

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-glucuronidase (Rattus norvegicus) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50335242 (3-Chloro-3'-((2-cyclopentyl-3-oxo-2,3-dihydrobenzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142192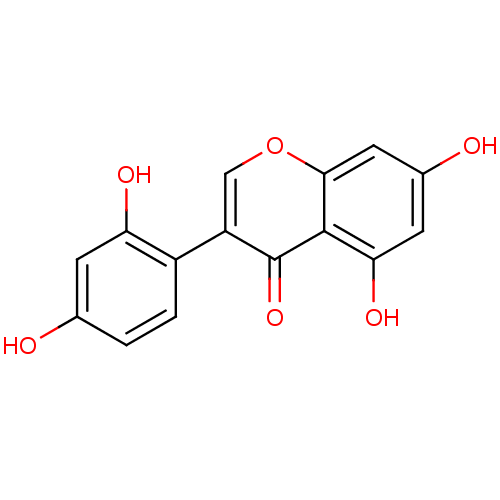 (3-(2,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-o...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50335242 (3-Chloro-3'-((2-cyclopentyl-3-oxo-2,3-dihydrobenzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50335253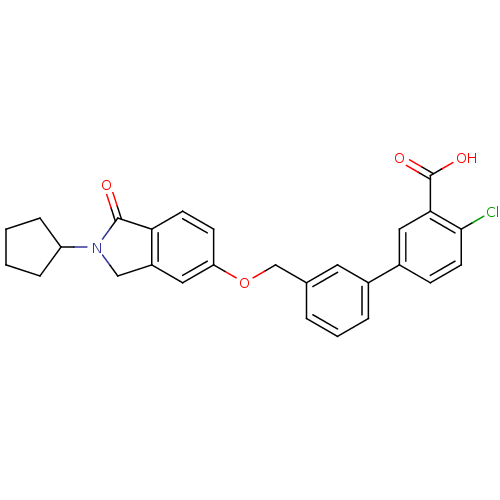 (4-Chloro-3'-((2-cyclopentyl-1-oxoisoindolin-5-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of radioligand binding to human histamine H1 receptor | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50335253 (4-Chloro-3'-((2-cyclopentyl-1-oxoisoindolin-5-ylox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of radioligand binding to human adrenergic alpha1a receptor | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50335253 (4-Chloro-3'-((2-cyclopentyl-1-oxoisoindolin-5-ylox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM79181 (10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142192 (3-(2,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50017268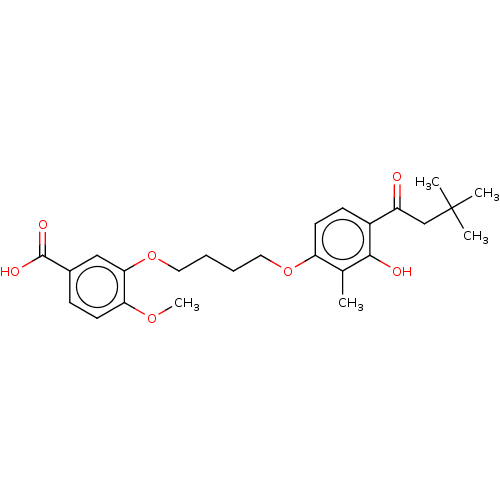 (CHEMBL3287723 | US10099993, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human histamine H4 receptor at 10 uM by radioligand displacement assay | J Med Chem 57: 4154-72 (2014) Article DOI: 10.1021/jm5000563 BindingDB Entry DOI: 10.7270/Q2GH9KH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50017268 (CHEMBL3287723 | US10099993, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human histamine H2 receptor at 10 uM by radioligand displacement assay | J Med Chem 57: 4154-72 (2014) Article DOI: 10.1021/jm5000563 BindingDB Entry DOI: 10.7270/Q2GH9KH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 4 (Rattus norvegicus (Rat)) | BDBM50017264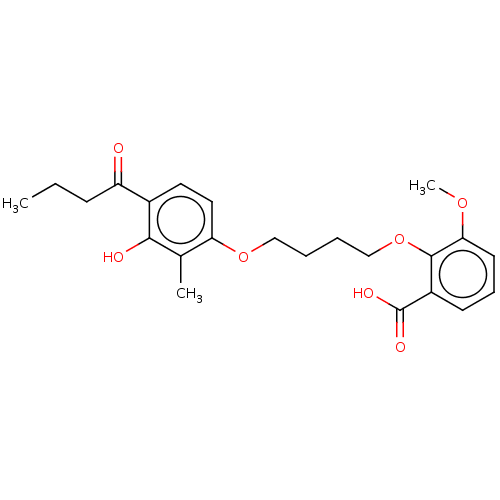 (CHEMBL3287718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGlu4 receptor expressed in HEK293 cells | J Med Chem 57: 4154-72 (2014) Article DOI: 10.1021/jm5000563 BindingDB Entry DOI: 10.7270/Q2GH9KH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50335253 (4-Chloro-3'-((2-cyclopentyl-1-oxoisoindolin-5-ylox...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of radioligand binding to human adrenergic alpha1d receptor | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50335242 (3-Chloro-3'-((2-cyclopentyl-3-oxo-2,3-dihydrobenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of radioligand binding to human histamine H1 receptor | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50017264 (CHEMBL3287718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at rat mGlu1 receptor expressed in HEK293 cells | J Med Chem 57: 4154-72 (2014) Article DOI: 10.1021/jm5000563 BindingDB Entry DOI: 10.7270/Q2GH9KH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50335242 (3-Chloro-3'-((2-cyclopentyl-3-oxo-2,3-dihydrobenzo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of radioligand binding to human dopamine D1 receptor | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50077323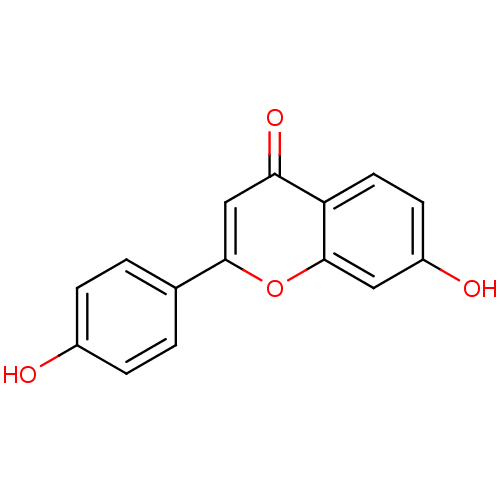 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50335242 (3-Chloro-3'-((2-cyclopentyl-3-oxo-2,3-dihydrobenzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes | J Med Chem 55: 9434-45 (2012) Article DOI: 10.1021/jm3005306 BindingDB Entry DOI: 10.7270/Q2RX9D78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142193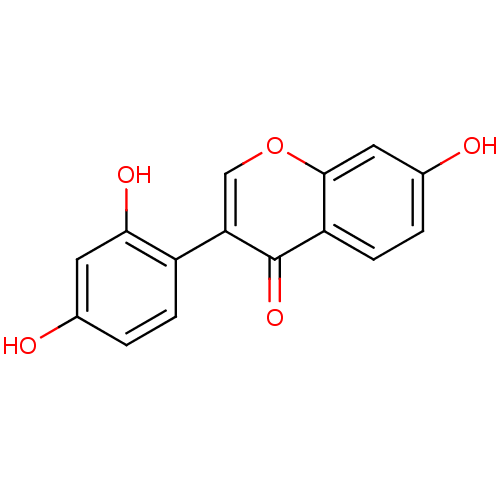 (2'-hydroxydaidzein | 3-(2,4-dihydroxyphenyl)-7-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142191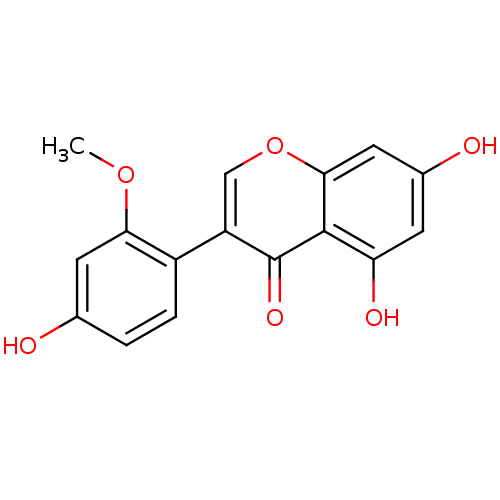 (5,7-Dihydroxy-3-(4-hydroxy-2-methoxy-phenyl)-chrom...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50077323 (7,4'-Dihydroxyflavone | 7-hydroxy-2-(4-hydroxyphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142190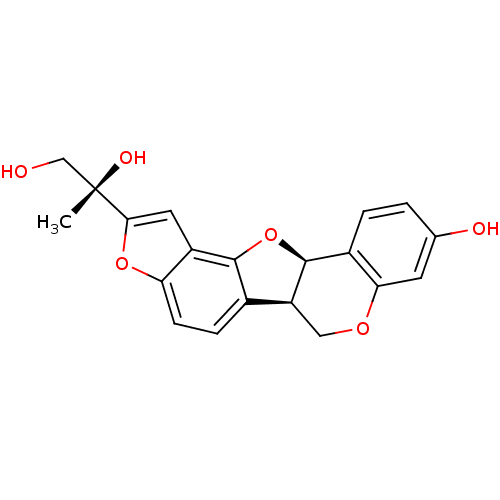 (2-((11bR,12R)-9-Hydroxy-5b,11b-dihydro-6H-3,7,12-t...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142191 (5,7-Dihydroxy-3-(4-hydroxy-2-methoxy-phenyl)-chrom...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50142190 (2-((11bR,12R)-9-Hydroxy-5b,11b-dihydro-6H-3,7,12-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50366927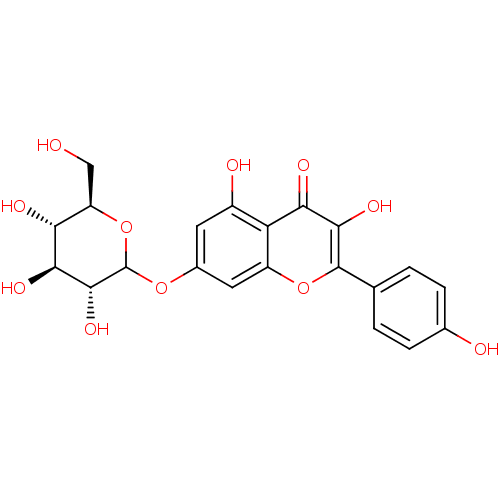 (CHEMBL1159471) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Rattus norvegicus) | BDBM50142193 (2'-hydroxydaidzein | 3-(2,4-dihydroxyphenyl)-7-hyd...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of Beta-glucuronidase in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysozyme C-1 (Rattus norvegicus) | BDBM50366927 (CHEMBL1159471) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kaohsiung Medical University Curated by ChEMBL | Assay Description Inhibitory effect of compound on the release of lysozyme in rat neutrophils stimulated with fMLP/CB | Bioorg Med Chem Lett 14: 1011-4 (2004) Article DOI: 10.1016/j.bmcl.2003.11.074 BindingDB Entry DOI: 10.7270/Q2C829VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295965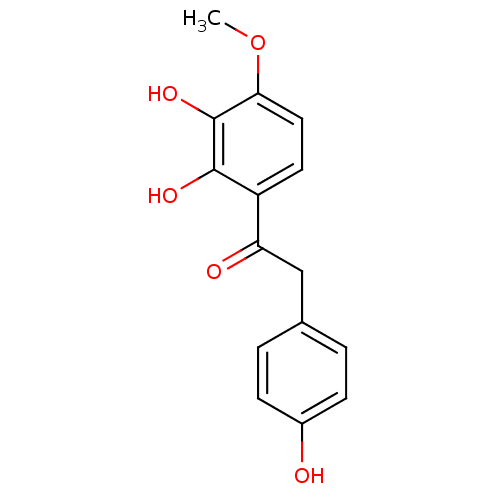 (2,3,4'-Trihydroxy-4-methoxydeoxybenzoin | CHEMBL54...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295965 (2,3,4'-Trihydroxy-4-methoxydeoxybenzoin | CHEMBL54...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295965 (2,3,4'-Trihydroxy-4-methoxydeoxybenzoin | CHEMBL54...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295964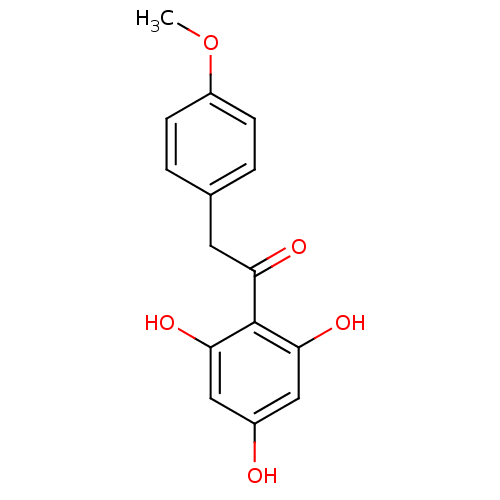 (2,4,6-Trihydroxy-4'-methoxydeoxybenzoin | CHEMBL55...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295962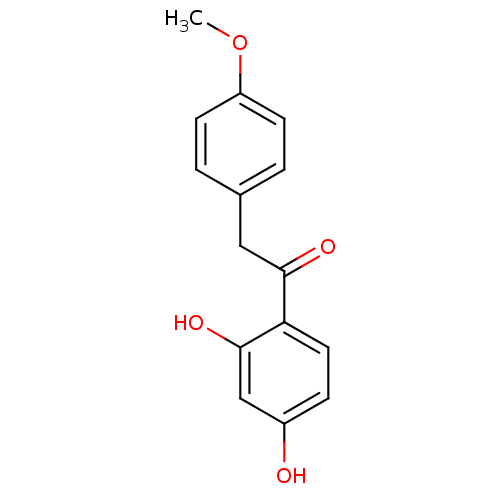 (1-(2,4-dihydroxyphenyl)-2-(4-methoxyphenyl)ethanon...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295969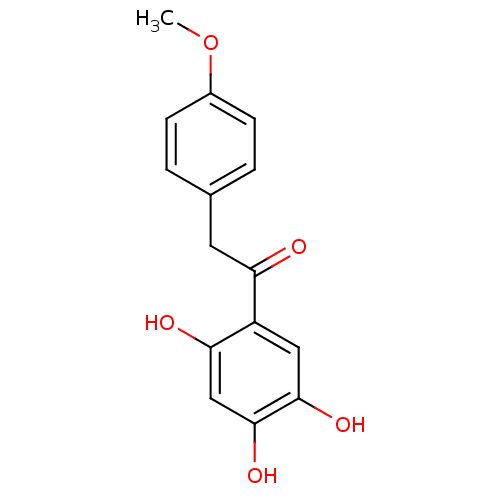 (2,4,5-Trihydroxy-4'-methoxydeoxybenzoin | CHEMBL55...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295961 (2,4-Dihydroxy-3',4'-dimethoxydeoxybenzoin | CHEMBL...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295966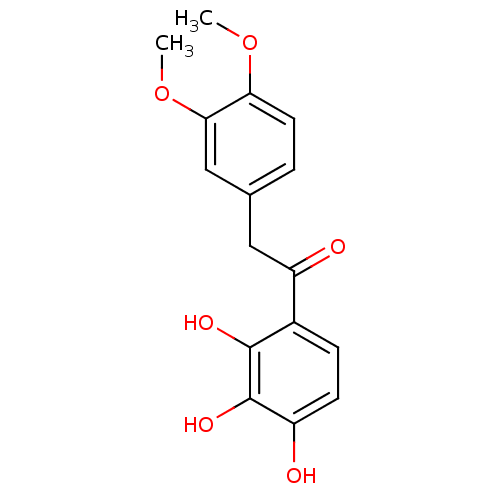 (2,3,4-Trihydroxy-3',4'-dimethoxydeoxybenzoin | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50166034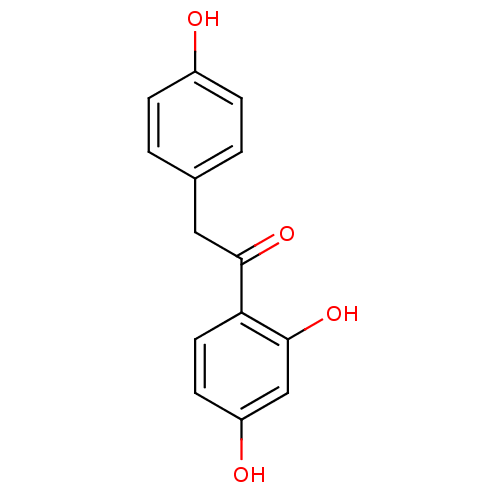 (1-(2,4-Dihydroxy-phenyl)-2-(4-hydroxy-phenyl)-etha...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295966 (2,3,4-Trihydroxy-3',4'-dimethoxydeoxybenzoin | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295969 (2,4,5-Trihydroxy-4'-methoxydeoxybenzoin | CHEMBL55...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295962 (1-(2,4-dihydroxyphenyl)-2-(4-methoxyphenyl)ethanon...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295960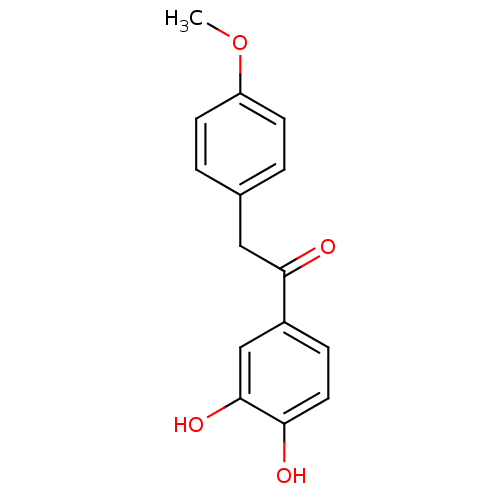 (3,4-Dihydroxy-4'-methoxydeoxybenzoin | CHEMBL55018...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50166034 (1-(2,4-Dihydroxy-phenyl)-2-(4-hydroxy-phenyl)-etha...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tajen University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase after 30 mins | J Nat Prod 71: 1930-1933 (2008) Article DOI: 10.1021/np800564z BindingDB Entry DOI: 10.7270/Q2SB45TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295960 (3,4-Dihydroxy-4'-methoxydeoxybenzoin | CHEMBL55018...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50166034 (1-(2,4-Dihydroxy-phenyl)-2-(4-hydroxy-phenyl)-etha...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 30 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295962 (1-(2,4-dihydroxyphenyl)-2-(4-methoxyphenyl)ethanon...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295969 (2,4,5-Trihydroxy-4'-methoxydeoxybenzoin | CHEMBL55...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 150 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50295968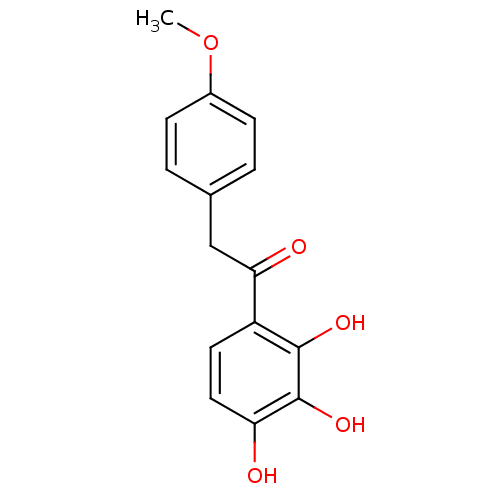 (2,3,4-Trihydroxy-4'-methoxydeoxybenzoin | CHEMBL26...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase activity after 90 mins by spectrophotometry | Bioorg Med Chem 17: 4360-6 (2009) Article DOI: 10.1016/j.bmc.2009.05.019 BindingDB Entry DOI: 10.7270/Q28W3DC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 228 total ) | Next | Last >> |