 Found 737 hits with Last Name = 'kubo' and Initial = 't'
Found 737 hits with Last Name = 'kubo' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50058163
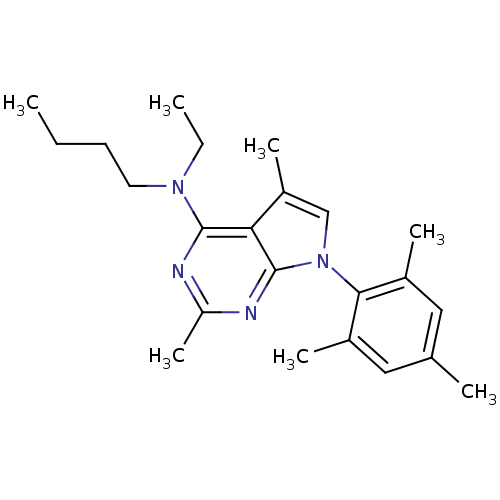
(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18656

((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccc(nc1)C(F)(F)F)c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5O/c1-12-11-32(19(33)30-15-4-6-18(29-9-15)21(25,26)27)13(2)10-31(12)16-5-3-14(8-28)17(7-16)20(22,23)24/h3-7,9,12-13H,10-11H2,1-2H3,(H,30,33)/t12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18656

((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccc(nc1)C(F)(F)F)c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5O/c1-12-11-32(19(33)30-15-4-6-18(29-9-15)21(25,26)27)13(2)10-31(12)16-5-3-14(8-28)17(7-16)20(22,23)24/h3-7,9,12-13H,10-11H2,1-2H3,(H,30,33)/t12-,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18525
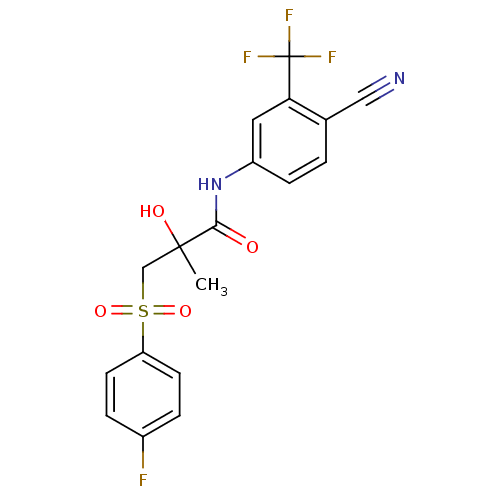
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50074456
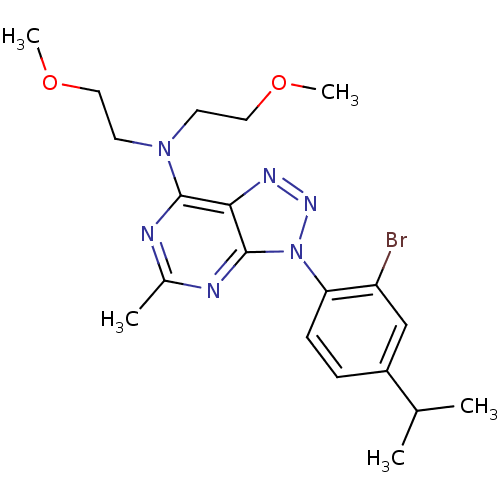
(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85397
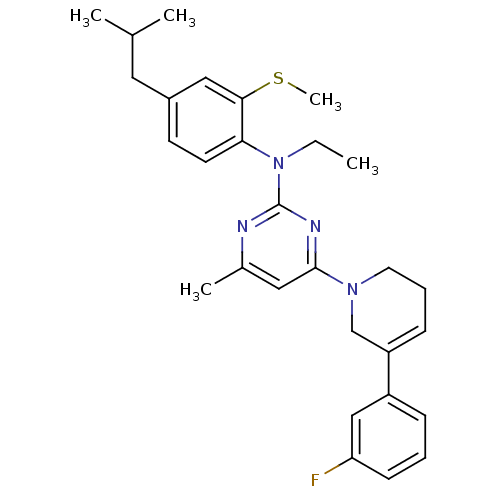
(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 15.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85398
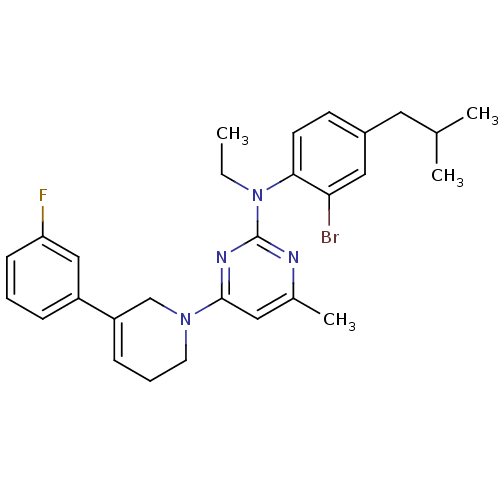
(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525

(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85397

(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 20.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50074456

(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM85398

(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 22.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Monkey) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(RAT) | BDBM86058

(MCL0129)Show SMILES COc1ccc2ccccc2c1CCCCN1CCN(C[C@@H](N2CCN(CC2)C(C)C)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C34H47FN4O/c1-27(2)38-22-24-39(25-23-38)33(29-11-14-30(35)15-12-29)26-37-20-18-36(19-21-37)17-7-6-10-32-31-9-5-4-8-28(31)13-16-34(32)40-3/h4-5,8-9,11-16,27,33H,6-7,10,17-26H2,1-3H3/t33-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 304: 818-26 (2003)
Article DOI: 10.1124/jpet.102.044826
BindingDB Entry DOI: 10.7270/Q2FQ9V5W |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Rattus norvegicus) | BDBM18656

((2R,5S)-4-[4-cyano-3-(trifluoromethyl)phenyl]-2,5-...)Show SMILES C[C@@H]1CN([C@@H](C)CN1C(=O)Nc1ccc(nc1)C(F)(F)F)c1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H19F6N5O/c1-12-11-32(19(33)30-15-4-6-18(29-9-15)21(25,26)27)13(2)10-31(12)16-5-3-14(8-28)17(7-16)20(22,23)24/h3-7,9,12-13H,10-11H2,1-2H3,(H,30,33)/t12-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Rattus norvegicus) | BDBM18525

(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd] where [L] is the concentration of labeled ligand a... |
J Med Chem 49: 716-26 (2006)
Article DOI: 10.1021/jm050293c
BindingDB Entry DOI: 10.7270/Q2P84952 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM85397

(CRA1000)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1SC |c:14| Show InChI InChI=1S/C29H35FN4S/c1-6-34(26-13-12-22(15-20(2)3)17-27(26)35-5)29-31-21(4)16-28(32-29)33-14-8-10-24(19-33)23-9-7-11-25(30)18-23/h7,9-13,16-18,20H,6,8,14-15,19H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM50074456

(CHEMBL10504 | SC241 | [3-(2-Bromo-4-isopropyl-phen...)Show SMILES COCCN(CCOC)c1nc(C)nc2n(nnc12)-c1ccc(cc1Br)C(C)C Show InChI InChI=1S/C20H27BrN6O2/c1-13(2)15-6-7-17(16(21)12-15)27-20-18(24-25-27)19(22-14(3)23-20)26(8-10-28-4)9-11-29-5/h6-7,12-13H,8-11H2,1-5H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM85398

(CRA1001)Show SMILES CCN(c1nc(C)cc(n1)N1CCC=C(C1)c1cccc(F)c1)c1ccc(CC(C)C)cc1Br |c:14| Show InChI InChI=1S/C28H32BrFN4/c1-5-34(26-12-11-21(14-19(2)3)16-25(26)29)28-31-20(4)15-27(32-28)33-13-7-9-23(18-33)22-8-6-10-24(30)17-22/h6,8-12,15-17,19H,5,7,13-14,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(RAT) | BDBM50058163

(Butyl-[2,5-dimethyl-7-(2,4,6-trimethyl-phenyl)-7H-...)Show SMILES CCCCN(CC)c1nc(C)nc2n(cc(C)c12)-c1c(C)cc(C)cc1C |(-10.62,2.69,;-9.29,3.46,;-7.95,2.7,;-6.62,3.48,;-5.28,2.71,;-3.95,3.49,;-3.96,5.03,;-5.27,1.17,;-6.6,.4,;-6.6,-1.14,;-7.94,-1.91,;-5.27,-1.91,;-3.92,-1.14,;-2.44,-1.61,;-1.54,-.35,;-2.46,.9,;-1.99,2.37,;-3.93,.41,;-1.96,-3.07,;-2.99,-4.22,;-4.49,-3.91,;-2.5,-5.68,;-.99,-5.99,;-.51,-7.46,;.03,-4.83,;-.46,-3.38,;.56,-2.22,)| Show InChI InChI=1S/C23H32N4/c1-8-10-11-26(9-2)22-20-18(6)14-27(23(20)25-19(7)24-22)21-16(4)12-15(3)13-17(21)5/h12-14H,8-11H2,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 289: 926-35 (1999)
BindingDB Entry DOI: 10.7270/Q2GM85VG |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649820
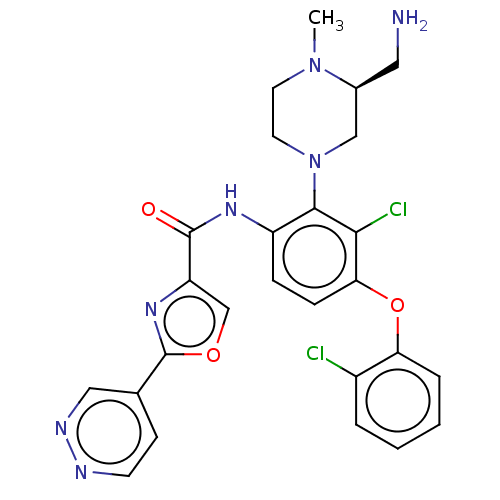
(US20240043403, Example 57)Show SMILES CN1CCN(C[C@@H]1CN)c1c(NC(=O)c2coc(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649819
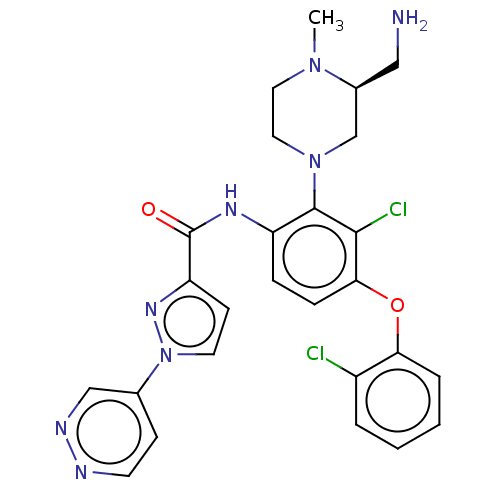
(US20240043403, Example 56)Show SMILES CN1CCN(C[C@@H]1CN)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol kinase delta
(Human) | BDBM649841
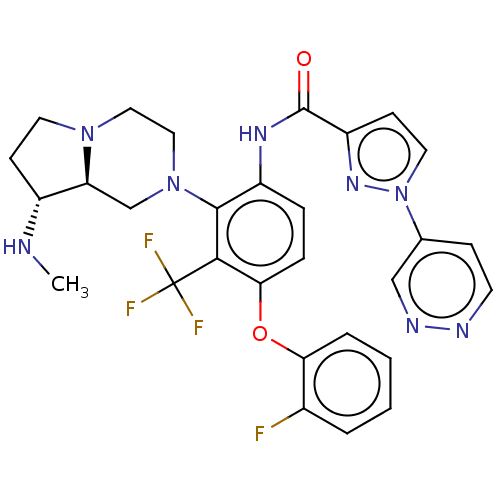
(US20240043403, Example 78)Show SMILES CN[C@@H]1CCN2CCN(C[C@@H]12)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649881
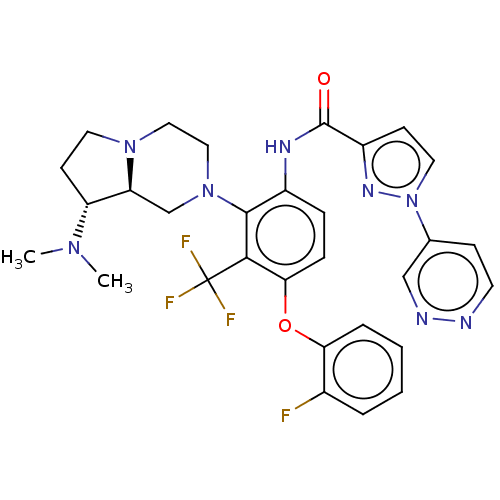
(US20240043403, Example 118)Show SMILES CN(C)[C@@H]1CCN2CCN(C[C@@H]12)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649857
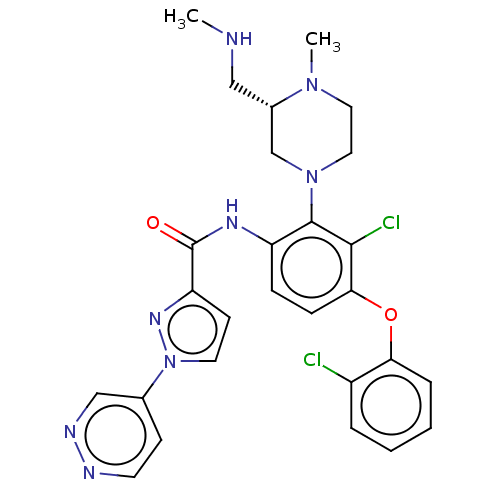
(US20240043403, Example 94)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649849
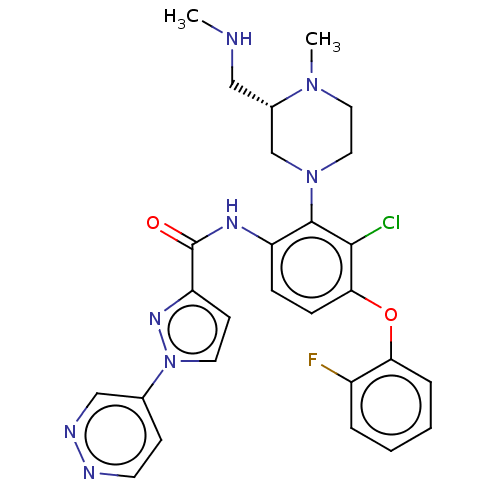
(US20240043403, Example 125 | US20240043403, Exampl...)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649847
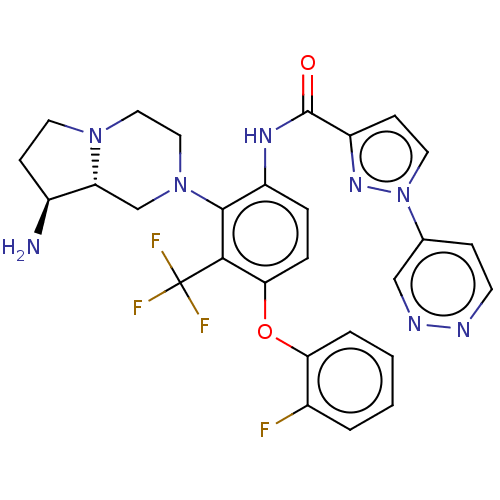
(US20240043403, Example 84)Show SMILES N[C@H]1CCN2CCN(C[C@H]12)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012951

(4-Cyclohexyl-2-hydroxy-3-[3-(1H-imidazol-4-yl)-2-(...)Show SMILES CC(C)OC(=O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](CC(=O)N1CCOCC1)Cc1cccc2ccccc12 Show InChI InChI=1S/C38H51N5O7/c1-25(2)50-38(48)35(45)32(19-26-9-4-3-5-10-26)41-37(47)33(22-30-23-39-24-40-30)42-36(46)29(21-34(44)43-15-17-49-18-16-43)20-28-13-8-12-27-11-6-7-14-31(27)28/h6-8,11-14,23-26,29,32-33,35,45H,3-5,9-10,15-22H2,1-2H3,(H,39,40)(H,41,47)(H,42,46)/t29-,32-,33-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibition of renin from human |
J Med Chem 33: 2707-14 (1990)
BindingDB Entry DOI: 10.7270/Q2N29XJZ |
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649856
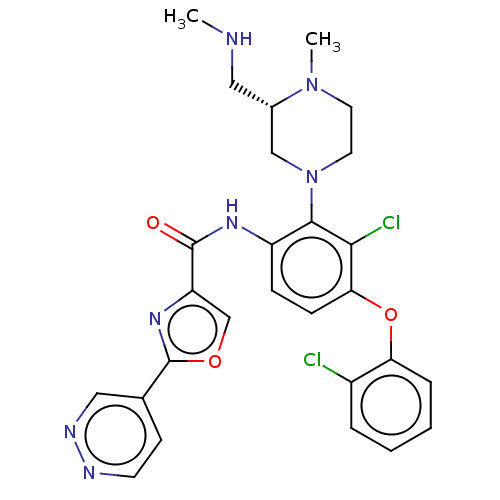
(US20240043403, Example 93)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2coc(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649849

(US20240043403, Example 125 | US20240043403, Exampl...)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649797
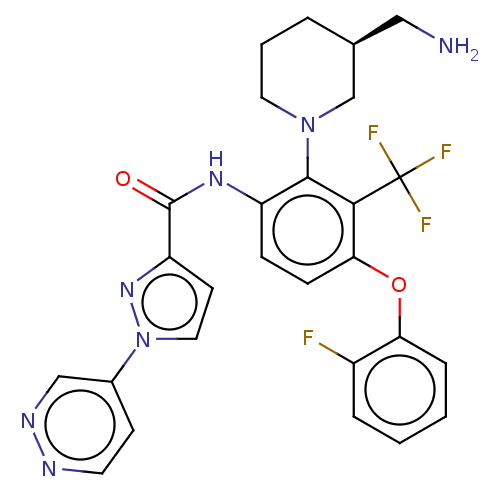
(US20240043403, Example 34)Show SMILES NC[C@@H]1CCCN(C1)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649809
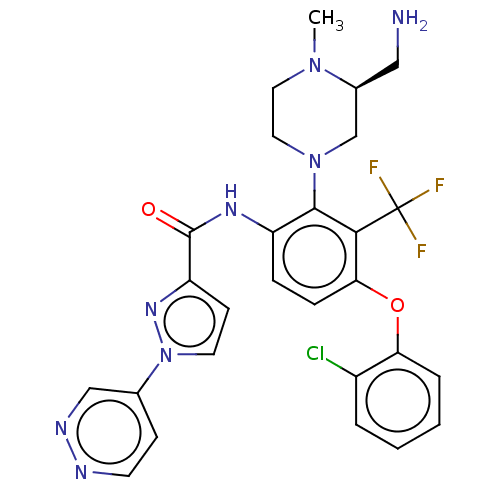
(US20240043403, Example 46)Show SMILES CN1CCN(C[C@@H]1CN)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649792

(US20240043403, Example 122 | US20240043403, Exampl...)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649846
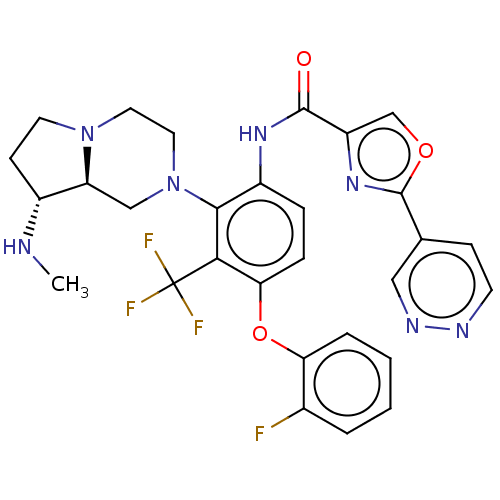
(US20240043403, Example 83)Show SMILES CN[C@@H]1CCN2CCN(C[C@@H]12)c1c(NC(=O)c2coc(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649858
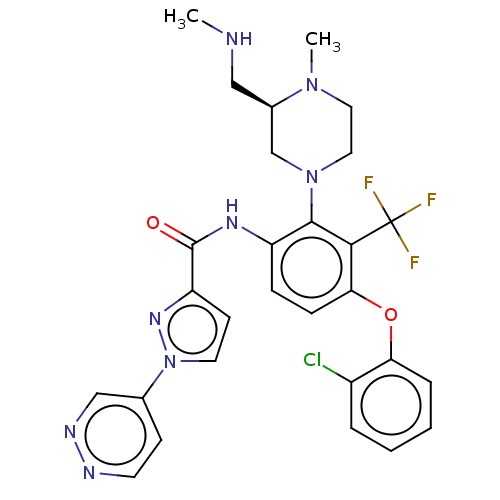
(US20240043403, Example 95)Show SMILES CNC[C@@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649811
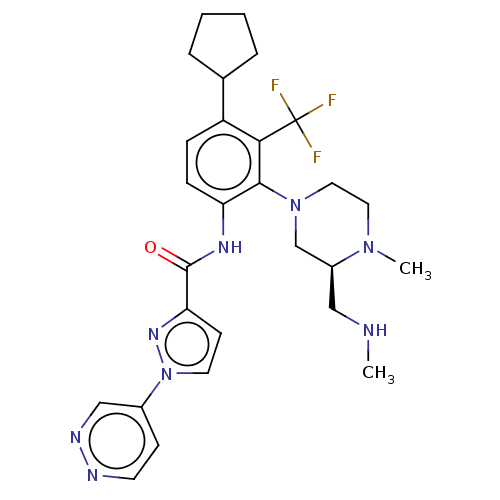
(US20240043403, Example 124 | US20240043403, Exampl...)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(C2CCCC2)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649810
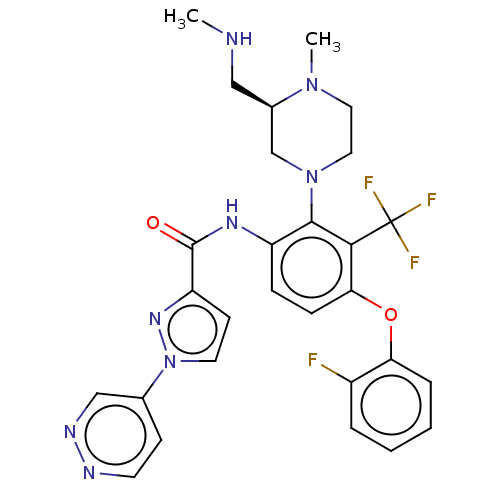
(US20240043403, Example 123 | US20240043403, Exampl...)Show SMILES CNC[C@@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2F)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Diacylglycerol kinase delta
(Human) | BDBM649850
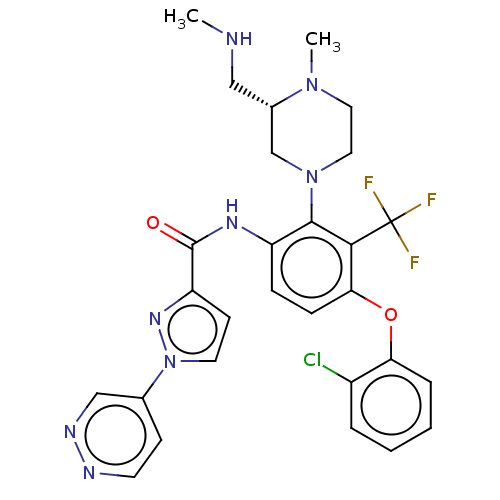
(US20240043403, Example 87)Show SMILES CNC[C@H]1CN(CCN1C)c1c(NC(=O)c2ccn(n2)-c2ccnnc2)ccc(Oc2ccccc2Cl)c1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data