

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106898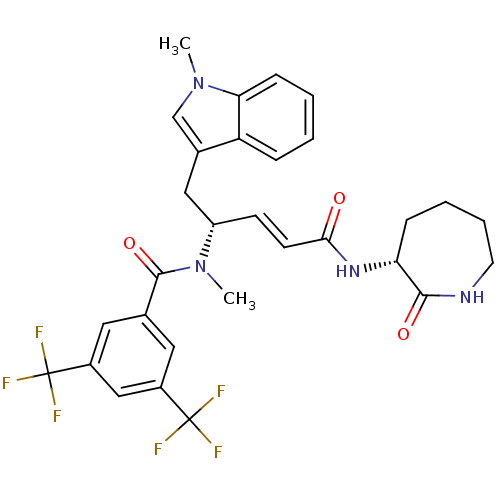 (CHEMBL317632 | N-Methyl-N-[(E)-(R)-1-(1-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106901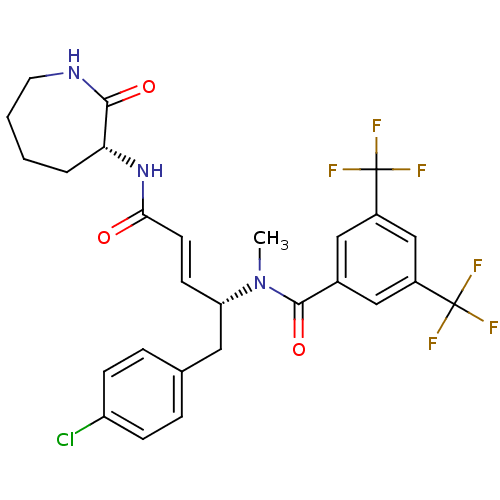 (CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106899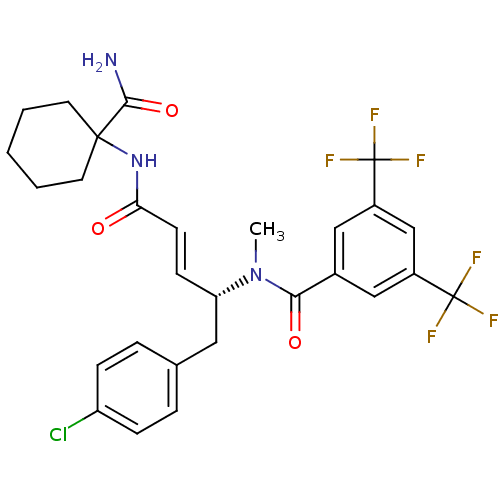 (CHEMBL105600 | N-[(E)-(R)-3-(1-Carbamoyl-cyclohexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106900 (CHEMBL103979 | N-Methyl-N-[(E)-(R)-1-naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106891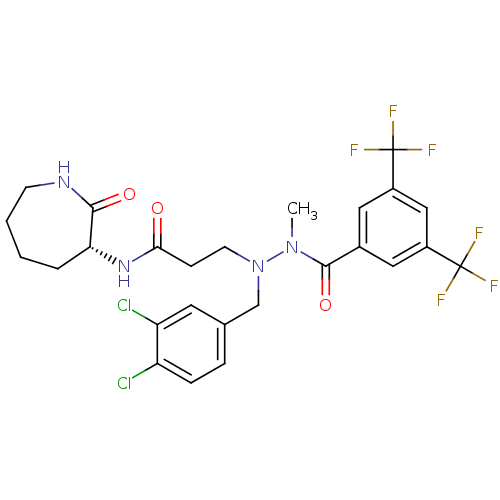 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(3,4-dic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106896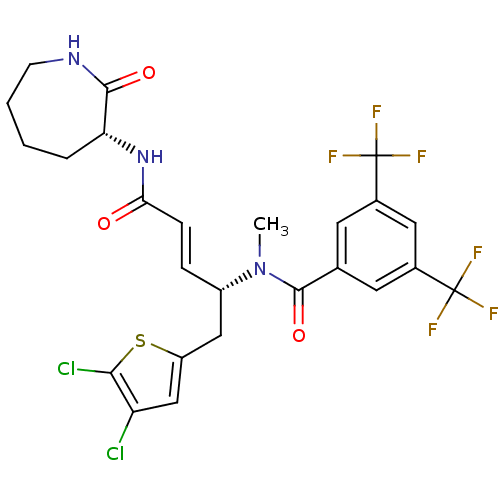 (CHEMBL104708 | N-[(E)-(R)-1-(4,5-Dichloro-thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106892 (3-[4-(3,4-dichlorophenyl)-3-[3,5-di(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106892 (3-[4-(3,4-dichlorophenyl)-3-[3,5-di(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106891 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(3,4-dic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106895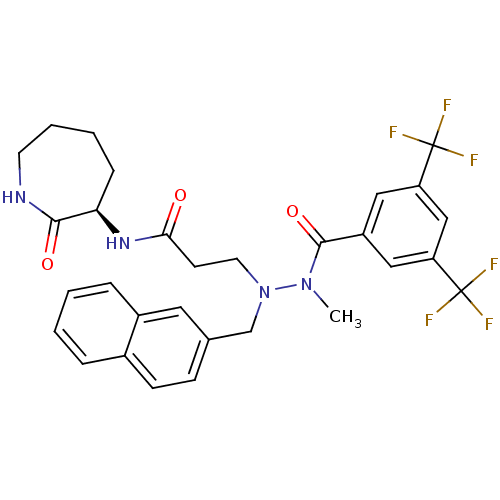 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N'-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106897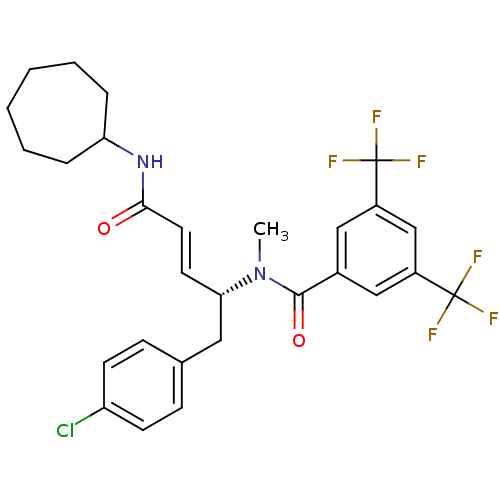 (CHEMBL105009 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218116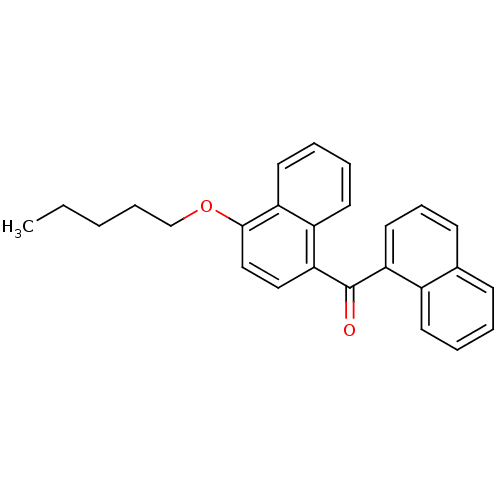 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106896 (CHEMBL104708 | N-[(E)-(R)-1-(4,5-Dichloro-thiophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106893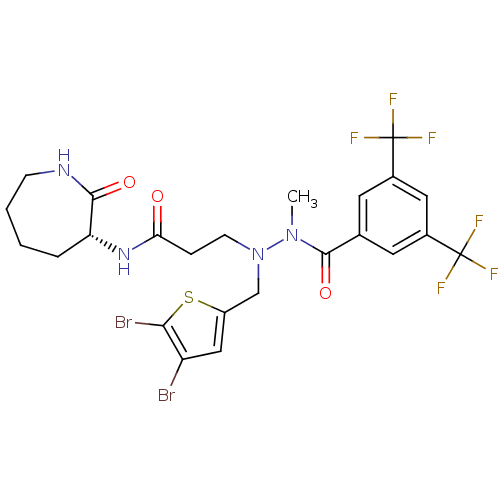 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(4,5-dib...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50106894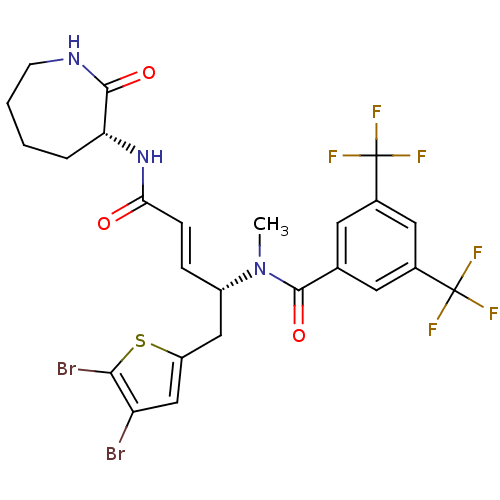 (CHEMBL102376 | N-[(E)-(R)-1-(4,5-Dibromo-thiophen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 1 of bovine retinal membranes using [3H]-Sar9-substance P as radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106893 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N-(4,5-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat CB1 receptor | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106898 (CHEMBL317632 | N-Methyl-N-[(E)-(R)-1-(1-methyl-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106901 (CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218121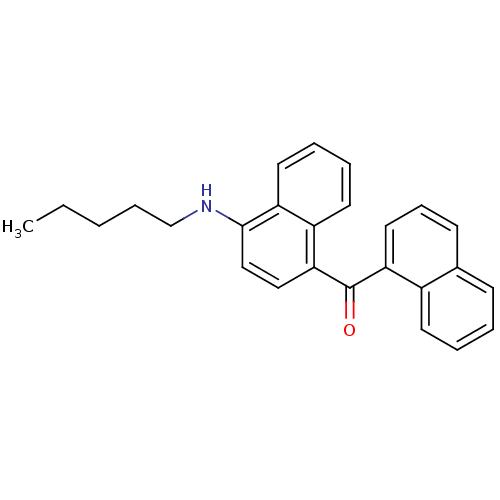 (CHEMBL390675 | naphthalen-1-yl-(4-pentylaminonapht...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106894 (CHEMBL102376 | N-[(E)-(R)-1-(4,5-Dibromo-thiophen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218123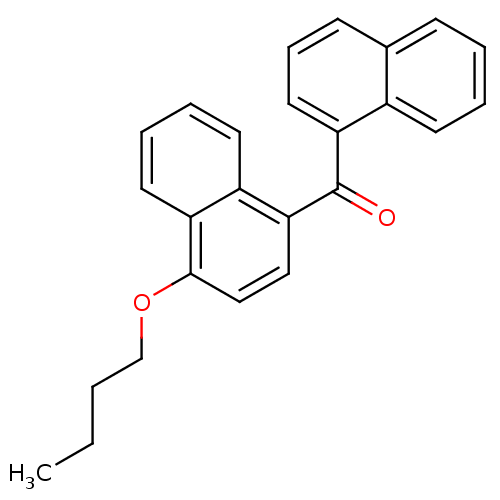 (CHEMBL244402 | naphthalen-1-yl-(4-butoxynaphthalen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106895 (3-[N'-(3,5-Bis-trifluoromethyl-benzoyl)-N'-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106900 (CHEMBL103979 | N-Methyl-N-[(E)-(R)-1-naphthalen-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106899 (CHEMBL105600 | N-[(E)-(R)-3-(1-Carbamoyl-cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218123 (CHEMBL244402 | naphthalen-1-yl-(4-butoxynaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218120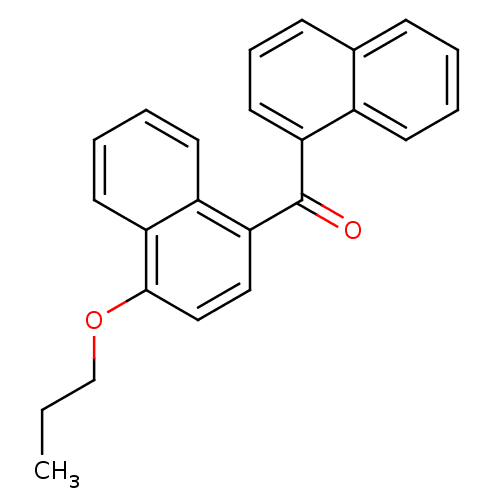 (CHEMBL243334 | naphthalen-1-yl-(4-propoxynaphthale...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218121 (CHEMBL390675 | naphthalen-1-yl-(4-pentylaminonapht...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218119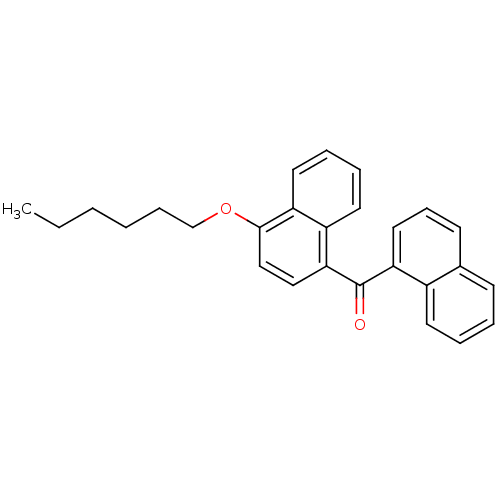 ((4-hexyloxynaphthalen-1-yl)naphthalen-1-ylmethanon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50106897 (CHEMBL105009 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity for Tachykinin receptor 2 of human-CHO cells using 125 I-NKA radioligand | Bioorg Med Chem Lett 11: 3081-4 (2001) BindingDB Entry DOI: 10.7270/Q2V40THD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218120 (CHEMBL243334 | naphthalen-1-yl-(4-propoxynaphthale...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218119 ((4-hexyloxynaphthalen-1-yl)naphthalen-1-ylmethanon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218122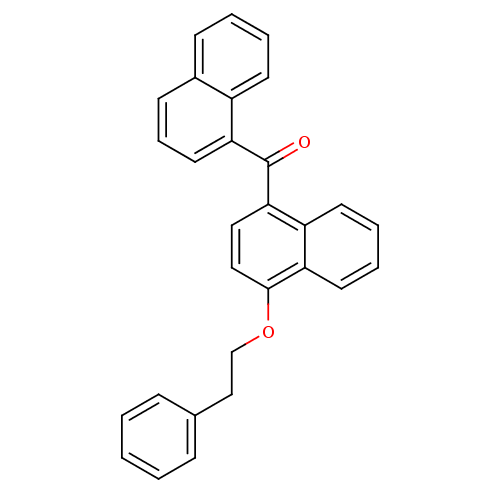 (CHEMBL244607 | naphthalen-1-yl-(4-phenethyloxynaph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218118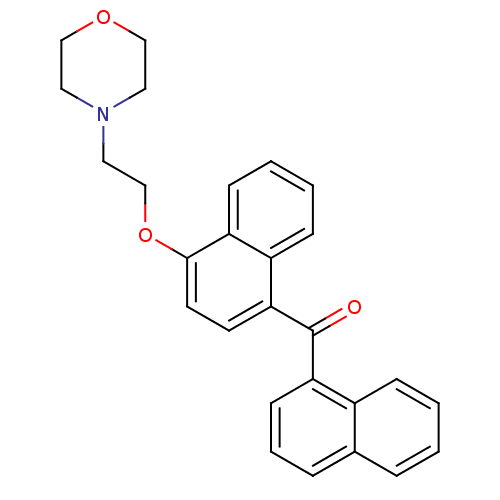 (CHEMBL244189 | [4-(2-morpholin-4-ylethoxy)naphthal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218122 (CHEMBL244607 | naphthalen-1-yl-(4-phenethyloxynaph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50218118 (CHEMBL244189 | [4-(2-morpholin-4-ylethoxy)naphthal...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as reversal of forskolin-evoked cAMP accumulation | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50218116 (CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as reversal of forskolin-evoked cAMP accumulation | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |