 Found 2140 hits with Last Name = 'kettle' and Initial = 'jg'
Found 2140 hits with Last Name = 'kettle' and Initial = 'jg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548115

(CHEMBL4778773)Show SMILES CNC(=O)c1cnc2cc(OC)c(OCN3CCN(C)CC3)cc2c1Nc1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548110

(CHEMBL4747532) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548117
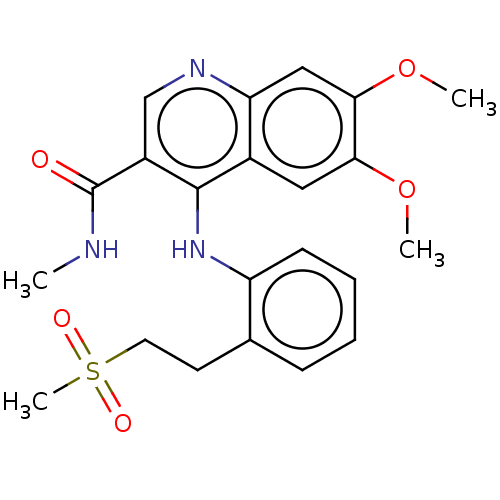
(CHEMBL4776026)Show SMILES CNC(=O)c1cnc2cc(OC)c(OC)cc2c1Nc1ccccc1CCS(C)(=O)=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50162074

(CHEMBL3792684)Show InChI InChI=1S/C19H19N3O3/c1-20-19(23)14-11-21-15-10-17(25-3)16(24-2)9-13(15)18(14)22-12-7-5-4-6-8-12/h4-11H,1-3H3,(H,20,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50162075
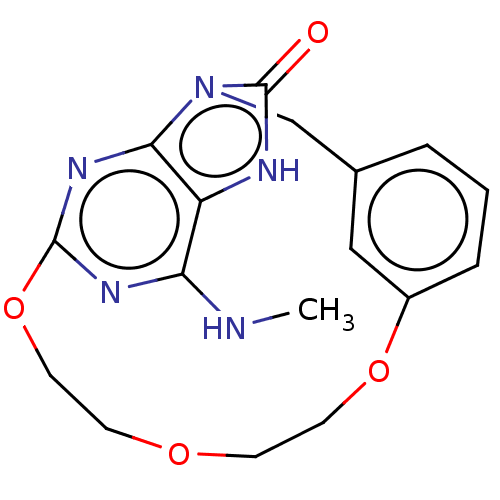
(CHEMBL3794167)Show InChI InChI=1S/C17H19N5O4/c1-18-14-13-15-21-16(20-14)26-8-6-24-5-7-25-12-4-2-3-11(9-12)10-22(15)17(23)19-13/h2-4,9H,5-8,10H2,1H3,(H,19,23)(H,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548124

(CHEMBL4784602) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548109
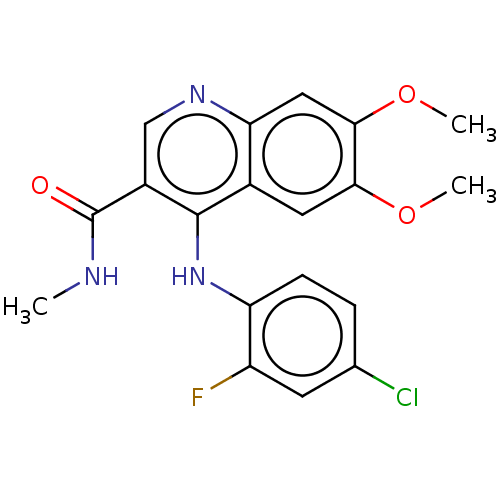
(CHEMBL4783492)Show SMILES CNC(=O)c1cnc2cc(OC)c(OC)cc2c1Nc1ccc(Cl)cc1F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50373856

(CHEMBL271705)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCOCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O3/c24-18-13-16(4-5-19(18)34-14-17-3-1-2-6-25-17)28-21-20-22(27-15-26-21)29-30-23(20)33-12-9-31-7-10-32-11-8-31/h1-6,13,15H,7-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293243
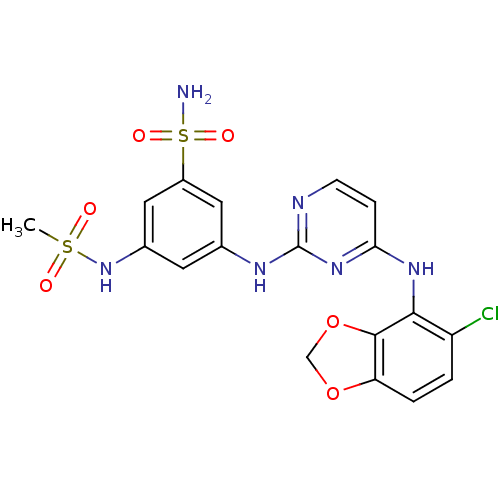
(3-(4-(5-chlorobenzo[d][1,3]dioxol-4-ylamino)pyrimi...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H17ClN6O6S2/c1-32(26,27)25-11-6-10(7-12(8-11)33(20,28)29)22-18-21-5-4-15(24-18)23-16-13(19)2-3-14-17(16)31-9-30-14/h2-8,25H,9H2,1H3,(H2,20,28,29)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190001
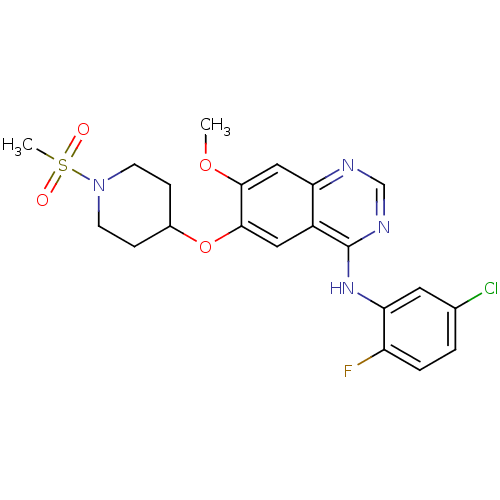
(CHEMBL213007 | N-(5-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cc(Cl)ccc3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-19-11-17-15(10-20(19)31-14-5-7-27(8-6-14)32(2,28)29)21(25-12-24-17)26-18-9-13(22)3-4-16(18)23/h3-4,9-12,14H,5-8H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081185
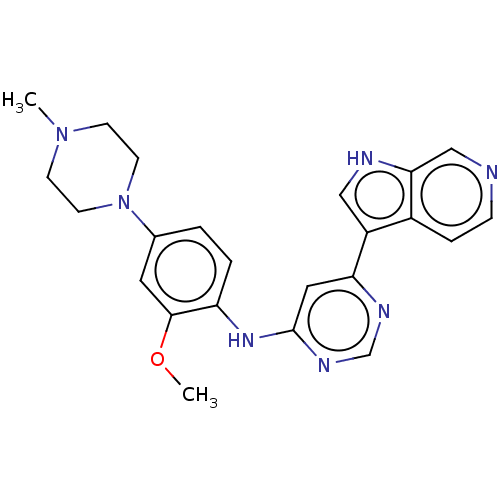
(CHEMBL3421963)Show SMILES COc1cc(ccc1Nc1cc(ncn1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-7-9-30(10-8-29)16-3-4-19(22(11-16)31-2)28-23-12-20(26-15-27-23)18-13-25-21-14-24-6-5-17(18)21/h3-6,11-15,25H,7-10H2,1-2H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373853
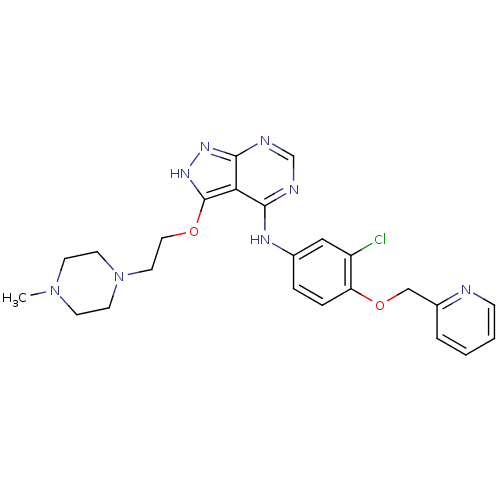
(CHEMBL258282)Show SMILES CN1CCN(CCOc2[nH]nc3ncnc(Nc4ccc(OCc5ccccn5)c(Cl)c4)c23)CC1 Show InChI InChI=1S/C24H27ClN8O2/c1-32-8-10-33(11-9-32)12-13-34-24-21-22(27-16-28-23(21)30-31-24)29-17-5-6-20(19(25)14-17)35-15-18-4-2-3-7-26-18/h2-7,14,16H,8-13,15H2,1H3,(H2,27,28,29,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599596
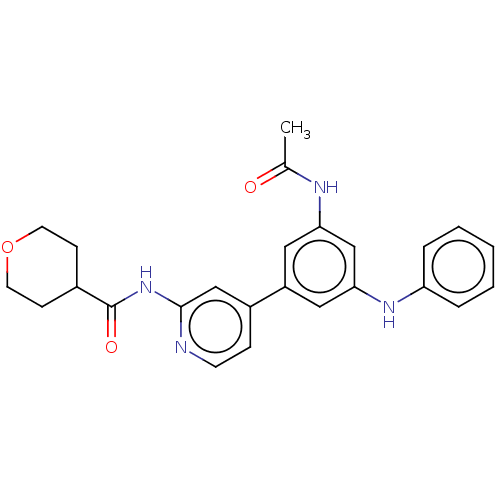
(CHEMBL5191668)Show SMILES CC(=O)Nc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548121
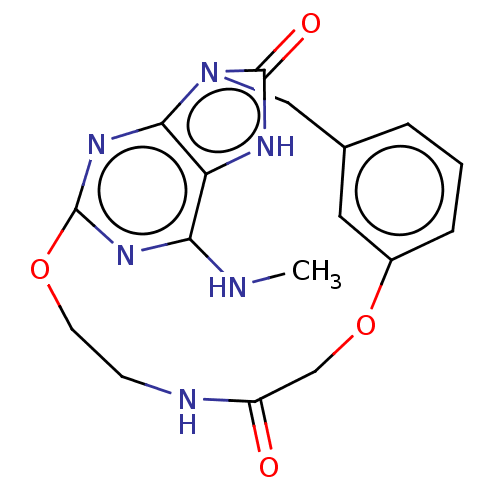
(CHEMBL4754128)Show SMILES CNc1nc2OCCNC(=O)COc3cccc(Cn4c(n2)c1[nH]c4=O)c3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
(Homo sapiens (Human)) | BDBM50599618
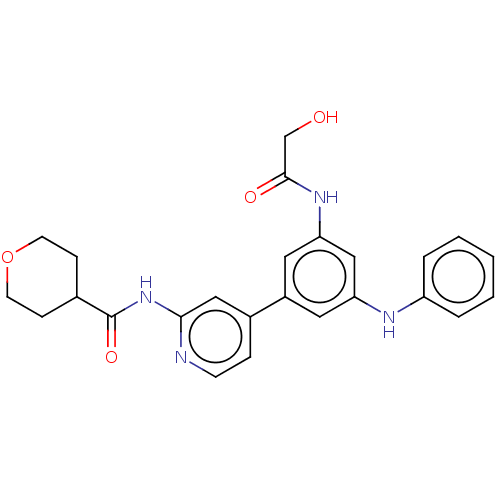
(CHEMBL5182242)Show SMILES OCC(=O)Nc1cc(Nc2ccccc2)cc(c1)-c1ccnc(NC(=O)C2CCOCC2)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116557
BindingDB Entry DOI: 10.7270/Q23J3J18 |
More data for this
Ligand-Target Pair | |
Oxidized purine nucleoside triphosphate hydrolase
(Homo sapiens (Human)) | BDBM50548119
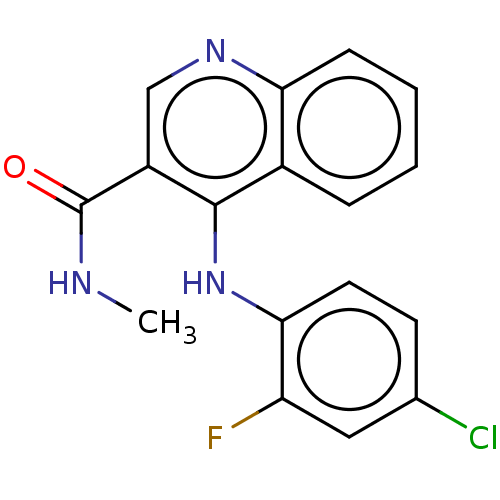
(CHEMBL4749068) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.5b01760
BindingDB Entry DOI: 10.7270/Q2TB1BHR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081186
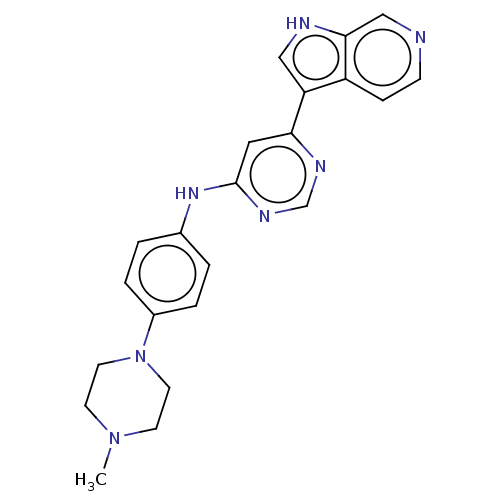
(CHEMBL3421962)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(ncn2)-c2c[nH]c3cnccc23)cc1 Show InChI InChI=1S/C22H23N7/c1-28-8-10-29(11-9-28)17-4-2-16(3-5-17)27-22-12-20(25-15-26-22)19-13-24-21-14-23-7-6-18(19)21/h2-7,12-15,24H,8-11H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50427336
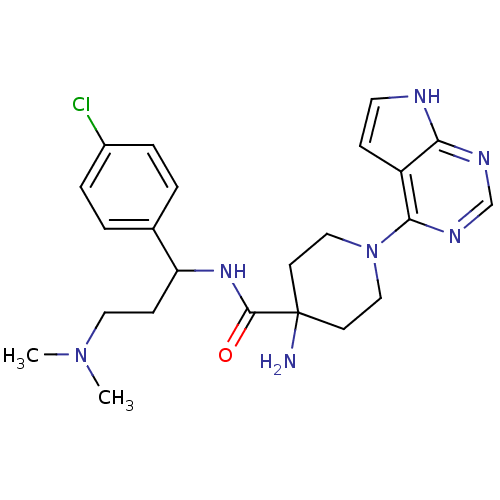
(CHEMBL2325992 | US10654855, Example 11 | US1123609...)Show SMILES CN(C)CCC(NC(=O)C1(N)CCN(CC1)c1ncnc2[nH]ccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H30ClN7O/c1-30(2)12-8-19(16-3-5-17(24)6-4-16)29-22(32)23(25)9-13-31(14-10-23)21-18-7-11-26-20(18)27-15-28-21/h3-7,11,15,19H,8-10,12-14,25H2,1-2H3,(H,29,32)(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... |
J Med Chem 56: 2059-73 (2013)
Article DOI: 10.1021/jm301762v
BindingDB Entry DOI: 10.7270/Q2QR4ZFN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340566

(CHEMBL1762535 | N2-(3,5-dimorpholinophenyl)-N4-(5-...)Show SMILES COc1cncc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H31N7O3/c1-30(22-16-23(33-2)18-26-17-22)24-3-4-27-25(29-24)28-19-13-20(31-5-9-34-10-6-31)15-21(14-19)32-7-11-35-12-8-32/h3-4,13-18H,5-12H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340570

((2-chloro-5-((2-(3,5-dimorpholinophenylamino)pyrim...)Show SMILES CN(c1ccc(Cl)c(CO)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H31ClN6O3/c1-31(21-2-3-24(27)19(14-21)18-34)25-4-5-28-26(30-25)29-20-15-22(32-6-10-35-11-7-32)17-23(16-20)33-8-12-36-13-9-33/h2-5,14-17,34H,6-13,18H2,1H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340572
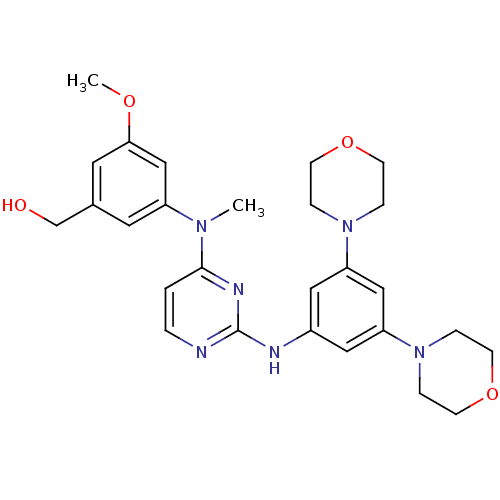
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES COc1cc(CO)cc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O4/c1-31(22-13-20(19-34)14-25(18-22)35-2)26-3-4-28-27(30-26)29-21-15-23(32-5-9-36-10-6-32)17-24(16-21)33-7-11-37-12-8-33/h3-4,13-18,34H,5-12,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340576

(CHEMBL1762525 | N2-(3,5-dimorpholinophenyl)-N4-(3-...)Show SMILES COc1cccc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(21-4-3-5-24(19-21)33-2)25-6-7-27-26(29-25)28-20-16-22(31-8-12-34-13-9-31)18-23(17-20)32-10-14-35-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340551
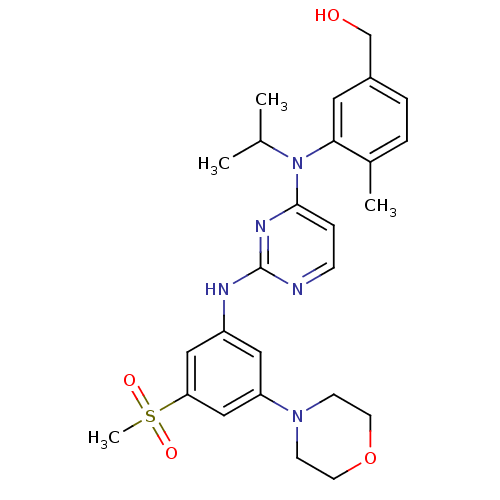
((3-(isopropyl(2-(3-(methylsulfonyl)-5-morpholinoph...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N5O4S/c1-18(2)31(24-13-20(17-32)6-5-19(24)3)25-7-8-27-26(29-25)28-21-14-22(30-9-11-35-12-10-30)16-23(15-21)36(4,33)34/h5-8,13-16,18,32H,9-12,17H2,1-4H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50371358
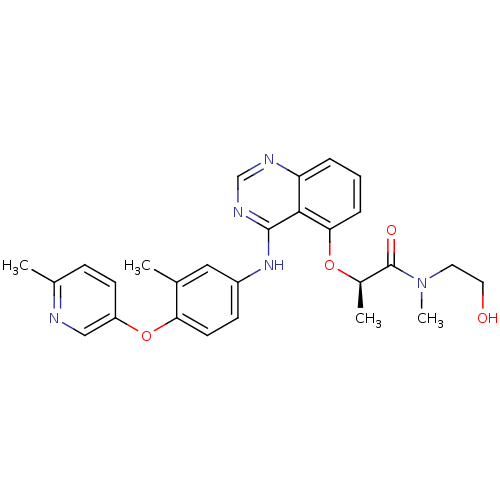
(CHEMBL257478)Show SMILES C[C@@H](Oc1cccc2ncnc(Nc3ccc(Oc4ccc(C)nc4)c(C)c3)c12)C(=O)N(C)CCO Show InChI InChI=1S/C27H29N5O4/c1-17-14-20(9-11-23(17)36-21-10-8-18(2)28-15-21)31-26-25-22(29-16-30-26)6-5-7-24(25)35-19(3)27(34)32(4)12-13-33/h5-11,14-16,19,33H,12-13H2,1-4H3,(H,29,30,31)/t19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of ebrB2 |
Bioorg Med Chem Lett 18: 674-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.052
BindingDB Entry DOI: 10.7270/Q2736RSH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189995

(CHEMBL213874 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-8-6-14(7-9-28)12-30-20-10-15-18(11-19(20)29-2)25-13-26-22(15)27-17-5-3-4-16(23)21(17)24/h3-5,10-11,13-14H,6-9,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190000

(CHEMBL214798 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(C)CC1 Show InChI InChI=1S/C21H22ClFN4O2/c1-27-8-6-13(7-9-27)29-19-10-14-17(11-18(19)28-2)24-12-25-21(14)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50190002

(CHEMBL387265 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C21H22ClFN4O4S/c1-30-18-11-17-14(10-19(18)31-13-6-8-27(9-7-13)32(2,28)29)21(25-12-24-17)26-16-5-3-4-15(22)20(16)23/h3-5,10-13H,6-9H2,1-2H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189993

(2-(4-(4-(3-chloro-2-fluorophenylamino)-7-methoxyqu...)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC1CCN(CC(N)=O)CC1 Show InChI InChI=1S/C22H23ClFN5O3/c1-31-18-10-17-14(9-19(18)32-13-5-7-29(8-6-13)11-20(25)30)22(27-12-26-17)28-16-4-2-3-15(23)21(16)24/h2-4,9-10,12-13H,5-8,11H2,1H3,(H2,25,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222489

((R)-N-(2-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](CN(C)C(=O)CO)Oc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C26H26ClN5O4/c1-17(13-32(2)24(34)14-33)36-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)35-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50222495

((S)-1-(2-((4-(3-chloro-4-(pyridin-2-ylmethoxy)phen...)Show SMILES OCC(=O)N1CCC[C@H]1COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12 Show InChI InChI=1S/C27H26ClN5O4/c28-21-13-18(9-10-23(21)36-15-19-5-1-2-11-29-19)32-27-26-22(30-17-31-27)7-3-8-24(26)37-16-20-6-4-12-33(20)25(35)14-34/h1-3,5,7-11,13,17,20,34H,4,6,12,14-16H2,(H,30,31,32)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 kinase |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293242
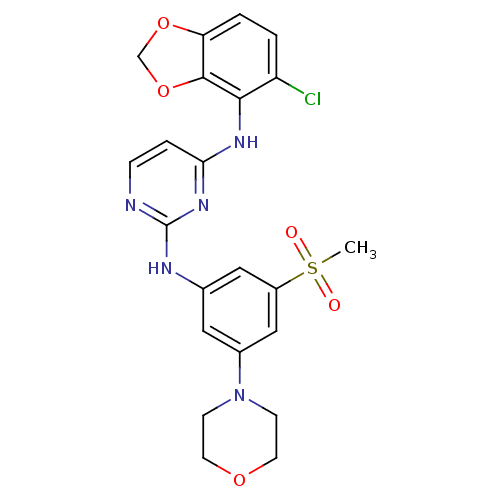
(CHEMBL497198 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES CS(=O)(=O)c1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H22ClN5O5S/c1-34(29,30)16-11-14(10-15(12-16)28-6-8-31-9-7-28)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)33-13-32-18/h2-5,10-12H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293244

(CHEMBL523744 | N-(3-(4-(5-chlorobenzo[d][1,3]dioxo...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H23ClN6O5S/c1-35(30,31)28-15-10-14(11-16(12-15)29-6-8-32-9-7-29)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)34-13-33-18/h2-5,10-12,28H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
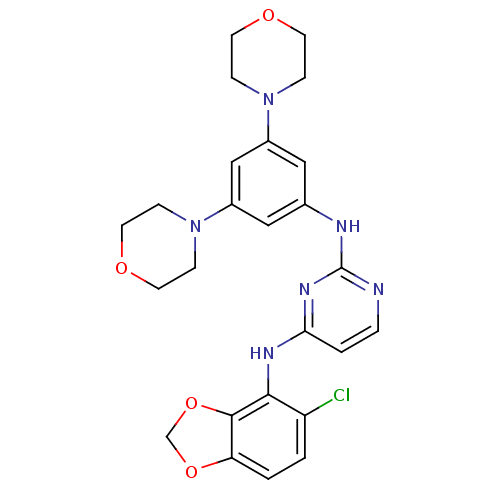
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50373862

(CHEMBL402553)Show SMILES Clc1cc(Nc2ncnc3n[nH]c(OCCN4CCCC4)c23)ccc1OCc1ccccn1 Show InChI InChI=1S/C23H24ClN7O2/c24-18-13-16(6-7-19(18)33-14-17-5-1-2-8-25-17)28-21-20-22(27-15-26-21)29-30-23(20)32-12-11-31-9-3-4-10-31/h1-2,5-8,13,15H,3-4,9-12,14H2,(H2,26,27,28,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of erbB2 |
Bioorg Med Chem Lett 18: 959-62 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.035
BindingDB Entry DOI: 10.7270/Q21N81ZG |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50605537

(CHEMBL5183988)Show SMILES [H][C@]12CN([C@H](C)CN1c1ncnc3c(F)c(c(Cl)c(OCC2)c13)-c1c(O)cccc1F)C(=O)C=C |r,wD:4.4,1.0,(2.77,3.02,;2.46,1.44,;3.89,1.99,;5.09,1.02,;4.85,-.5,;6.05,-1.47,;3.42,-1.05,;2.22,-.09,;1.01,-1.04,;1.67,-2.43,;.79,-3.65,;-.74,-3.57,;-1.4,-2.18,;-2.94,-2.1,;-3.81,-3.32,;-3.6,-.67,;-2.72,.6,;-3.38,1.99,;-1.19,.52,;-1.24,2.3,;.1,3.07,;1.6,2.71,;-.53,-.96,;-5.14,-.59,;-6.01,-1.81,;-5.35,-3.2,;-7.55,-1.73,;-8.2,-.29,;-7.33,.93,;-5.79,.85,;-4.92,2.12,;6.53,1.58,;7.73,.61,;6.77,3.1,;8.2,3.65,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00369
BindingDB Entry DOI: 10.7270/Q2W09B1P |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081188
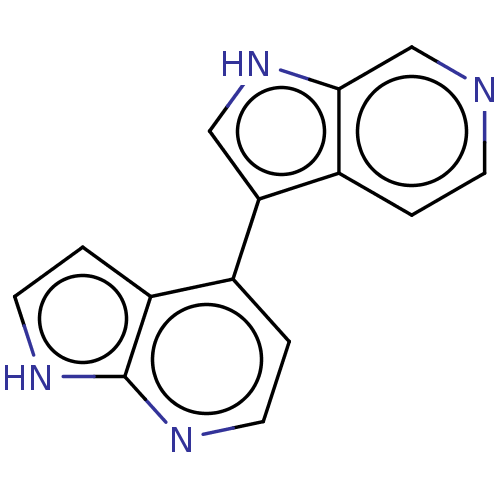
(CHEMBL3421981)Show InChI InChI=1S/C14H10N4/c1-4-15-8-13-10(1)12(7-18-13)9-2-5-16-14-11(9)3-6-17-14/h1-8,18H,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081174
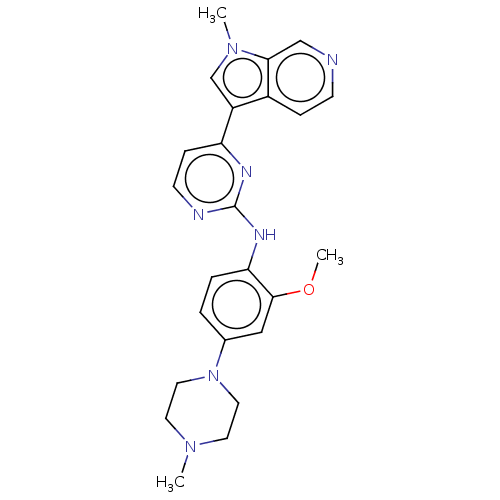
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50222493

((R)-N-(1-(4-(3-chloro-4-(pyridin-2-ylmethoxy)pheny...)Show SMILES C[C@H](COc1cccc2ncnc(Nc3ccc(OCc4ccccn4)c(Cl)c3)c12)N(C)C(=O)CO Show InChI InChI=1S/C26H26ClN5O4/c1-17(32(2)24(34)13-33)14-35-23-8-5-7-21-25(23)26(30-16-29-21)31-18-9-10-22(20(27)12-18)36-15-19-6-3-4-11-28-19/h3-12,16-17,33H,13-15H2,1-2H3,(H,29,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 17: 6326-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.073
BindingDB Entry DOI: 10.7270/Q2CF9PTZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189991

(CHEMBL214857 | N-(3-chloro-2-fluorophenyl)-7-metho...)Show SMILES COCCN1CCC(CC1)Oc1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC Show InChI InChI=1S/C23H26ClFN4O3/c1-30-11-10-29-8-6-15(7-9-29)32-21-12-16-19(13-20(21)31-2)26-14-27-23(16)28-18-5-3-4-17(24)22(18)25/h3-5,12-15H,6-11H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50189990

(CHEMBL215786 | N-(3-chloro-4-fluorophenyl)-7-metho...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24ClFN4O2/c1-28-7-5-14(6-8-28)12-30-21-10-16-19(11-20(21)29-2)25-13-26-22(16)27-15-3-4-18(24)17(23)9-15/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 16: 4908-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.054
BindingDB Entry DOI: 10.7270/Q2R78DTM |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM50082118
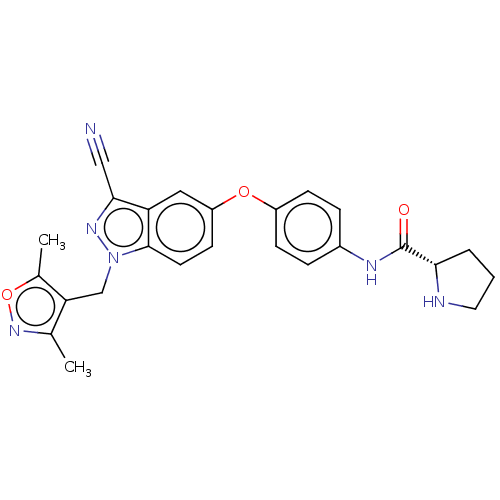
(CHEMBL3422678)Show SMILES Cc1noc(C)c1Cn1nc(C#N)c2cc(Oc3ccc(NC(=O)[C@@H]4CCCN4)cc3)ccc12 |r| Show InChI InChI=1S/C25H24N6O3/c1-15-21(16(2)34-30-15)14-31-24-10-9-19(12-20(24)23(13-26)29-31)33-18-7-5-17(6-8-18)28-25(32)22-4-3-11-27-22/h5-10,12,22,27H,3-4,11,14H2,1-2H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PFKFB3 using fructose 6 phosphate as substrate assessed as ADP generation after 1 hr by ADP Glo assay |
J Med Chem 58: 3611-25 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00352
BindingDB Entry DOI: 10.7270/Q2571DQB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50179758
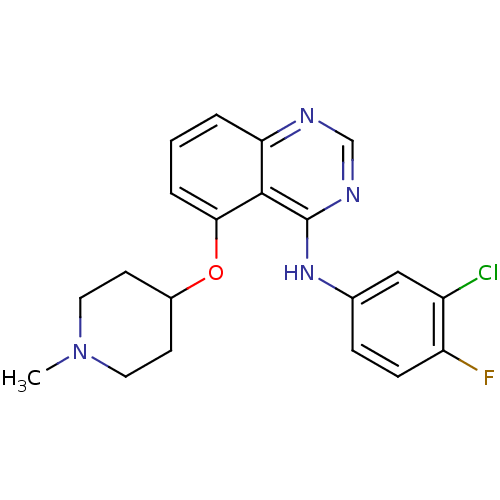
(CHEMBL203725 | N-(3-chloro-4-fluorophenyl)-5-(1-me...)Show SMILES CN1CCC(CC1)Oc1cccc2ncnc(Nc3ccc(F)c(Cl)c3)c12 Show InChI InChI=1S/C20H20ClFN4O/c1-26-9-7-14(8-10-26)27-18-4-2-3-17-19(18)20(24-12-23-17)25-13-5-6-16(22)15(21)11-13/h2-6,11-12,14H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibitory activity against EGFR |
Bioorg Med Chem Lett 16: 1633-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.028
BindingDB Entry DOI: 10.7270/Q2P84BGH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM264225
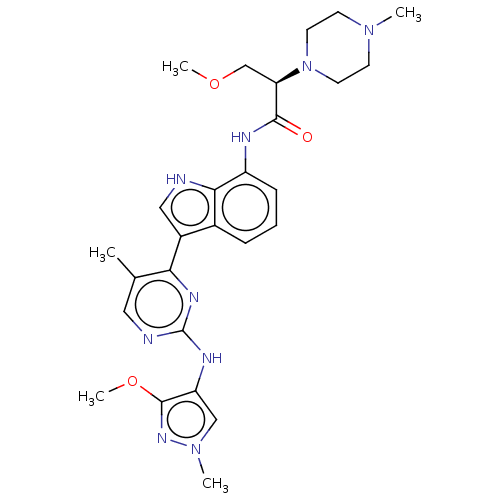
((2R)-3-methoxy-N- (3-{2-[(3-methoxy- 1-methyl-1H-p...)Show SMILES COC[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cn(C)nc2OC)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29G5R1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM264233

((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...)Show SMILES COC[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cn(C)nc2C)ncc1F |r| Show InChI InChI=1S/C26H32FN9O2/c1-16-21(14-35(3)33-16)31-26-29-13-19(27)23(32-26)18-12-28-24-17(18)6-5-7-20(24)30-25(37)22(15-38-4)36-10-8-34(2)9-11-36/h5-7,12-14,22,28H,8-11,15H2,1-4H3,(H,30,37)(H,29,31,32)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US9714236 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34PC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM489174

((R)-2-((R)-2,4- dimethylpiperazin-1- yl)-N-(3-(5-f...)Show SMILES COC[C@@H](N1CCN(C)C[C@H]1C)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM264233

((2R)-N-(3-{2- [(1,3-dimethyl-1H- pyrazol-4-yl)amin...)Show SMILES COC[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cn(C)nc2C)ncc1F |r| Show InChI InChI=1S/C26H32FN9O2/c1-16-21(14-35(3)33-16)31-26-29-13-19(27)23(32-26)18-12-28-24-17(18)6-5-7-20(24)30-25(37)22(15-38-4)36-10-8-34(2)9-11-36/h5-7,12-14,22,28H,8-11,15H2,1-4H3,(H,30,37)(H,29,31,32)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29G5R1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM264231
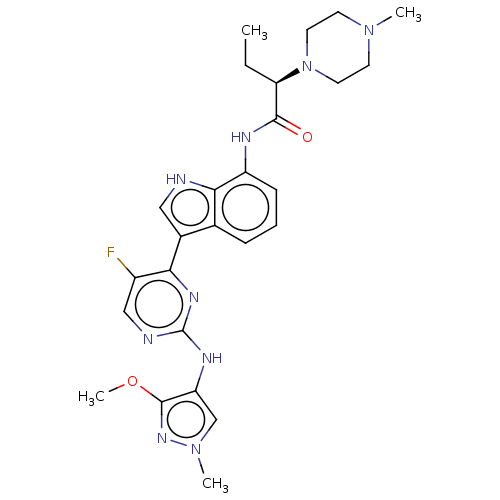
((2R)-N-(3-{5-fluoro- 2-[(3-methoxy-1- methyl-1H-py...)Show SMILES CC[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cn(C)nc2OC)ncc1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q29G5R1P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488661

((R)-N-(3-(2-((2-fluoro- 3- (methylsulfonyl)phenyl)...)Show SMILES C[C@@H](N1CCN(CCO)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488662

((R)-N-(3-(2-(2-fluoro-3- (methylsulfonyl)phenyl am...)Show SMILES C[C@@H](N1CCN(C)CC1)C(=O)Nc1cccc2c(c[nH]c12)-c1nc(Nc2cccc(c2F)S(C)(=O)=O)ncc1C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1 [866-1154]
(Homo sapiens (Human)) | BDBM488663
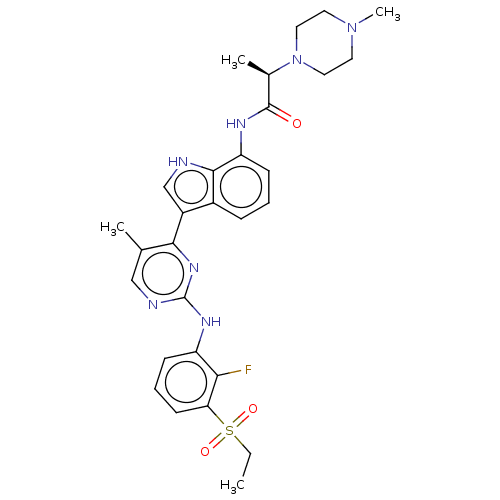
((R)-N-(3-(2-(3- (ethylsulfonyl)-2- fluorophenylami...)Show SMILES CCS(=O)(=O)c1cccc(Nc2ncc(C)c(n2)-c2c[nH]c3c(NC(=O)[C@@H](C)N4CCN(C)CC4)cccc23)c1F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB
US Patent
| Assay Description
Enzyme inhibition studies were performed using recombinant JAK1 (amino acids 866-1154, Life Technologies, #PV4774, Carlsbad, Calif.), JAK2 (amino aci... |
US Patent US10961228 (2021)
BindingDB Entry DOI: 10.7270/Q20G3P8F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data