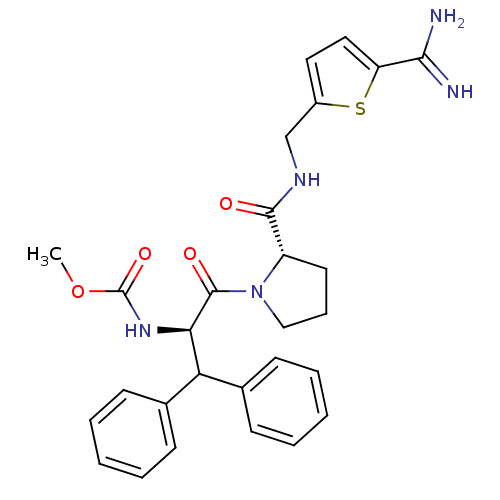 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131792 ((1-Benzhydryl-2-{2-[(6-carbamimidoyl-pyridin-3-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131782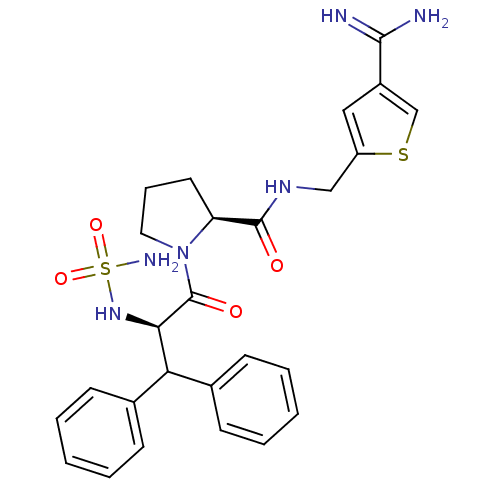 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131798 (1-(2-Methylamino-3,3-diphenyl-propionyl)-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50111122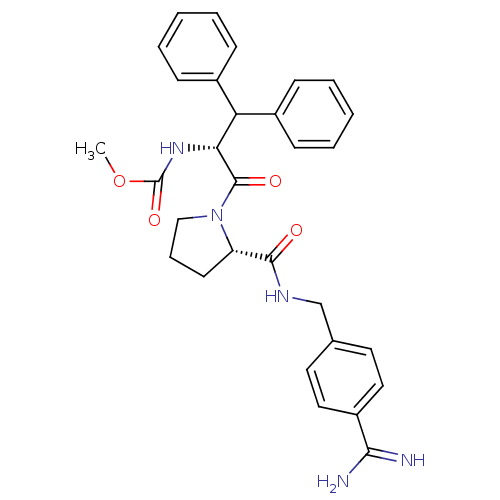 (2N-(4-Benzamidinemethyl)-1-[2-Carbamicacidmethyles...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131787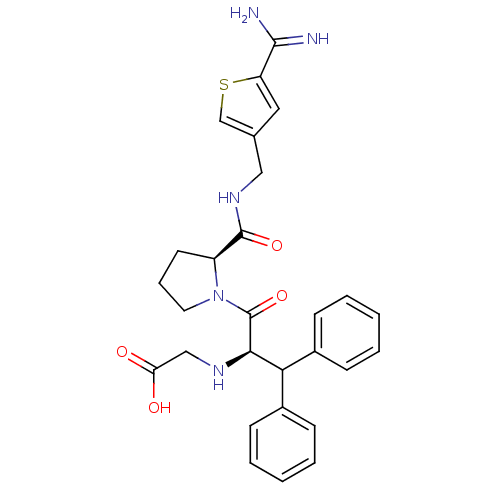 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131781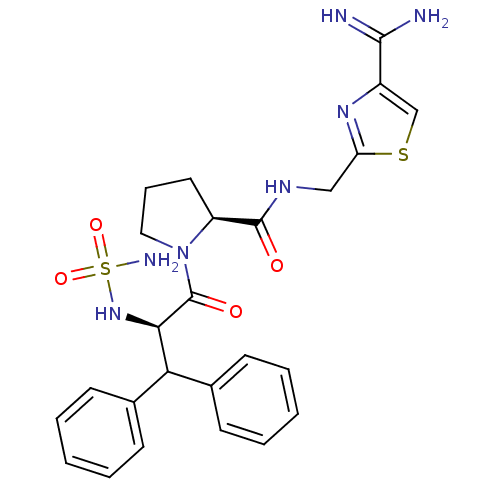 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442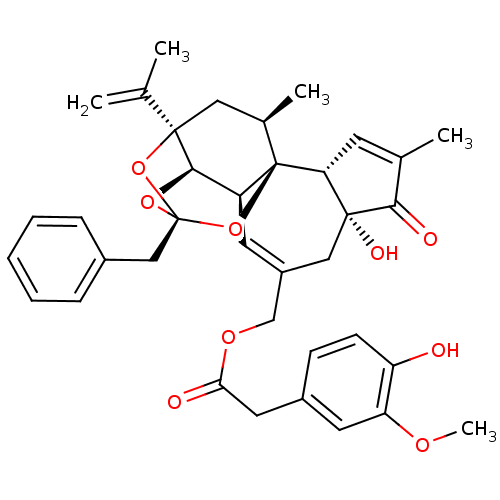 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131797 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131779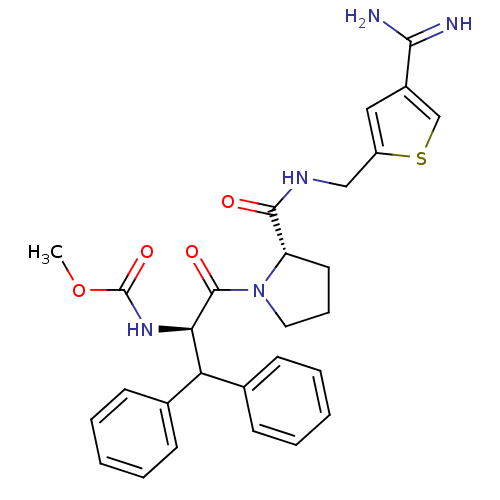 ((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1/Trypsin-2 (Homo sapiens (Human)) | BDBM50131780 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-2-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human human trypsin was determined | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50232114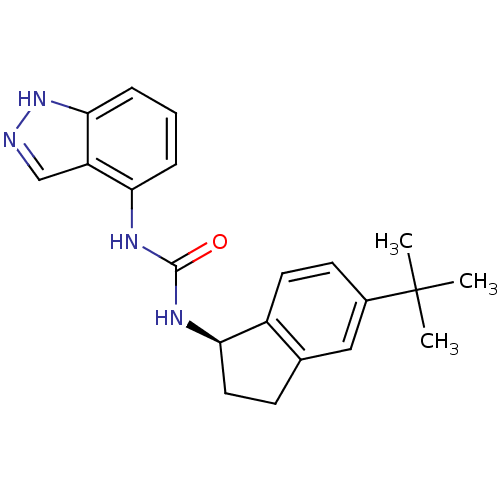 ((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564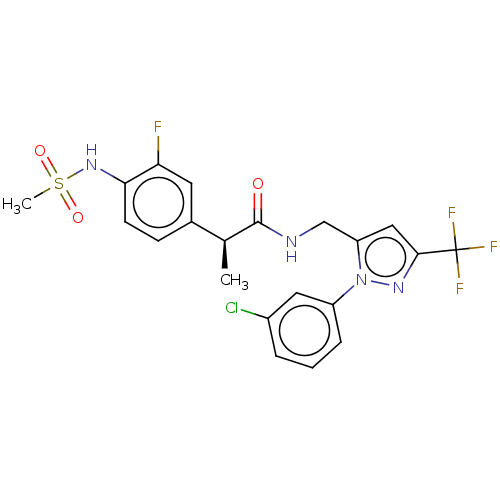 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555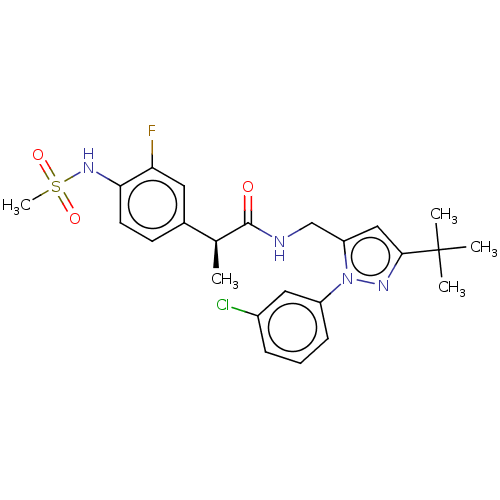 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553855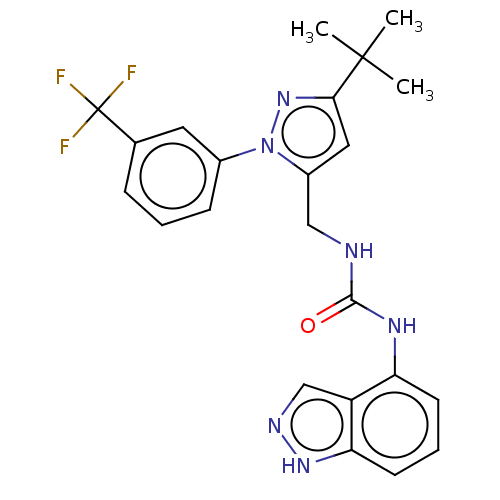 (CHEMBL4782906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553837 (CHEMBL4752231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131784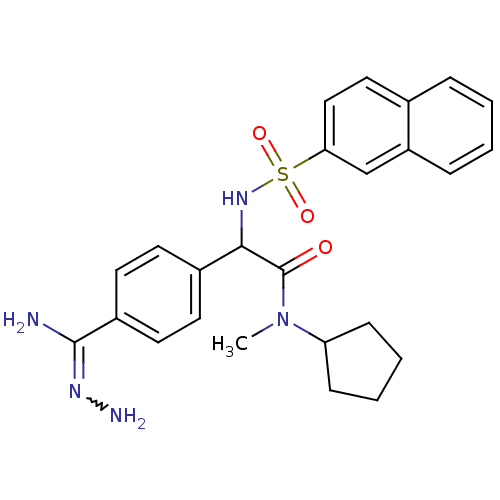 (2-{4-[(Z)-amino(hydrazono)methyl]phenyl}-N-cyclope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Chemical Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | Bioorg Med Chem Lett 10: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2FT8K8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131783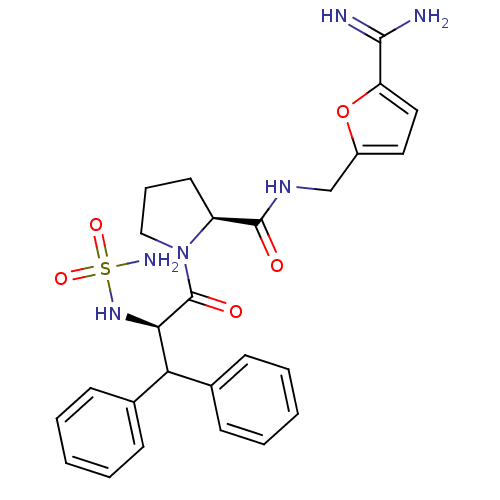 (1-(2-sulfonamide-amino-3,3-diphenyl-propionyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553860 (CHEMBL4745407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553861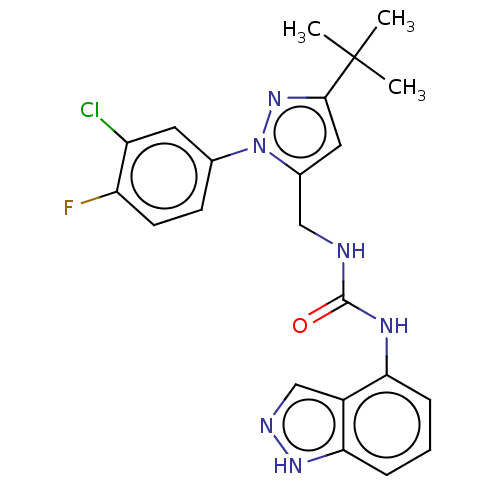 (CHEMBL4792344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553862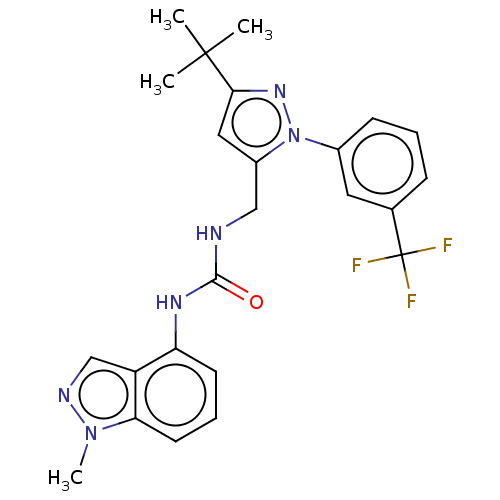 (CHEMBL4756342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553868 (CHEMBL4763969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553843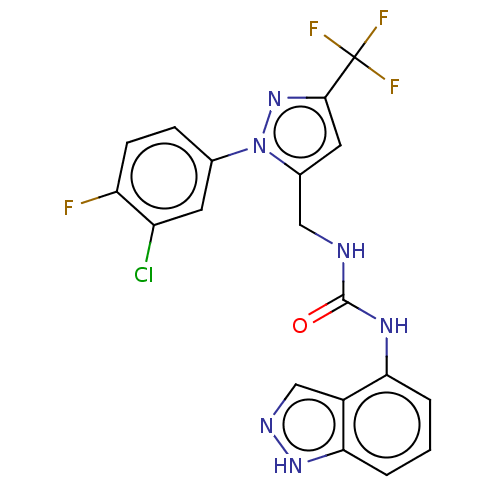 (CHEMBL4763915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553836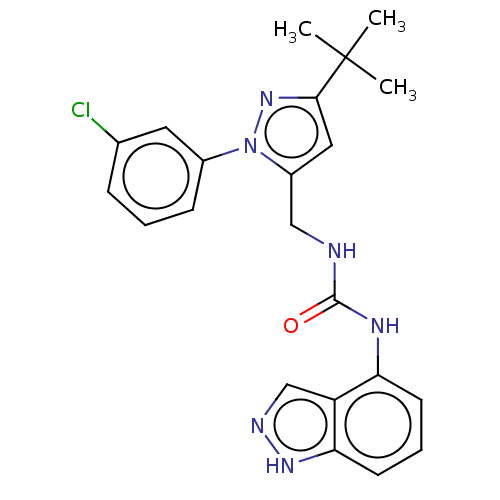 (CHEMBL4753881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131788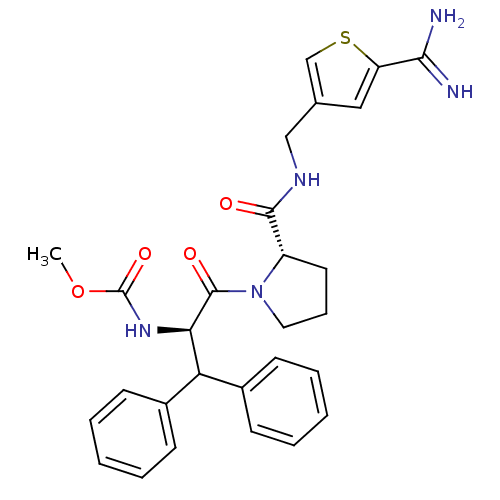 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-thiophen-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131793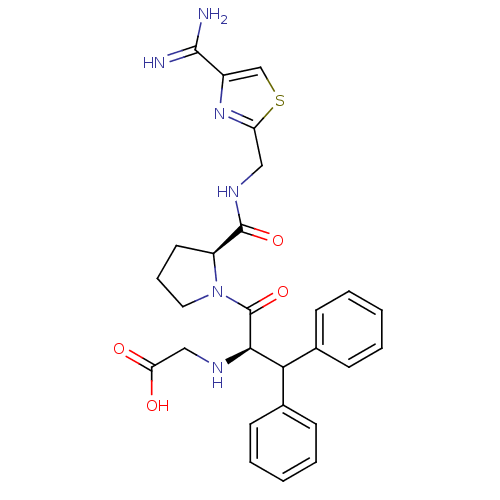 ((1-Benzhydryl-2-{2-[(4-carbamimidoyl-thiazol-2-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50095188 (1-{3,3-diphenyl-(2R)-2-[(aminosulfonyl)amino]propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Chemical Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | Bioorg Med Chem Lett 10: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2FT8K8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553833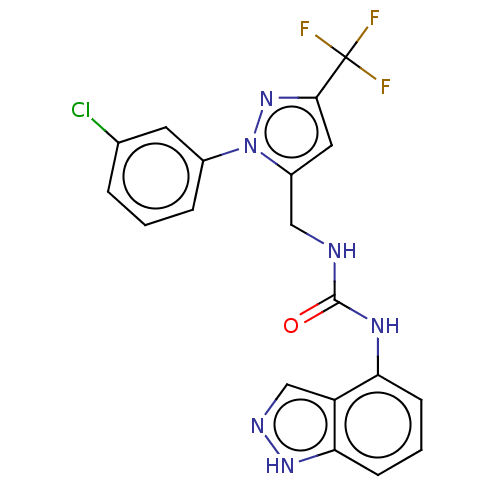 (CHEMBL4752959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553867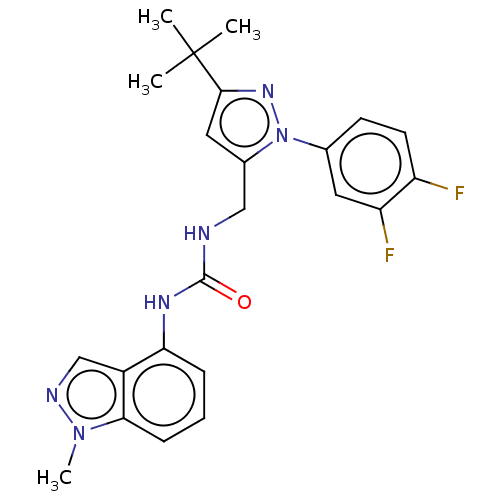 (CHEMBL4790987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553854 (CHEMBL4798584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553839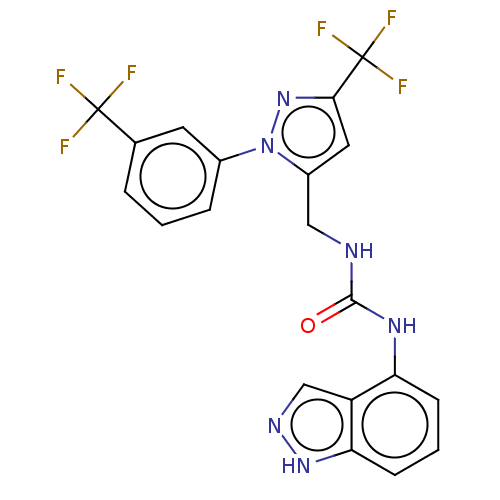 (CHEMBL4778103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50131777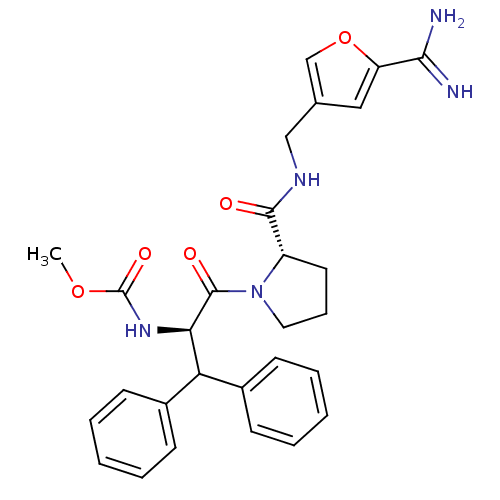 ((1-Benzhydryl-2-{2-[(5-carbamimidoyl-furan-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Life Sciences Ltd. Curated by ChEMBL | Assay Description In vitro inhibition constant (Ki) against human thrombin | J Med Chem 46: 3612-22 (2003) Article DOI: 10.1021/jm030025j BindingDB Entry DOI: 10.7270/Q28K79T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553846 (CHEMBL4740674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553852 (CHEMBL4764786) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553856 (CHEMBL4762740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553834 (CHEMBL4752981) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553840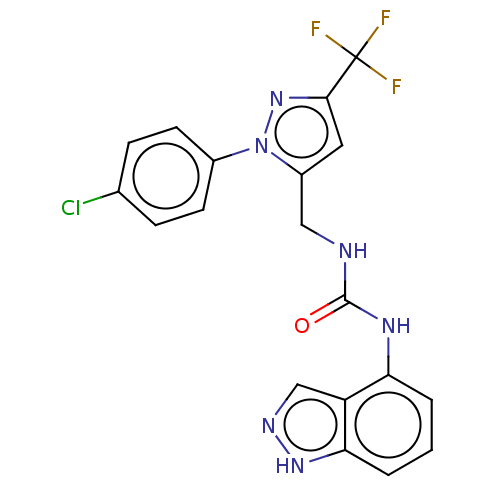 (CHEMBL4752186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50095190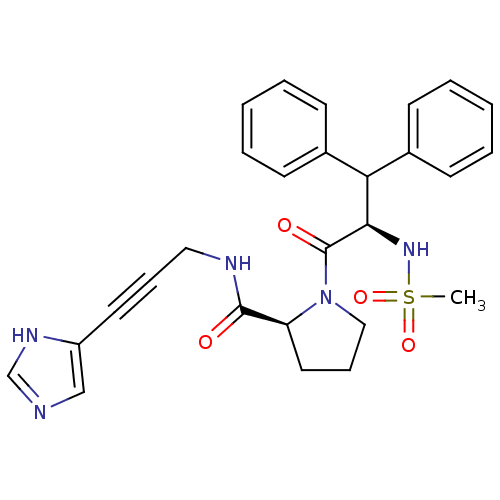 ((S)-1-((R)-2-Methanesulfonylamino-3,3-diphenyl-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LG Chemical Ltd Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | Bioorg Med Chem Lett 10: 2775-8 (2000) BindingDB Entry DOI: 10.7270/Q2FT8K8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1166 total ) | Next | Last >> |