

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277555 (Benzyl 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277951 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277914 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(2-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277949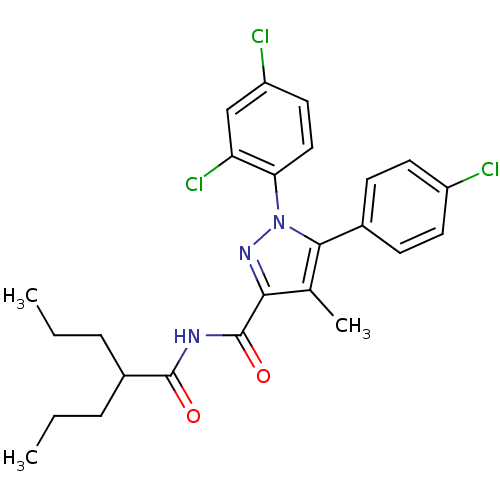 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278049 (5-(4-Chlorophenyl)-N-(cyclohexanecarbonyl)-1-(2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278000 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278002 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-hexano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277950 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(2-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278003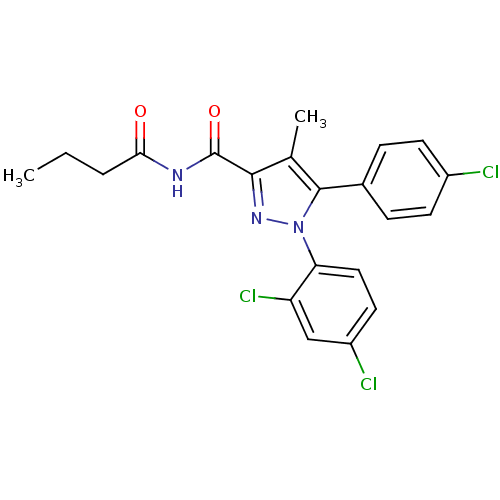 (CHEMBL484401 | N-Butyryl-5-(4-chlorophenyl)-1-(2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277764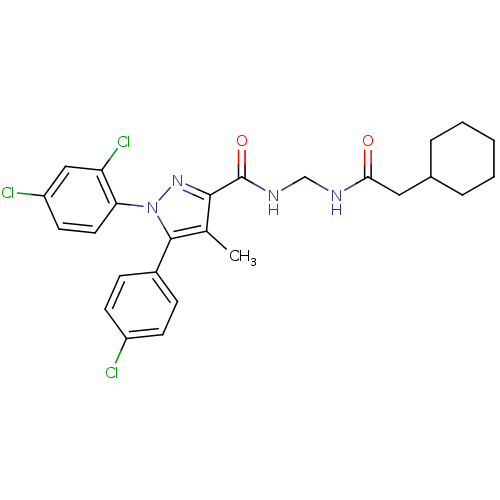 (5-(4-Chlorophenyl)-N-((2-cyclohexylacetamido)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277724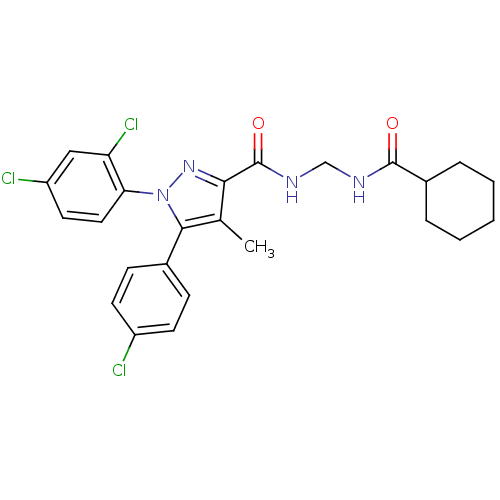 (5-(4-Chlorophenyl)-N-(cyclohexanecarboxamidomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251131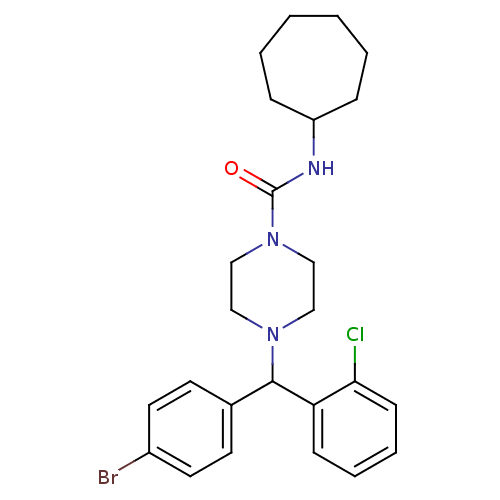 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-cycloh...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277952 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(3,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251130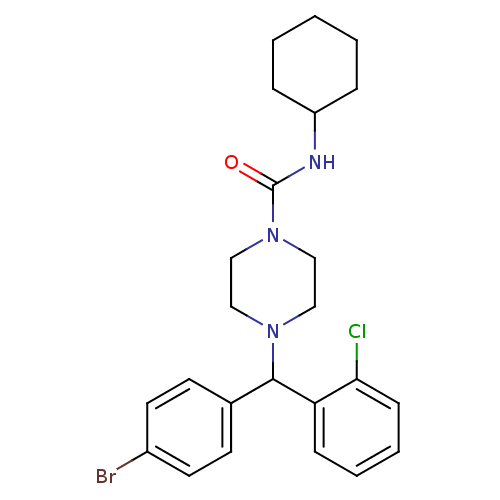 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-Ncyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242549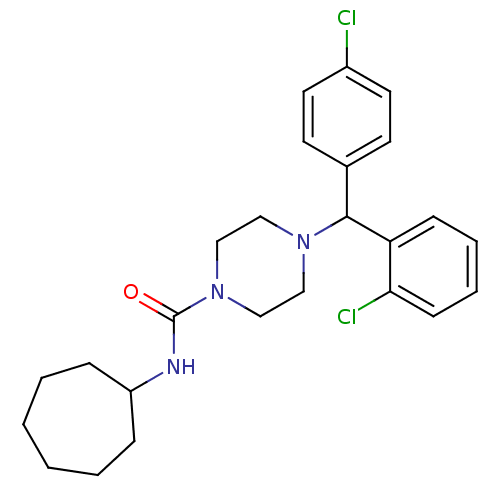 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278050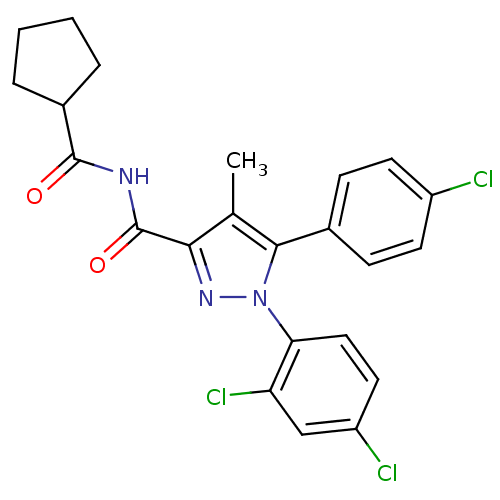 (5-(4-Chlorophenyl)-N-(cyclopentanecarbonyl)-1-(2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 67.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242548 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277725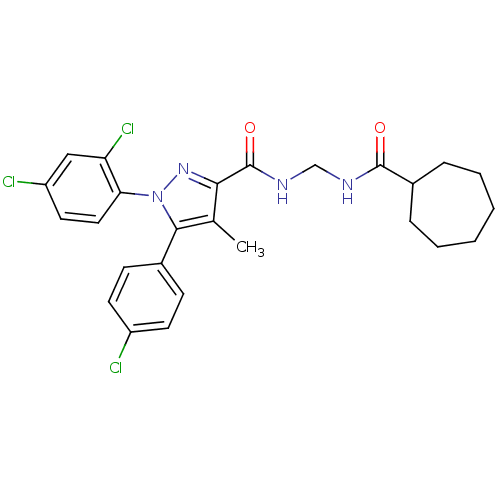 (5-(4-Chlorophenyl)-N-(cycloheptanecarboxamidomethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251131 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-cycloh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277592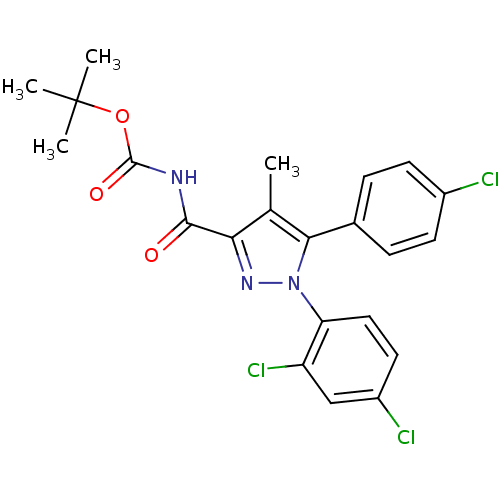 (CHEMBL485340 | tert-Butyl 5-(4-chlorophenyl)-1-(2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242557 (4-((4-Chlorophenyl)(2,4-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277591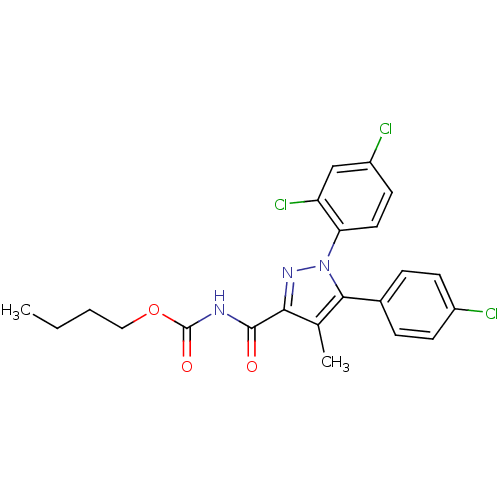 (Butyl 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 99.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277726 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251130 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-Ncyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242582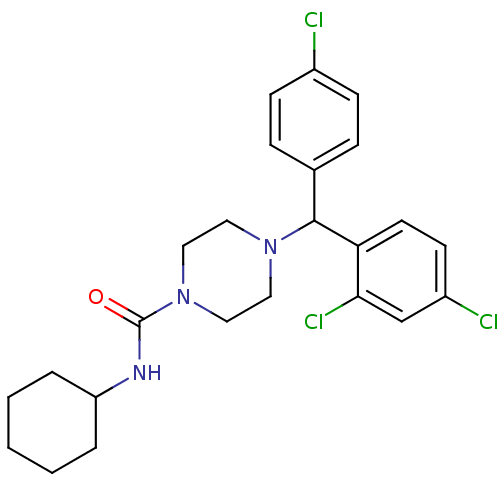 (4-((4-Chlorophenyl)(2,4-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242566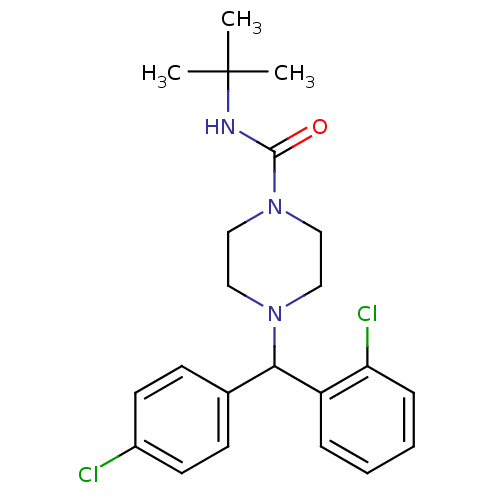 (CHEMBL519584 | N-tert-Butyl-4-((2-chlorophenyl)(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278052 (5-(4-Chlorophenyl)-N-(cyclopropanecarbonyl)-1-(2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278051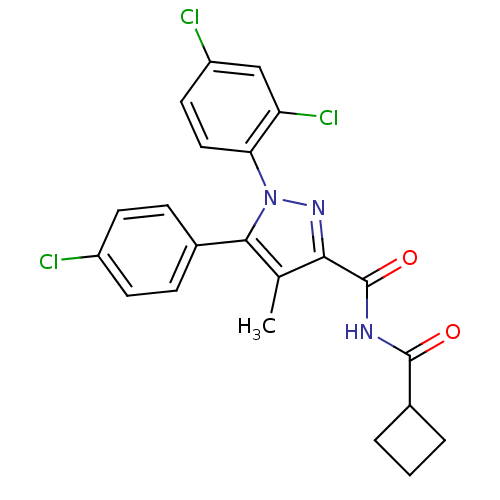 (5-(4-Chlorophenyl)-N-(cyclobutanecarbonyl)-1-(2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50251132 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-(cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242548 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-cyclo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277456 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-(hexyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277763 (5-(4-Chlorophenyl)-N-((2-cyclopentylacetamido)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242601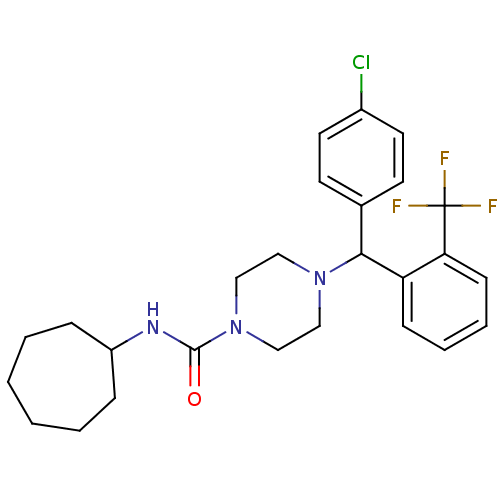 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50251132 (4-((4-Bromophenyl)(2-chlorophenyl)methyl)-N-(cyclo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277554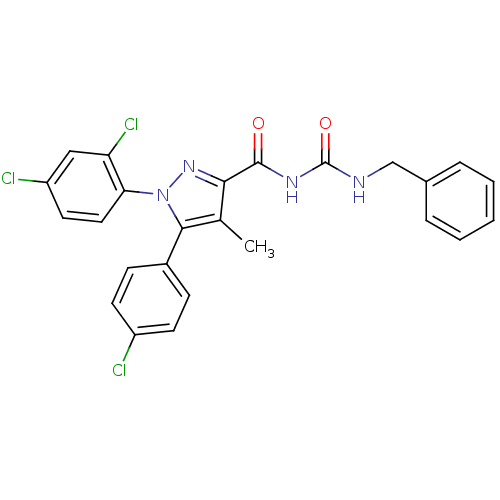 (CHEMBL520456 | N-(Benzylcarbamoyl)-5-(4-chlorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277723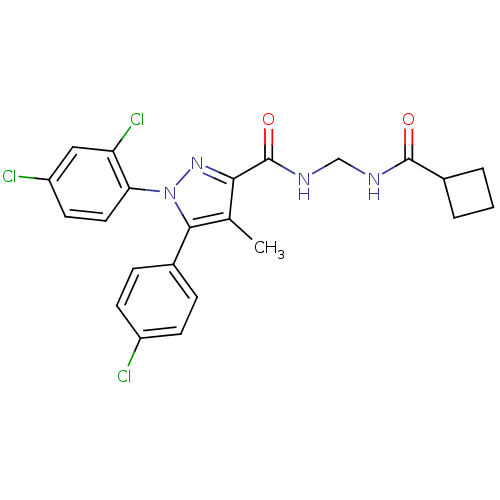 (5-(4-Chlorophenyl)-N-(cyclobutanecarboxamidomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242568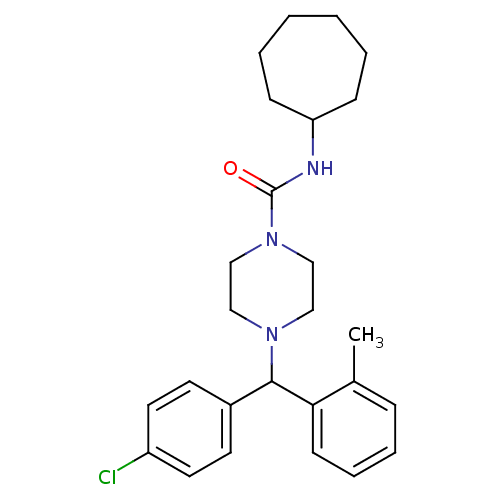 (4-((4-Chlorophenyl)(o-tolyl)methyl)-N-cycloheptylp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242537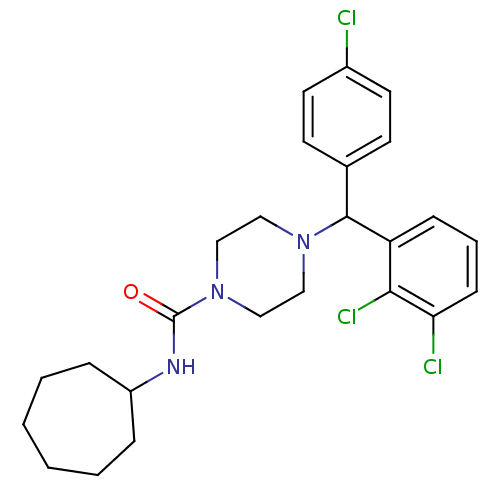 (4-((4-Chlorophenyl)(2,3-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50278001 (5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-N-isobut...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242600 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242568 (4-((4-Chlorophenyl)(o-tolyl)methyl)-N-cycloheptylp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50277684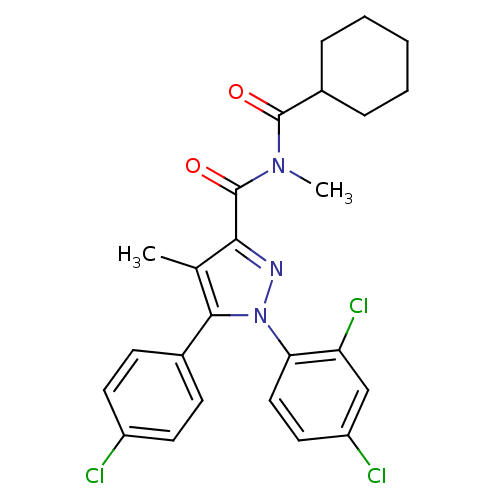 (5-(4-Chlorophenyl)-N-(cyclohexanecarbonyl)-1-(2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1R in Sprague-Dawley rat cerebellar membrane | Bioorg Med Chem 17: 3080-92 (2009) Article DOI: 10.1016/j.bmc.2009.03.006 BindingDB Entry DOI: 10.7270/Q22V2G0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242556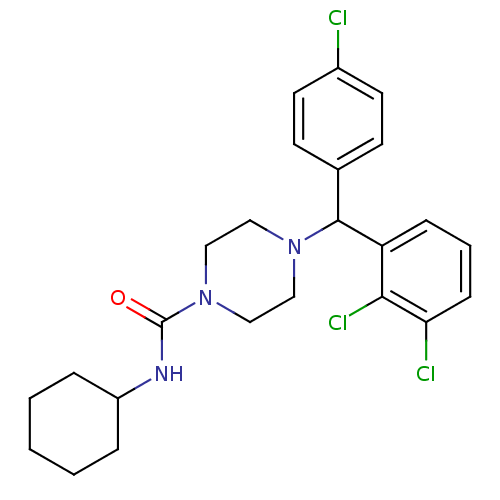 (4-((4-Chlorophenyl)(2,3-dichlorophenyl)methyl)-N-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242600 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50242565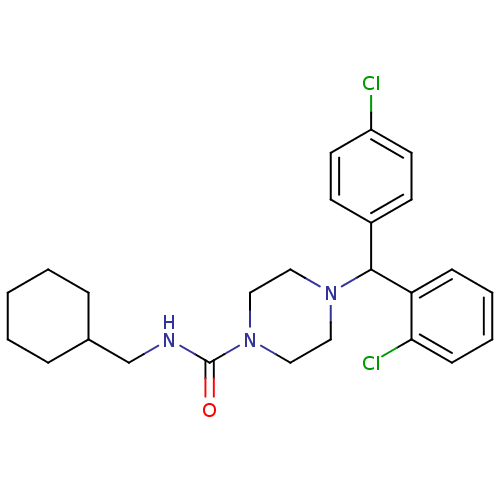 (4-((2-Chlorophenyl)(4-chlorophenyl)methyl)-N-(cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in Sprague-Dawley rat cerebella membrane | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242567 (4-((4-Chlorophenyl)(o-tolyl)methyl)-N-cyclohexylpi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50242601 (4-((4-Chlorophenyl)(2-(trifluoromethyl)phenyl)-met...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in CHOK1 cells by luciferase assay | Bioorg Med Chem 16: 4035-51 (2008) Article DOI: 10.1016/j.bmc.2008.01.023 BindingDB Entry DOI: 10.7270/Q2B56JH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 137 total ) | Next | Last >> |