

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109593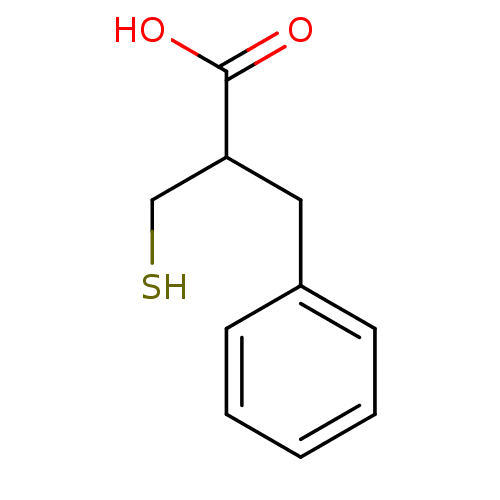 (2-Benzyl-3-mercapto-propionic acid | 2-Mercaptomet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109593 (2-Benzyl-3-mercapto-propionic acid | 2-Mercaptomet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279954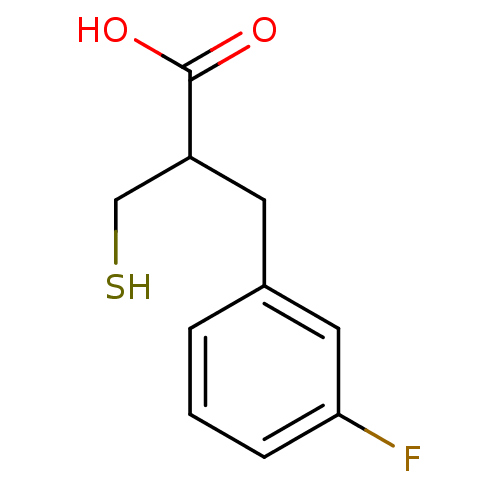 (3-(3-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279947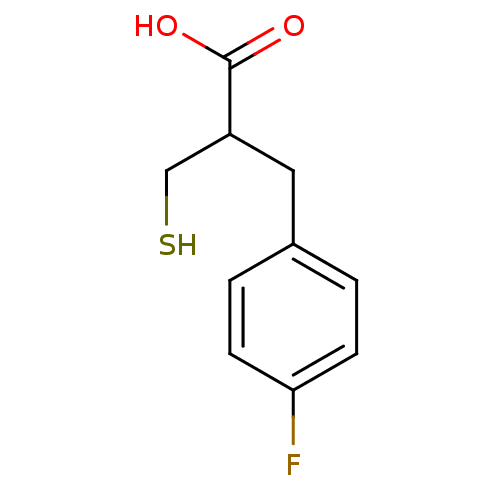 (3-(4-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279947 (3-(4-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279958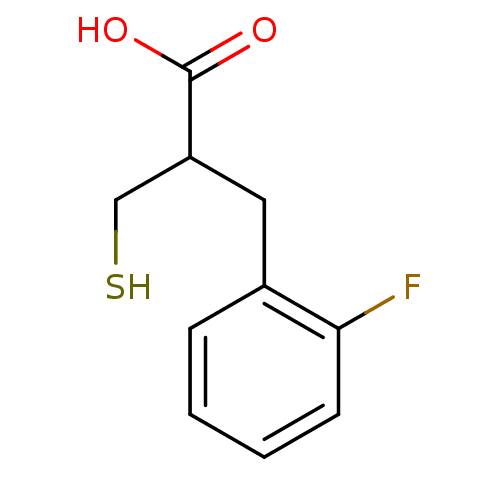 (3-(2-Fluoro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279960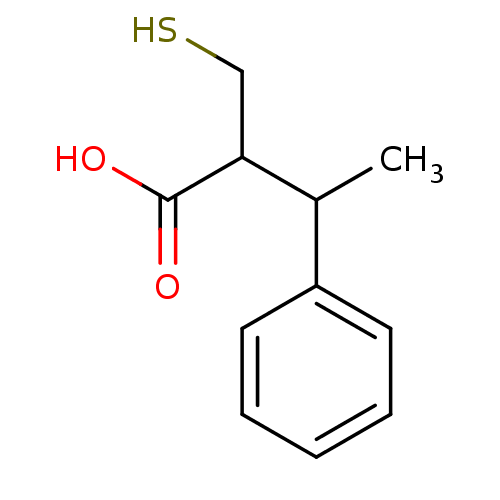 (2-Mercaptomethyl-3-phenyl-butyric acid | CHEMBL109...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279955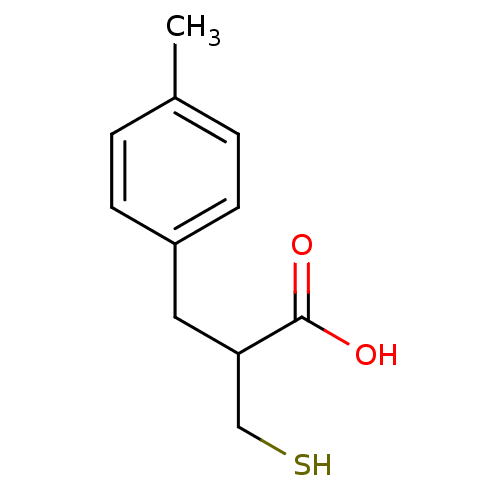 (2-Mercaptomethyl-3-p-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279955 (2-Mercaptomethyl-3-p-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279956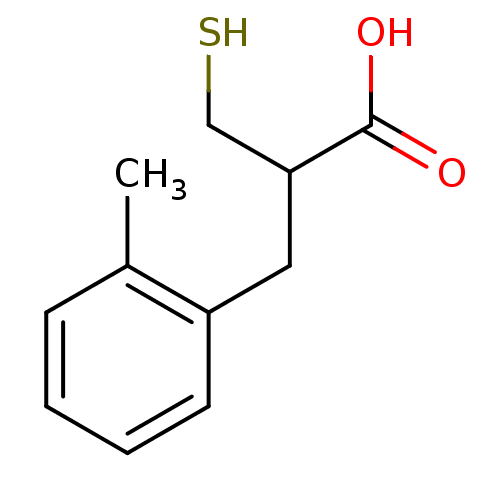 (2-Mercaptomethyl-3-o-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279961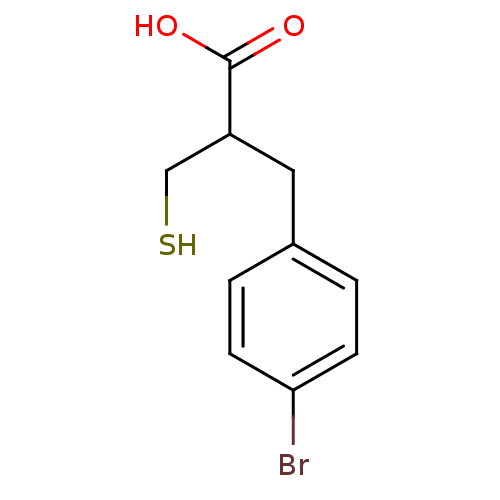 (3-(4-Bromo-phenyl)-2-mercaptomethyl-propionic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279963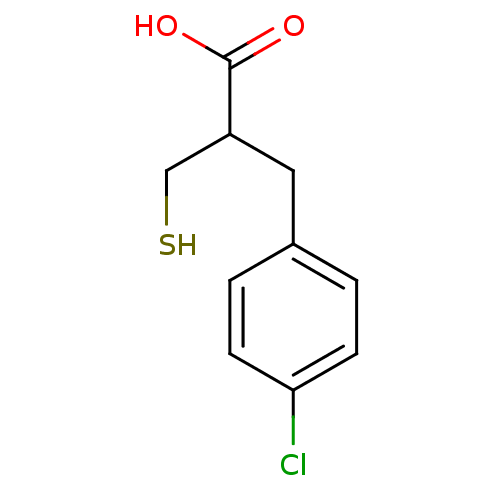 (3-(4-Chloro-phenyl)-2-mercaptomethyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279953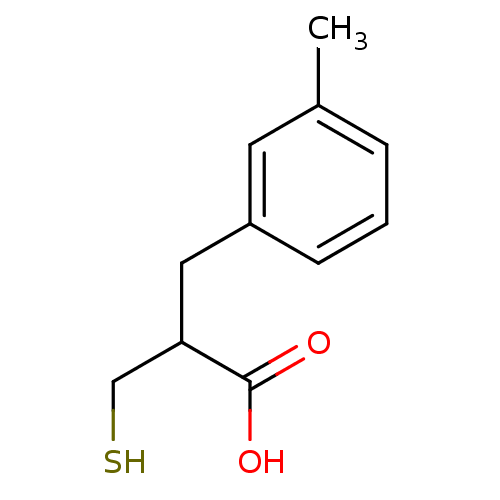 (2-Mercaptomethyl-3-m-tolyl-propionic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279949 (2-Mercaptomethyl-3-naphthalen-2-yl-propionic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279945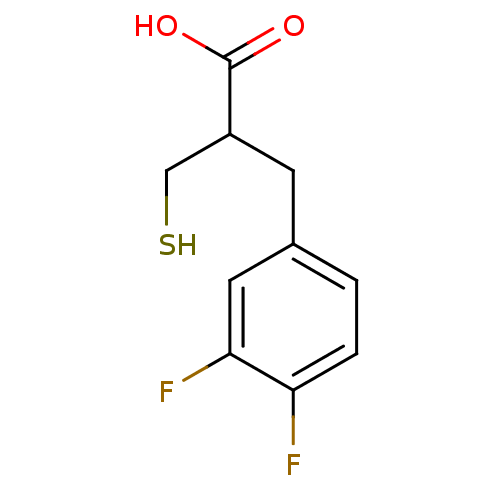 (3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279950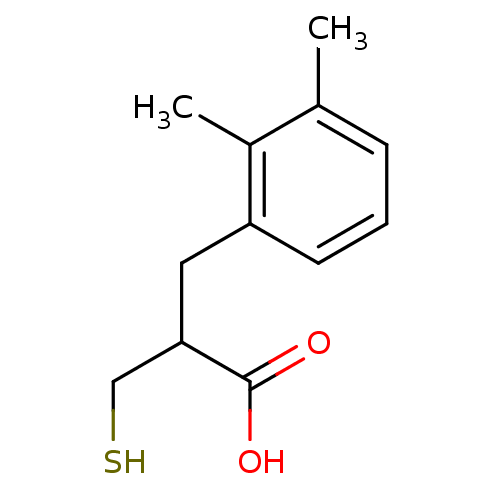 (3-(2,3-Dimethyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279957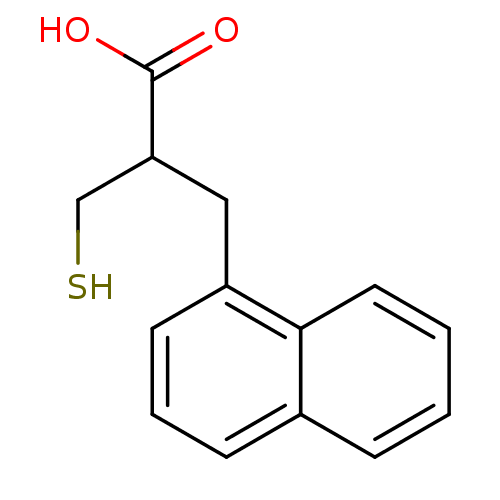 (2-Mercaptomethyl-3-naphthalen-1-yl-propionic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279952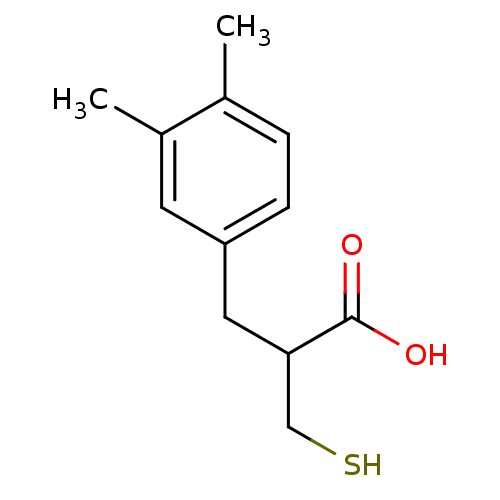 (3-(3,4-Dimethyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279946 (2-Mercaptomethyl-3-(3,4,5-trifluoro-phenyl)-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279962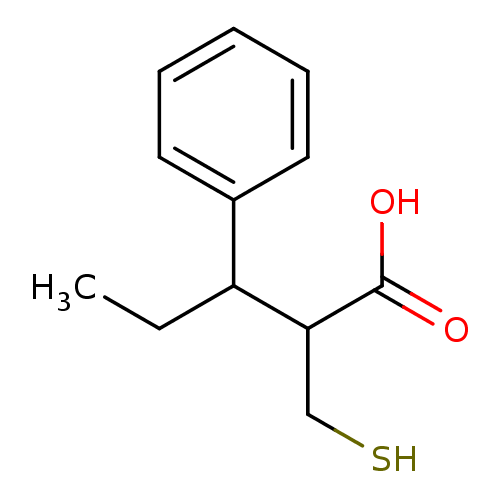 (2-Mercaptomethyl-3-phenyl-pentanoic acid | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against carboxypeptidase A (CPA), expressed as inhibitory constant (Ki) | Bioorg Med Chem Lett 1: 323-326 (1991) Article DOI: 10.1016/S0960-894X(01)80817-9 BindingDB Entry DOI: 10.7270/Q2QR4X1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279951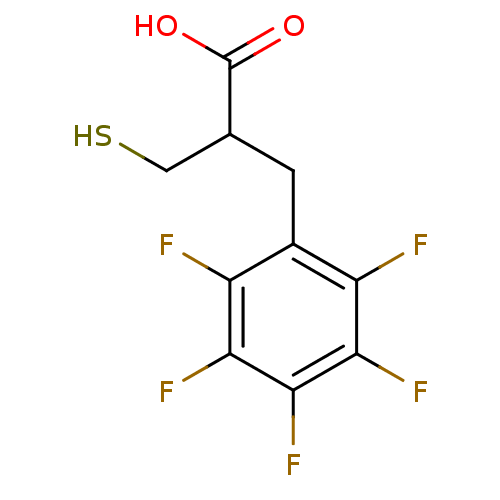 (2-Mercaptomethyl-3-pentafluorophenyl-propionic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109602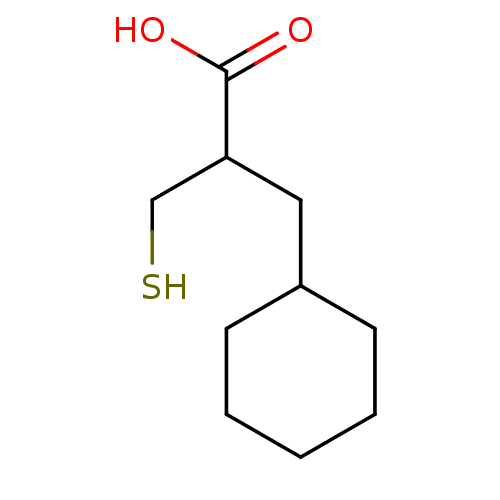 (3-Cyclohexyl-2-mercaptomethyl-propionic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279959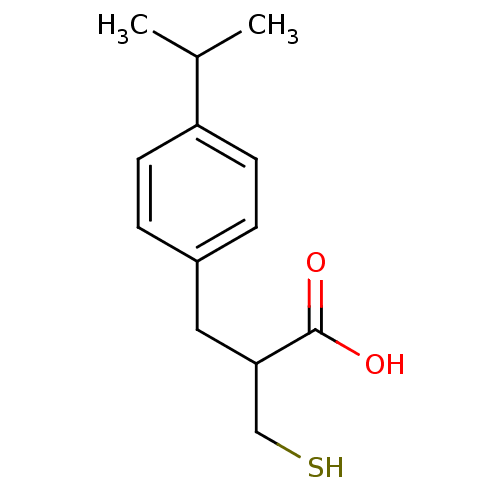 (3-(4-Isopropyl-phenyl)-2-mercaptomethyl-propionic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50279948 (3-(4-tert-Butyl-phenyl)-2-mercaptomethyl-propionic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against carboxypeptidase A using hippurylphenylalanine as substrate | Bioorg Med Chem Lett 1: 317-322 (1991) Article DOI: 10.1016/S0960-894X(01)80816-7 BindingDB Entry DOI: 10.7270/Q2VH5NR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099832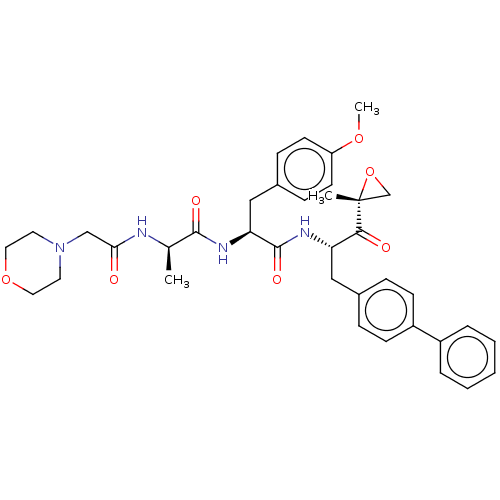 (CHEMBL3319587) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099830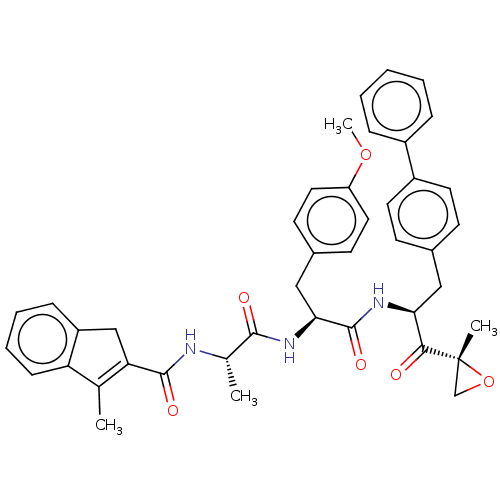 (CHEMBL3319585) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099659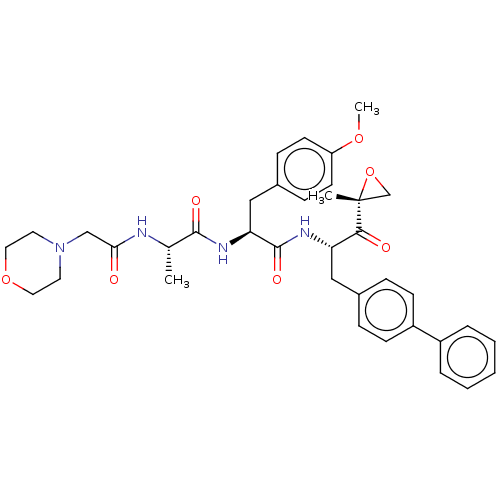 (CHEMBL3319478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099831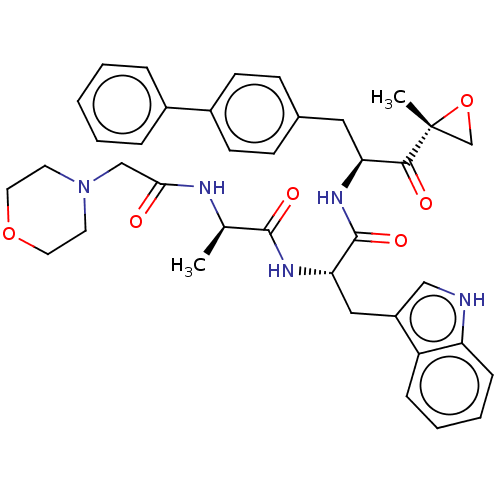 (CHEMBL3319586) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50524277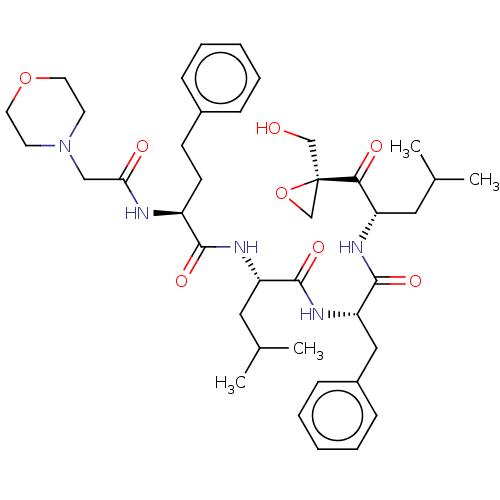 (CHEMBL4451741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human RPMI8226 cell lysate using Suc-LLVY-AMC as substrate pretreated for 1 hr followed by substrate ad... | J Med Chem 62: 4444-4455 (2019) Article DOI: 10.1021/acs.jmedchem.8b01943 BindingDB Entry DOI: 10.7270/Q2W95DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099663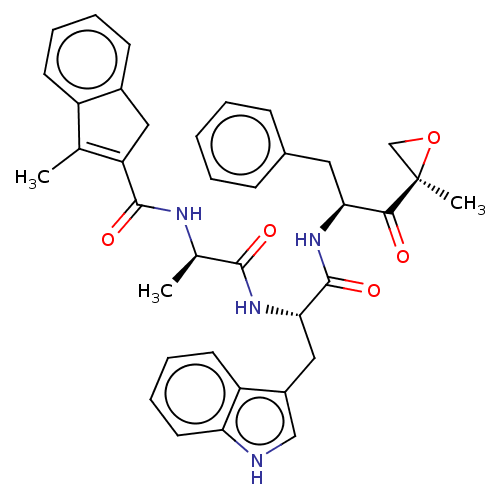 (CHEMBL3319482) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50277889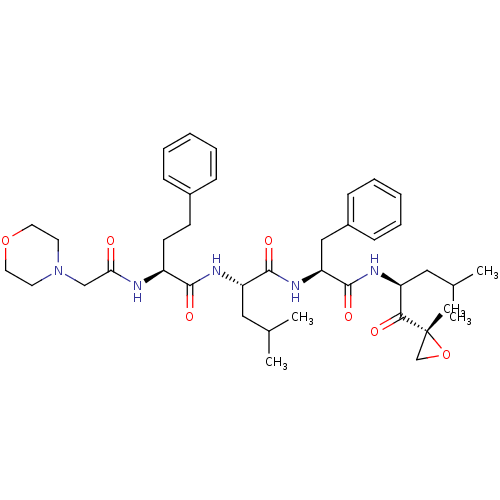 (CARFILZOMIB | CHEMBL451887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human RPMI8226 cell lysate using Suc-LLVY-AMC as substrate pretreated for 1 hr followed by substrate ad... | J Med Chem 62: 4444-4455 (2019) Article DOI: 10.1021/acs.jmedchem.8b01943 BindingDB Entry DOI: 10.7270/Q2W95DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099829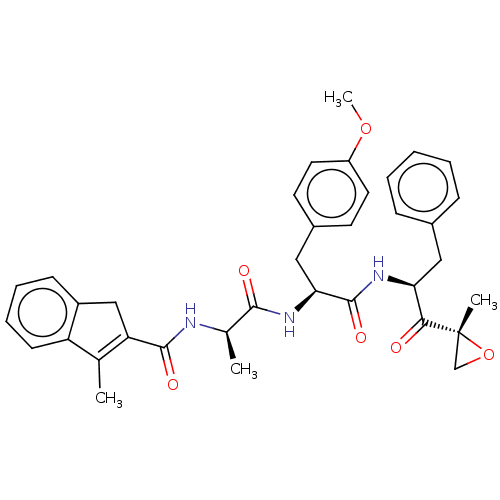 (CHEMBL3319584) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099685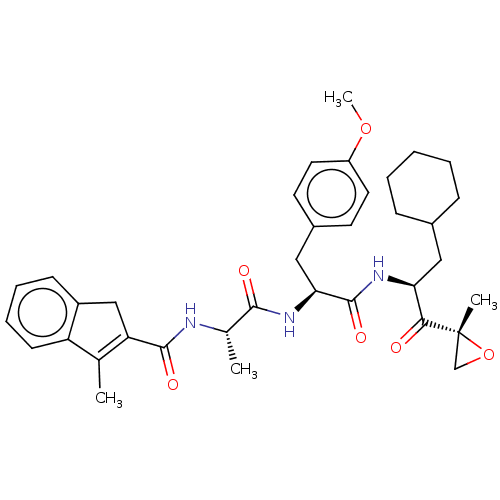 (CHEMBL3319578) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099660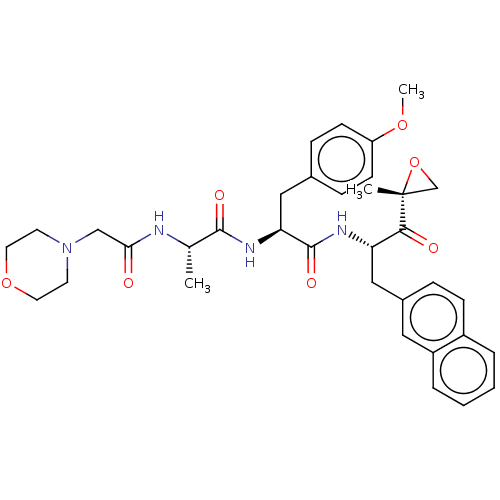 (CHEMBL3319479) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099833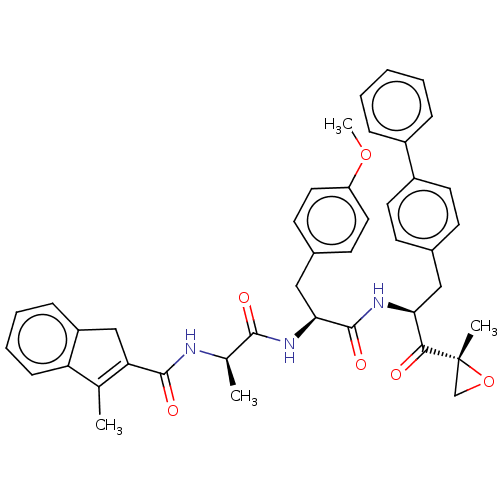 (CHEMBL3319588) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099683 (CHEMBL3319484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099687 (CHEMBL3319580) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50007203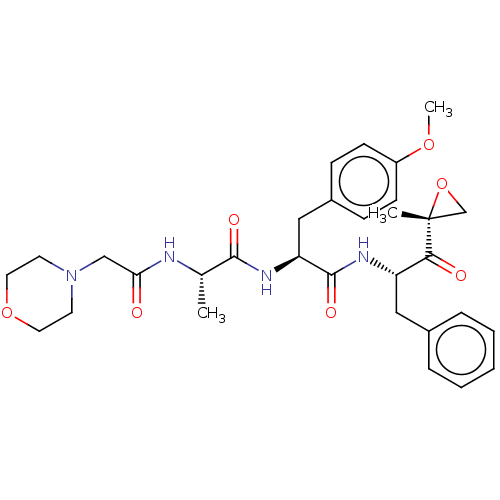 (CHEMBL3237875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099662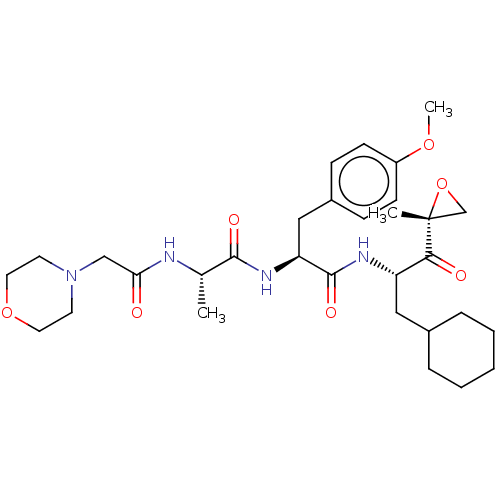 (CHEMBL3319481) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50277889 (CARFILZOMIB | CHEMBL451887) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S proteasome chymotrypsin-like activity in human RPMI-8226 cells using Suc-LLVY-AMC as fluorogenic substrate incubated for 3 hr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00291 BindingDB Entry DOI: 10.7270/Q2RX9GWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50524278 (CHEMBL4449204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human RPMI8226 cell lysate using Suc-LLVY-AMC as substrate pretreated for 1 hr followed by substrate ad... | J Med Chem 62: 4444-4455 (2019) Article DOI: 10.1021/acs.jmedchem.8b01943 BindingDB Entry DOI: 10.7270/Q2W95DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099664 (CHEMBL3319483) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099686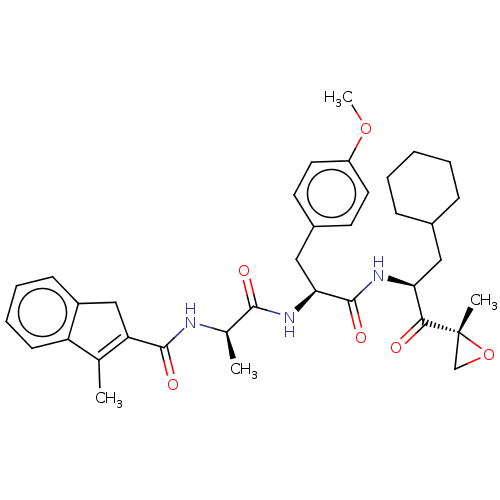 (CHEMBL3319579) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099827 (CHEMBL3319582) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099828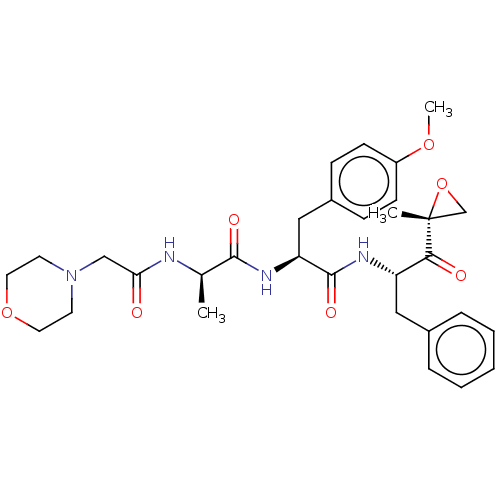 (CHEMBL3319583) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50007203 (CHEMBL3237875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human RPMI8226 cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50099945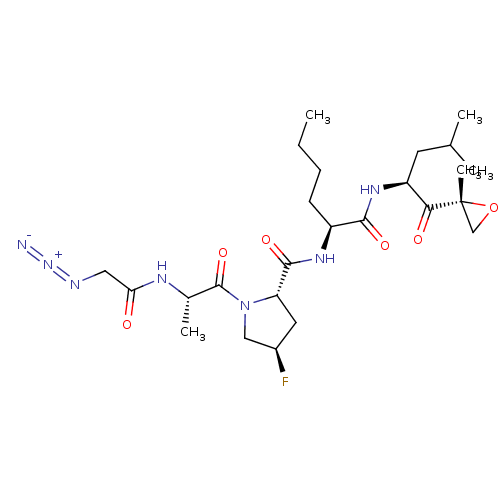 (CHEMBL3319599) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-1i in human Raji cells using BODIPY- epoxomicin by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50538158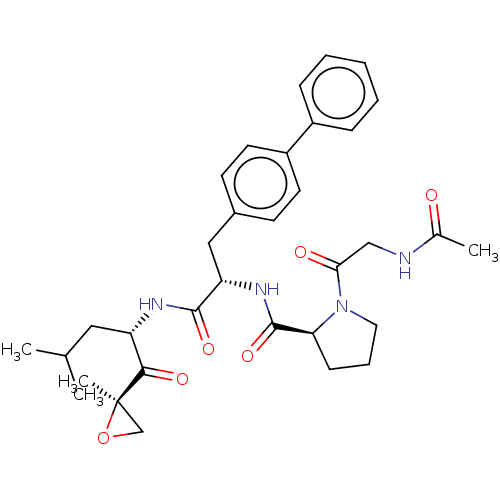 (CHEMBL4632784) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Inhibition of LMP2 in human 20S immunoproteasome using Ac-PAL-AMC as substrate after 1 hr by fluorescence based microplate reader analysis | J Med Chem 63: 3763-3783 (2020) Article DOI: 10.1021/acs.jmedchem.0c00416 BindingDB Entry DOI: 10.7270/Q2HH6PM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099663 (CHEMBL3319482) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human RPMI8226 cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-9 (Homo sapiens (Human)) | BDBM50573691 (CHEMBL4845896) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human 20S proteasome LMP2 activity in human RPMI-8226 cells using Ac-PAL-AMC as fluorogenic substrate incubated for 72 hrs by fluoresce... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00291 BindingDB Entry DOI: 10.7270/Q2RX9GWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 255 total ) | Next | Last >> |