

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145681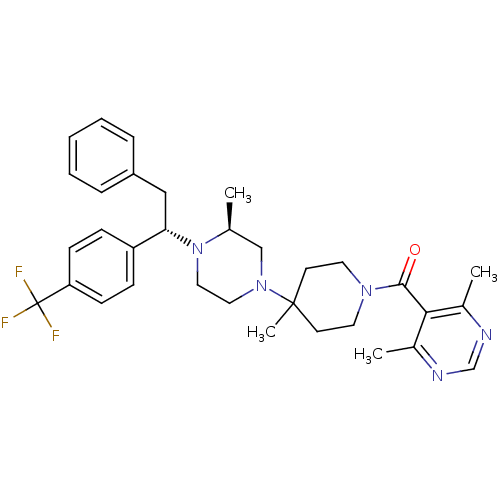 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685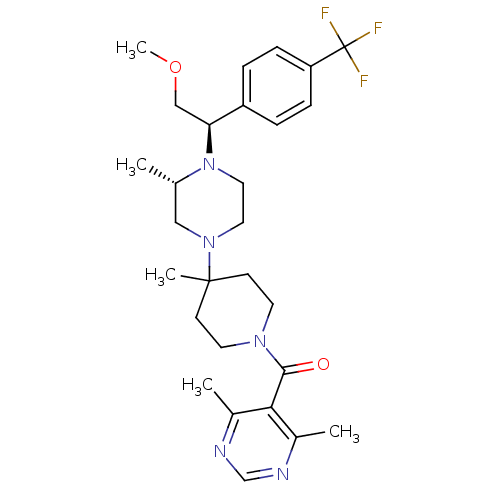 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145682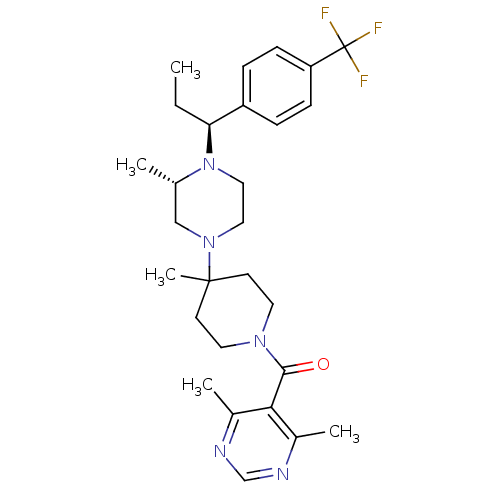 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 716 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145683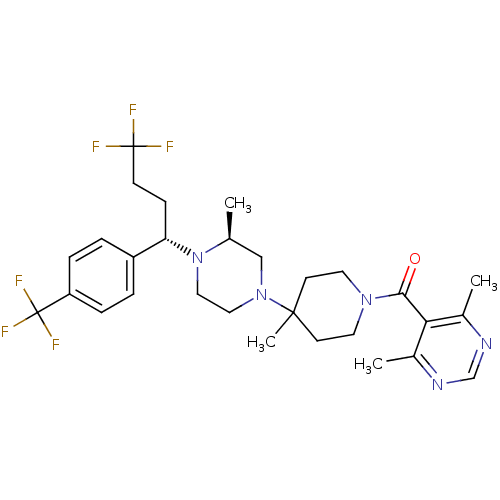 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145683 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM519420 ((4S,5R)-5-[3-chloro-5- (trifluoromethyl)phenyl]- 4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ST7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (Human) | BDBM518926 (N-cyclohexyl-N-ethyl-3-(2-(trans-4-ethylcyclohexyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The TarO biochemical enzymatic assay is a liquid chromatography-mass spectroscopy (LC-MS) based end point assay that measures C55-P-P-GlcNAc (LIPID I... | Citation and Details BindingDB Entry DOI: 10.7270/Q2XK8JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442673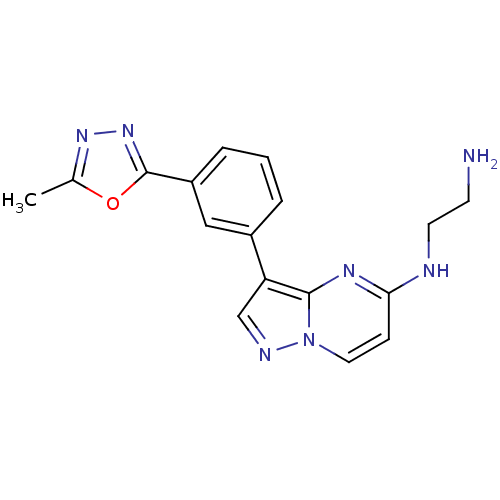 (CHEMBL2442291) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442690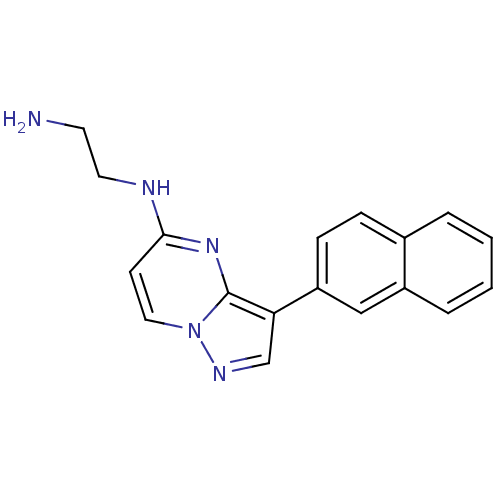 (CHEMBL2442296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442685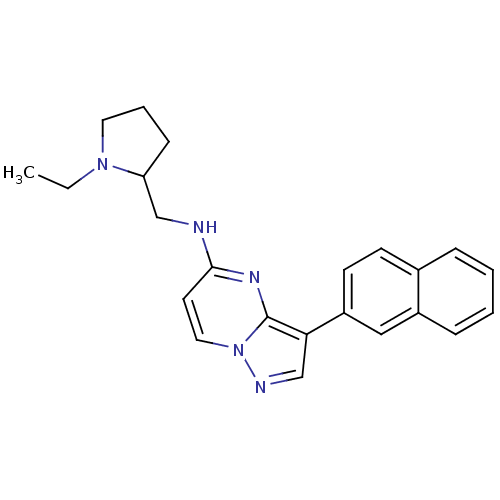 (CHEMBL2442301) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50442673 (CHEMBL2442291) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) using STK1 as substrate preincubated for 30 mins followed by substrate and ATP addition after 60 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442679 (CHEMBL2442302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585035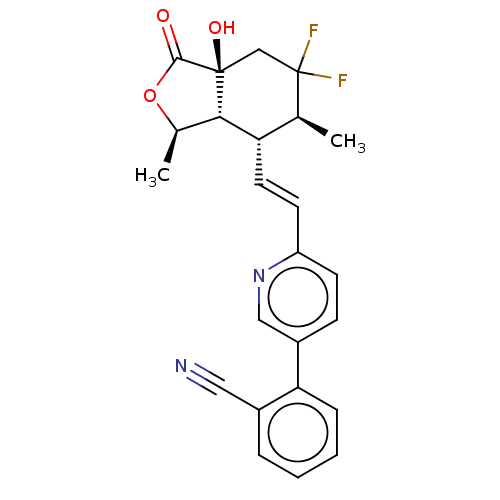 (CHEMBL5084411) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442692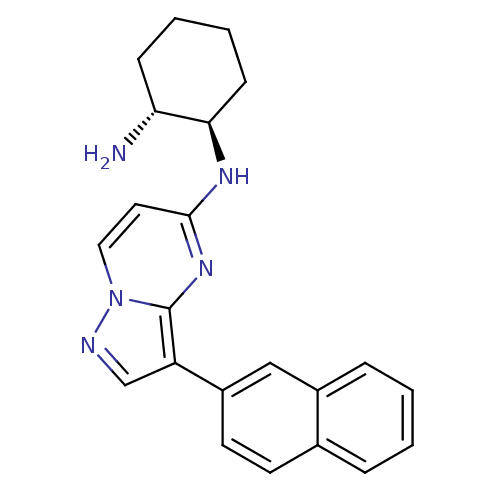 (CHEMBL2442317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585030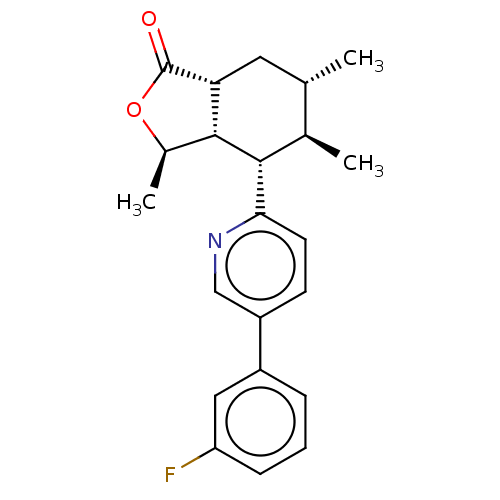 (CHEMBL5091232) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334854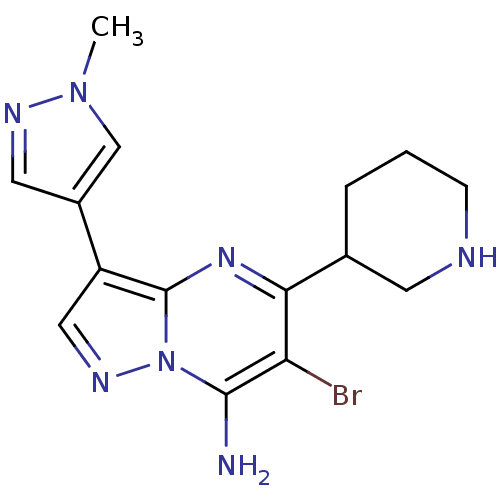 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334855 (6-iodo-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334853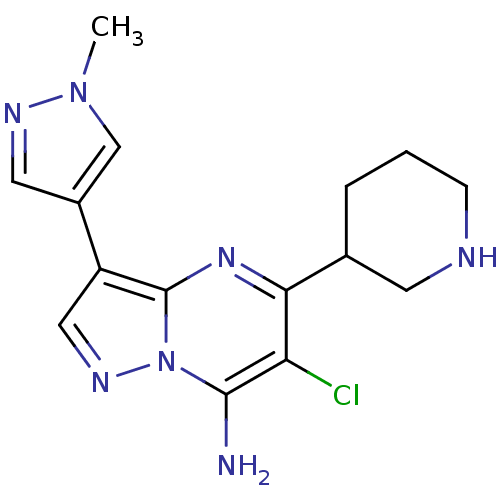 (6-chloro-3-(1-methyl-1H-pyrazol-4-yl)-5-(piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50223567 (3-bromo-5-(2-chlorophenyl)-N-(pyridin-3-ylmethyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585037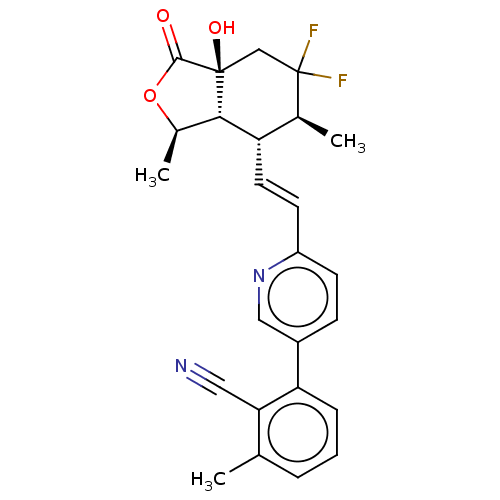 (CHEMBL5085562) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442693 (CHEMBL2442316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585042 (CHEMBL5069528) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442681 (CHEMBL2442290) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442686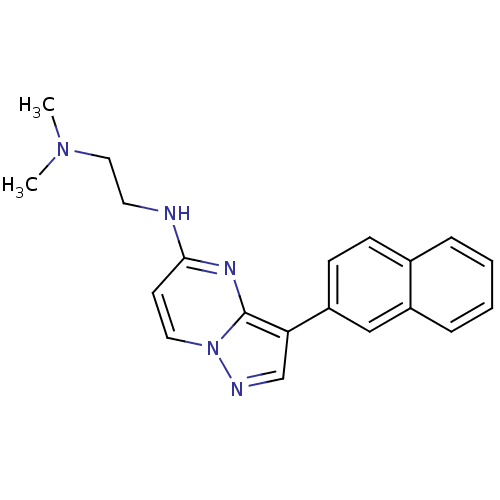 (CHEMBL2442300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585031 (CHEMBL5077302) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334849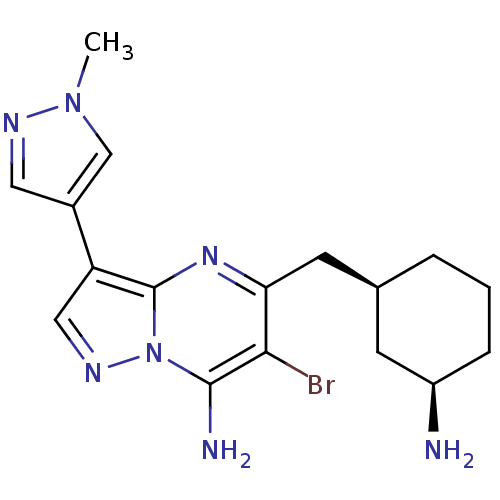 (CHEMBL1643236 | Syn-5-((3-aminocyclohexyl)methyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442687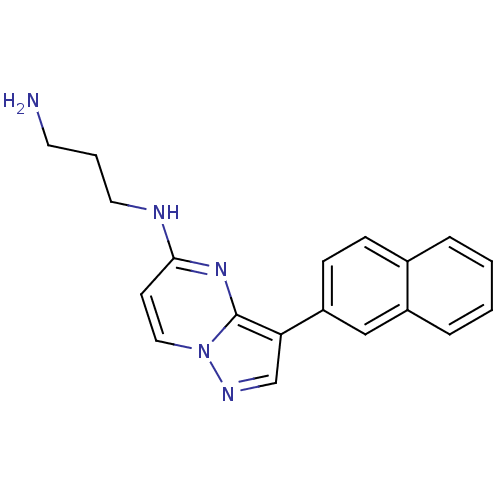 (CHEMBL2442299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50585039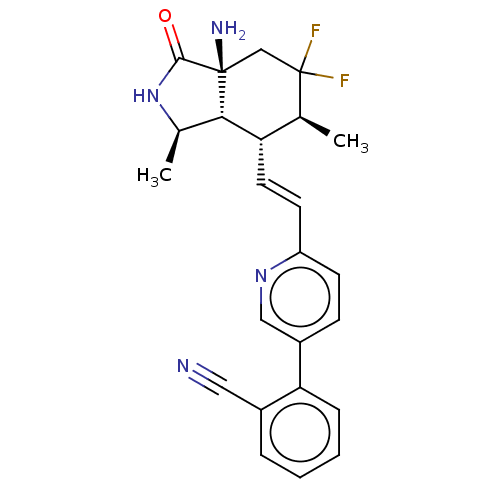 (CHEMBL5081773) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02048 BindingDB Entry DOI: 10.7270/Q2514342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM105239 (US8580782, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description CDK2 kinase assays (either cyclin A or cyclin E-dependent) were performed in low protein binding 96-well plates (Corning Inc., Corning, N.Y.). | US Patent US8580782 (2013) BindingDB Entry DOI: 10.7270/Q2VM49WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50334876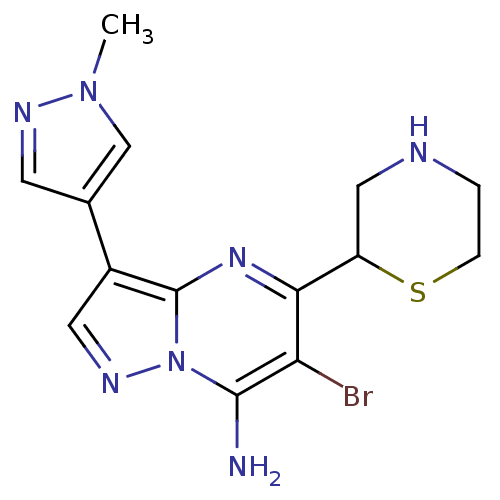 (6-bromo-3-(1-methyl-1H-pyrazol-4-yl)-5-(thiomorpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of histidine-tagged recombinant CHK1 expressed in baculovirus expression system after 2 hrs by scintillation proximity assay | Bioorg Med Chem Lett 21: 471-4 (2010) Article DOI: 10.1016/j.bmcl.2010.10.114 BindingDB Entry DOI: 10.7270/Q2P26ZC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50442691 (CHEMBL2442295) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Pim1 (unknown origin) using STK3 as substrate preincubated for 30 mins followed by substrate and ATP addition after 45 mins by HTRF ass... | Bioorg Med Chem Lett 23: 6178-82 (2013) Article DOI: 10.1016/j.bmcl.2013.08.110 BindingDB Entry DOI: 10.7270/Q2MS3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 420 total ) | Next | Last >> |