 Found 539 hits with Last Name = 'lafrance' and Initial = 'l'
Found 539 hits with Last Name = 'lafrance' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607602

(CHEMBL5220994)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CC(F)(F)C1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607596

(CHEMBL5221053)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607597
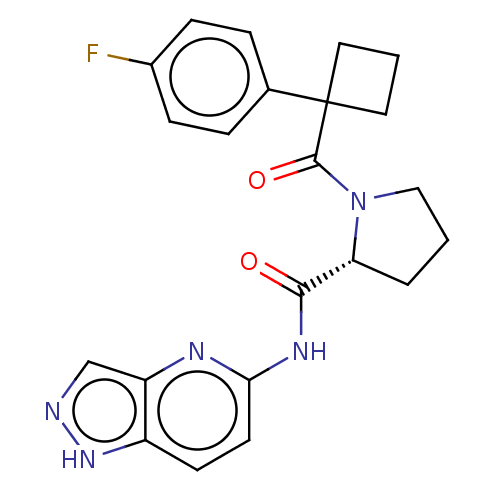
(CHEMBL5219157)Show SMILES Fc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607593
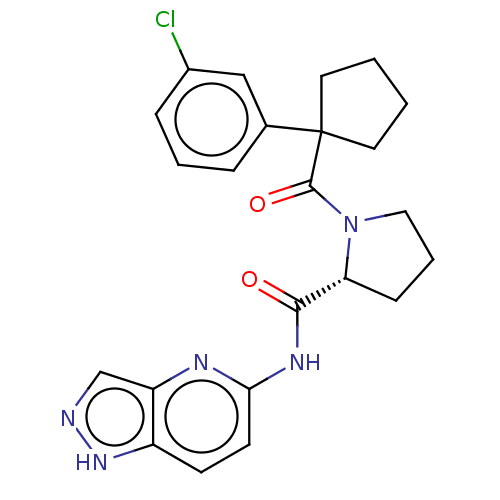
(CHEMBL5219693)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607595

(CHEMBL5219030)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607601
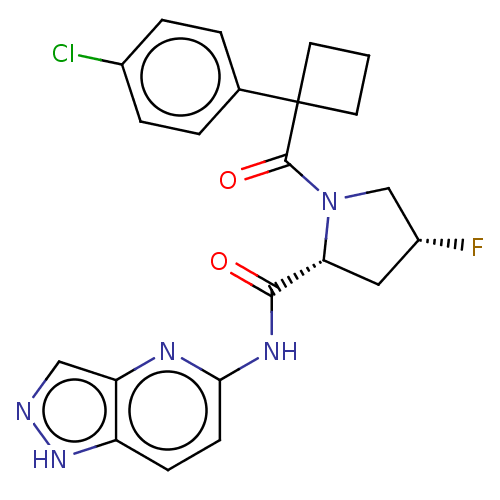
(CHEMBL5219667)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CCC1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607598
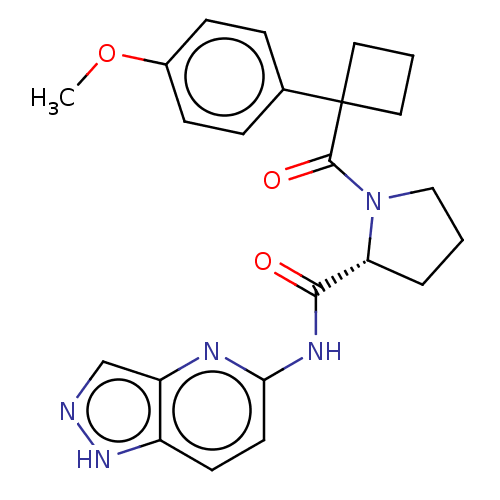
(CHEMBL5220447)Show SMILES COc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607599
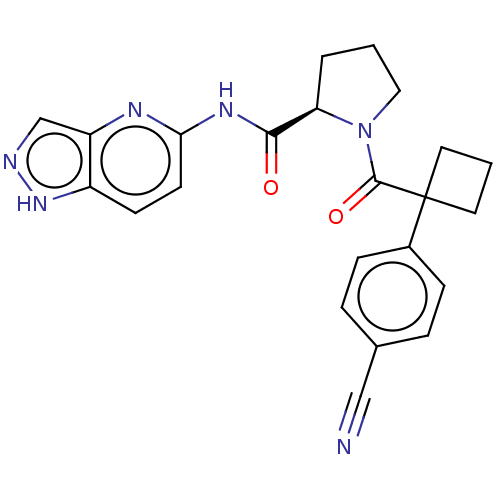
(CHEMBL5220732)Show SMILES O=C(Nc1ccc2[nH]ncc2n1)[C@H]1CCCN1C(=O)C1(CCC1)c1ccc(cc1)C#N |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607589
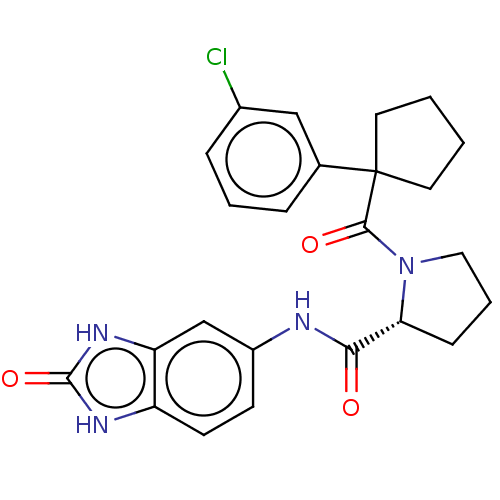
(CHEMBL5219678)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]c(=O)[nH]c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607585
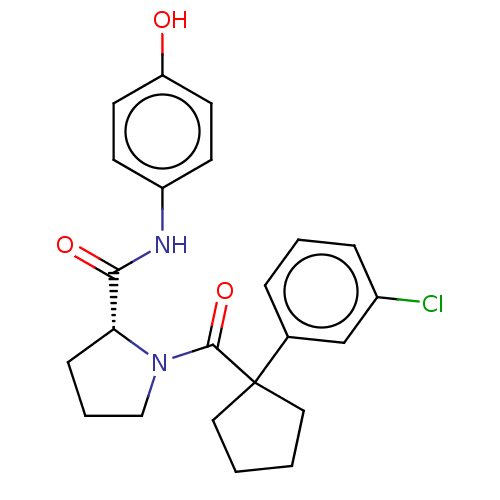
(CHEMBL5219512)Show SMILES Oc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607588
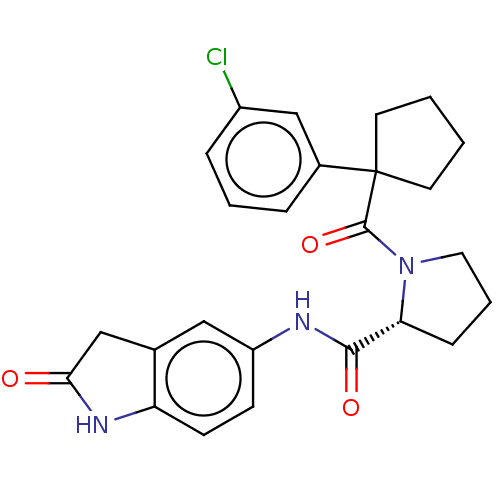
(CHEMBL5220546)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2NC(=O)Cc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607586
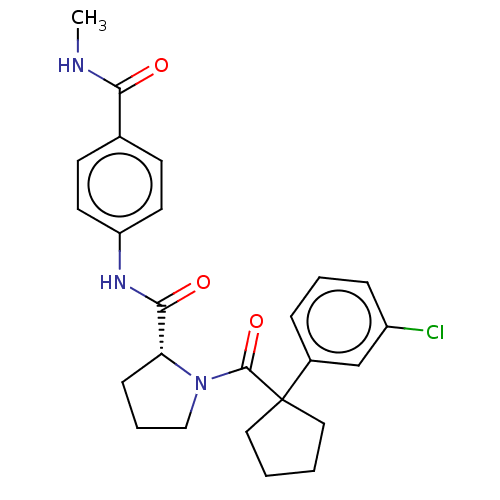
(CHEMBL5221030)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607590
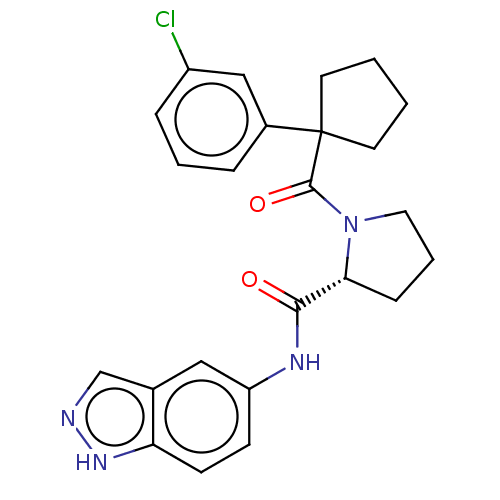
(CHEMBL5220332)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607605
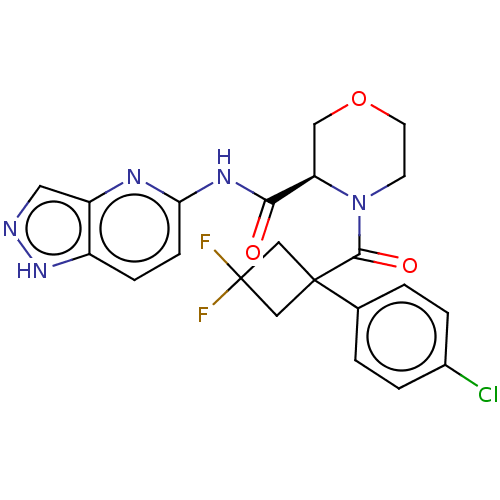
(CHEMBL5219177)Show SMILES FC1(F)CC(C1)(C(=O)N1CCOC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306154

((2S)-1-(1H-indol-3-yl)-3-(5-(3-methyl-1H-pyrazolo[...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25N7O2/c1-15-25-27(34-33-15)31-13-24(32-25)22-10-19(12-30-26(22)20-7-8-35-16(20)2)36-14-18(28)9-17-11-29-23-6-4-3-5-21(17)23/h3-8,10-13,18,29H,9,14,28H2,1-2H3,(H,31,33,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306155
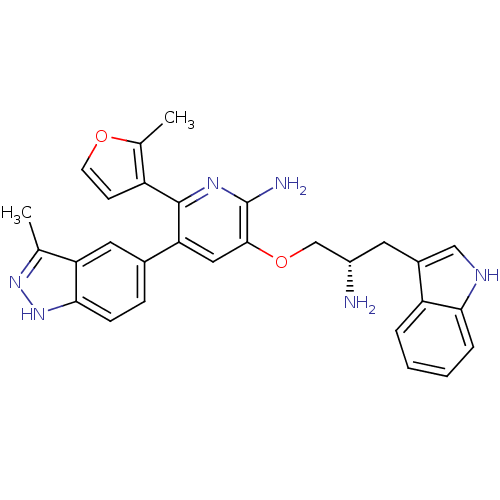
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C29H28N6O2/c1-16-23-12-18(7-8-26(23)35-34-16)24-13-27(29(31)33-28(24)21-9-10-36-17(21)2)37-15-20(30)11-19-14-32-25-6-4-3-5-22(19)25/h3-10,12-14,20,32H,11,15,30H2,1-2H3,(H2,31,33)(H,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306156
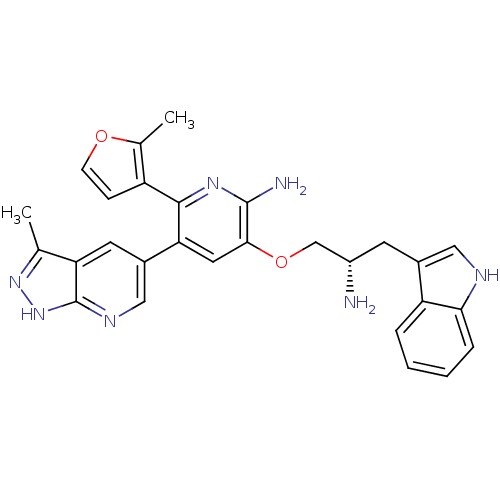
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-22-10-18(13-32-28(22)35-34-15)23-11-25(27(30)33-26(23)20-7-8-36-16(20)2)37-14-19(29)9-17-12-31-24-6-4-3-5-21(17)24/h3-8,10-13,19,31H,9,14,29H2,1-2H3,(H2,30,33)(H,32,34,35)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306157
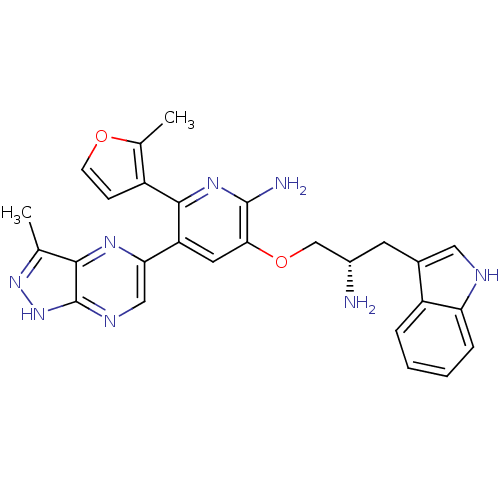
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H26N8O2/c1-14-24-27(35-34-14)31-12-22(32-24)20-10-23(26(29)33-25(20)18-7-8-36-15(18)2)37-13-17(28)9-16-11-30-21-6-4-3-5-19(16)21/h3-8,10-12,17,30H,9,13,28H2,1-2H3,(H2,29,33)(H,31,34,35)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25004
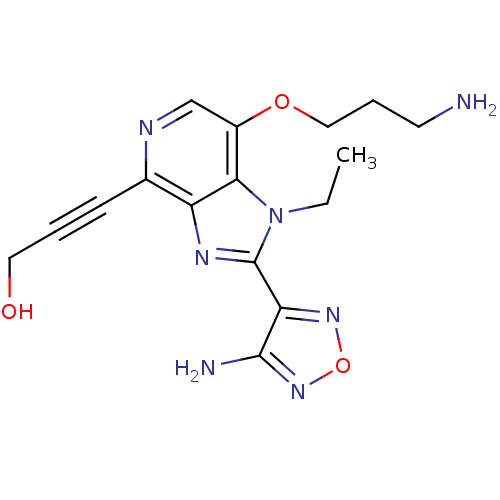
(3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...)Show InChI InChI=1S/C16H19N7O3/c1-2-23-14-11(25-8-4-6-17)9-19-10(5-3-7-24)12(14)20-16(23)13-15(18)22-26-21-13/h9,24H,2,4,6-8,17H2,1H3,(H2,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306163
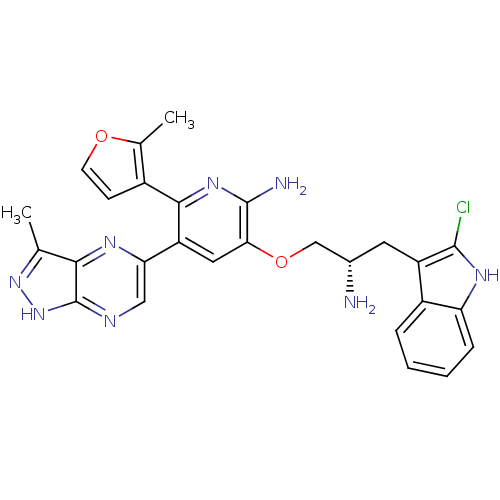
(3-((S)-2-amino-3-(2-chloro-1H-indol-3-yl)propoxy)-...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c(Cl)[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25ClN8O2/c1-13-23-27(36-35-13)31-11-21(32-23)19-10-22(26(30)34-24(19)16-7-8-37-14(16)2)38-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)28/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,34)(H,31,35,36)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306164
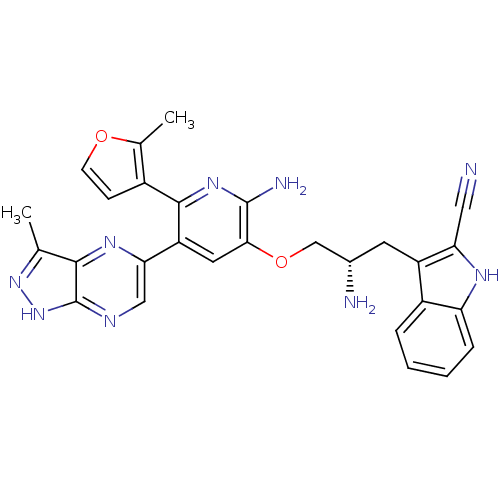
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C#N)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H25N9O2/c1-14-25-28(37-36-14)32-12-23(34-25)20-10-24(27(31)35-26(20)17-7-8-38-15(17)2)39-13-16(30)9-19-18-5-3-4-6-21(18)33-22(19)11-29/h3-8,10,12,16,33H,9,13,30H2,1-2H3,(H2,31,35)(H,32,36,37)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306164

(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C#N)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H25N9O2/c1-14-25-28(37-36-14)32-12-23(34-25)20-10-24(27(31)35-26(20)17-7-8-38-15(17)2)39-13-16(30)9-19-18-5-3-4-6-21(18)33-22(19)11-29/h3-8,10,12,16,33H,9,13,30H2,1-2H3,(H2,31,35)(H,32,36,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306165
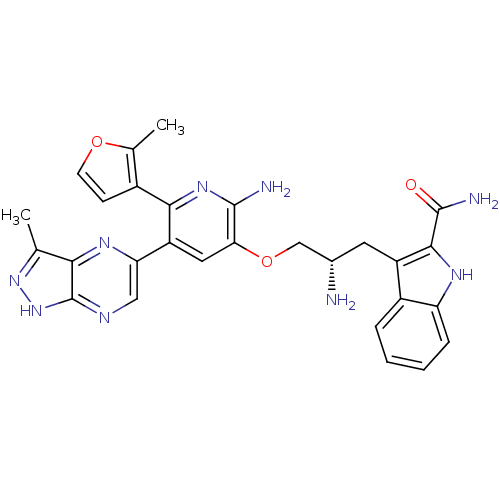
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C(N)=O)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N9O3/c1-13-23-28(37-36-13)32-11-21(34-23)19-10-22(26(30)35-24(19)16-7-8-39-14(16)2)40-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)27(31)38/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,36,37)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306165

(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C(N)=O)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N9O3/c1-13-23-28(37-36-13)32-11-21(34-23)19-10-22(26(30)35-24(19)16-7-8-39-14(16)2)40-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)27(31)38/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,36,37)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24994
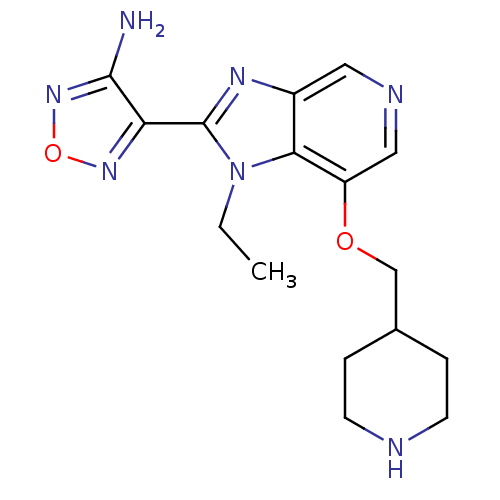
(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306158
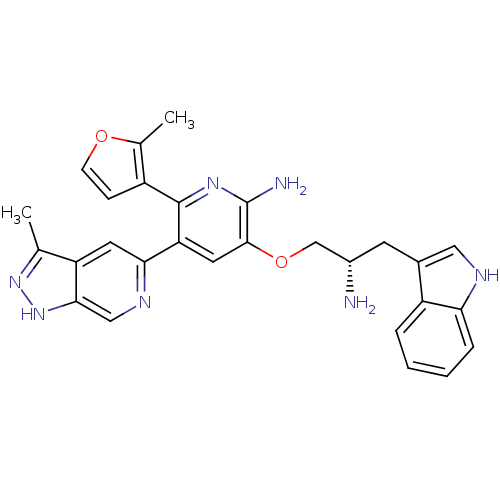
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2cnc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-21-10-24(32-13-25(21)35-34-15)22-11-26(28(30)33-27(22)19-7-8-36-16(19)2)37-14-18(29)9-17-12-31-23-6-4-3-5-20(17)23/h3-8,10-13,18,31H,9,14,29H2,1-2H3,(H2,30,33)(H,34,35)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607592
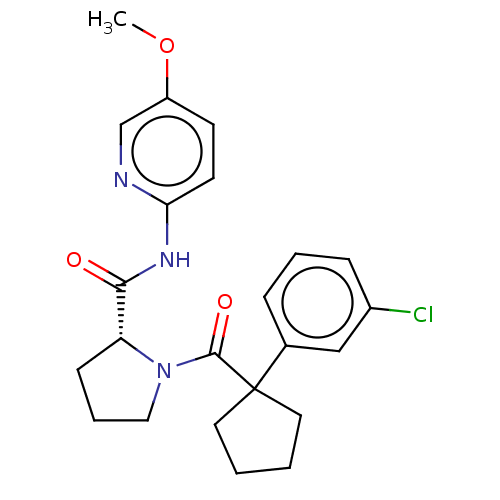
(CHEMBL5219466)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)nc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306157

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H26N8O2/c1-14-24-27(35-34-14)31-12-22(32-24)20-10-23(26(29)33-25(20)18-7-8-36-15(18)2)37-13-17(28)9-16-11-30-21-6-4-3-5-19(16)21/h3-8,10-12,17,30H,9,13,28H2,1-2H3,(H2,29,33)(H,31,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306156

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-22-10-18(13-32-28(22)35-34-15)23-11-25(27(30)33-26(23)20-7-8-36-16(20)2)37-14-19(29)9-17-12-31-24-6-4-3-5-21(17)24/h3-8,10-13,19,31H,9,14,29H2,1-2H3,(H2,30,33)(H,32,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
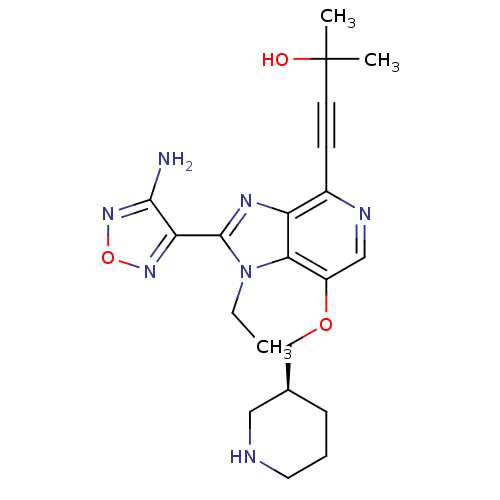
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306163

(3-((S)-2-amino-3-(2-chloro-1H-indol-3-yl)propoxy)-...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c(Cl)[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25ClN8O2/c1-13-23-27(36-35-13)31-11-21(32-23)19-10-22(26(30)34-24(19)16-7-8-37-14(16)2)38-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)28/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,34)(H,31,35,36)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25004

(3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...)Show InChI InChI=1S/C16H19N7O3/c1-2-23-14-11(25-8-4-6-17)9-19-10(5-3-7-24)12(14)20-16(23)13-15(18)22-26-21-13/h9,24H,2,4,6-8,17H2,1H3,(H2,18,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25009
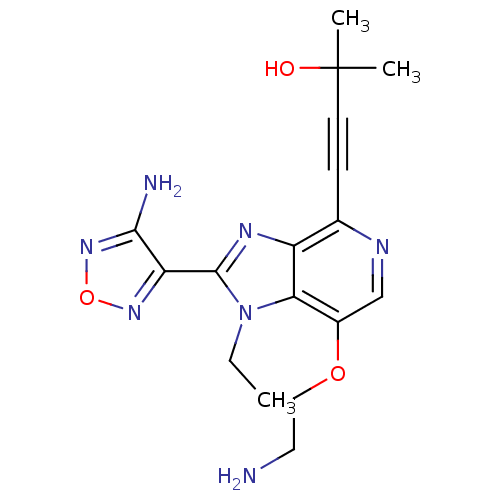
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(2-aminoetho...)Show SMILES CCn1c(nc2c(ncc(OCCN)c12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-14-11(26-8-7-18)9-20-10(5-6-17(2,3)25)12(14)21-16(24)13-15(19)23-27-22-13/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50161784
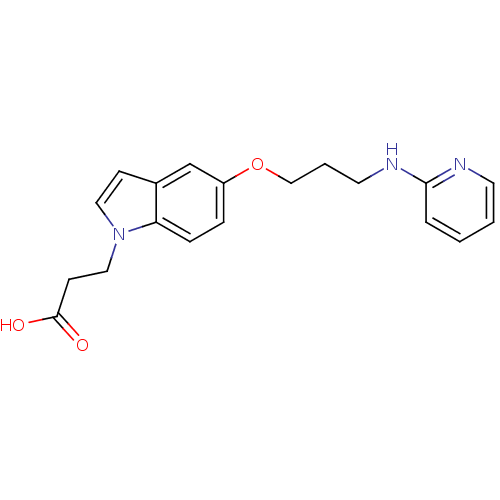
(3-{5-[3-(Pyridin-2-ylamino)-propoxy]-indol-1-yl}-p...)Show InChI InChI=1S/C19H21N3O3/c23-19(24)8-12-22-11-7-15-14-16(5-6-17(15)22)25-13-3-10-21-18-4-1-2-9-20-18/h1-2,4-7,9,11,14H,3,8,10,12-13H2,(H,20,21)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of human alphaV-beta5 integrin binding in ELISA |
J Med Chem 48: 926-34 (2005)
Article DOI: 10.1021/jm049725u
BindingDB Entry DOI: 10.7270/Q2W37VTG |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306155

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C29H28N6O2/c1-16-23-12-18(7-8-26(23)35-34-16)24-13-27(29(31)33-28(24)21-9-10-36-17(21)2)37-15-20(30)11-19-14-32-25-6-4-3-5-22(19)25/h3-10,12-14,20,32H,11,15,30H2,1-2H3,(H2,31,33)(H,34,35)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306158

(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2cnc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-21-10-24(32-13-25(21)35-34-15)22-11-26(28(30)33-27(22)19-7-8-36-16(19)2)37-14-18(29)9-17-12-31-23-6-4-3-5-20(17)23/h3-8,10-13,18,31H,9,14,29H2,1-2H3,(H2,30,33)(H,34,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306160
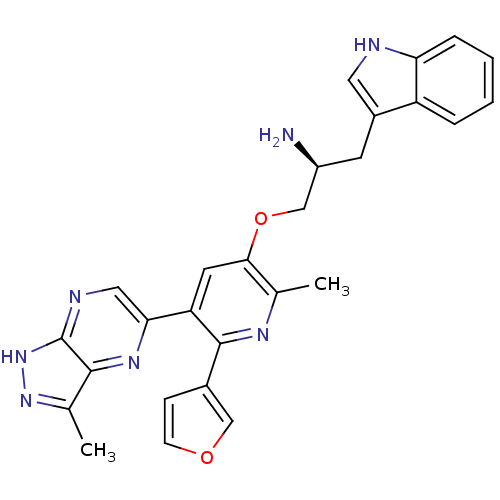
((S)-1-(6-(furan-3-yl)-2-methyl-5-(3-methyl-1H-pyra...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(C)nc1-c1ccoc1 |r| Show InChI InChI=1S/C27H25N7O2/c1-15-24(36-14-19(28)9-18-11-29-22-6-4-3-5-20(18)22)10-21(26(31-15)17-7-8-35-13-17)23-12-30-27-25(32-23)16(2)33-34-27/h3-8,10-13,19,29H,9,14,28H2,1-2H3,(H,30,33,34)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT1 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM24994

(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25014
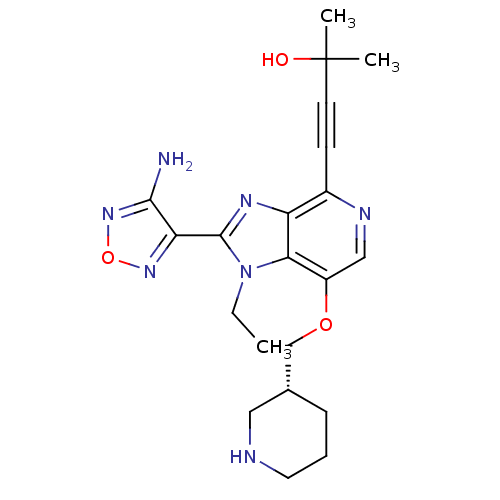
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...)Show SMILES CCn1c(nc2c(ncc(OC[C@@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25016
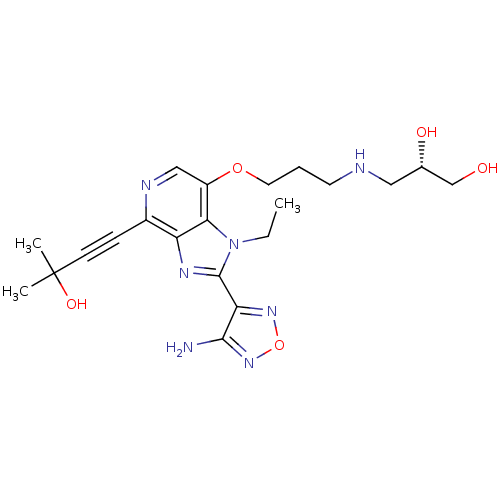
((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...)Show SMILES CCn1c(nc2c(ncc(OCCCNC[C@H](O)CO)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H29N7O5/c1-4-28-18-15(32-9-5-8-23-10-13(30)12-29)11-24-14(6-7-21(2,3)31)16(18)25-20(28)17-19(22)27-33-26-17/h11,13,23,29-31H,4-5,8-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24991
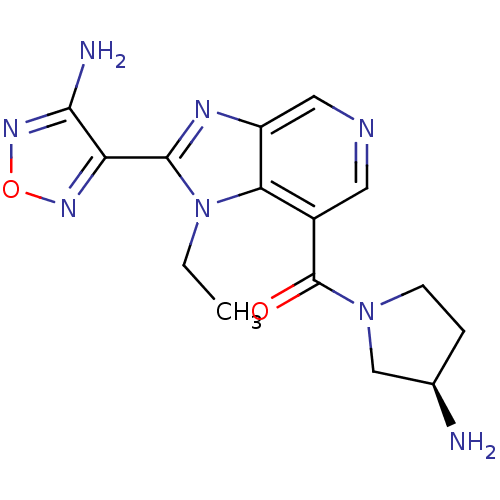
(4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@@H](N)C3)c12)-c1nonc1N |r| Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25010

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...)Show SMILES CCn1c(nc2c(ncc(OCC[C@@H](N)Cc3ccccc3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C25H29N7O3/c1-4-32-22-19(34-13-11-17(26)14-16-8-6-5-7-9-16)15-28-18(10-12-25(2,3)33)20(22)29-24(32)21-23(27)31-35-30-21/h5-9,15,17,33H,4,11,13-14,26H2,1-3H3,(H2,27,31)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25010

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...)Show SMILES CCn1c(nc2c(ncc(OCC[C@@H](N)Cc3ccccc3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C25H29N7O3/c1-4-32-22-19(34-13-11-17(26)14-16-8-6-5-7-9-16)15-28-18(10-12-25(2,3)33)20(22)29-24(32)21-23(27)31-35-30-21/h5-9,15,17,33H,4,11,13-14,26H2,1-3H3,(H2,27,31)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607584
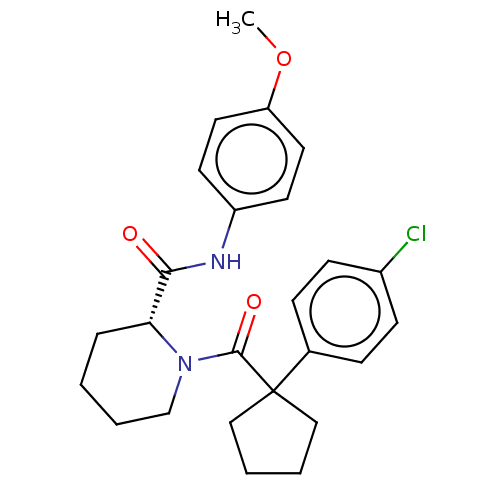
(CHEMBL5221088)Show SMILES COc1ccc(NC(=O)[C@H]2CCCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25015
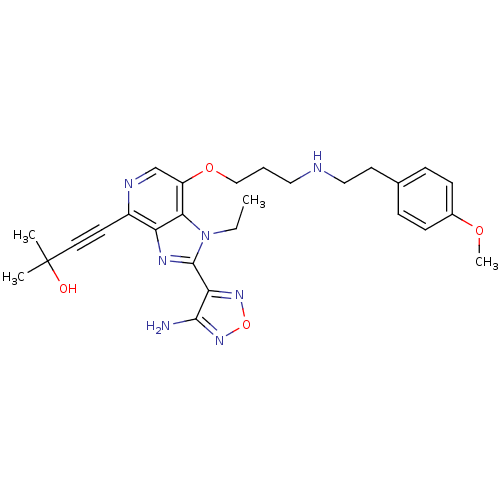
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(3-{...)Show SMILES CCn1c(nc2c(ncc(OCCCNCCc3ccc(OC)cc3)c12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C27H33N7O4/c1-5-34-24-21(37-16-6-14-29-15-12-18-7-9-19(36-4)10-8-18)17-30-20(11-13-27(2,3)35)22(24)31-26(34)23-25(28)33-38-32-23/h7-10,17,29,35H,5-6,12,14-16H2,1-4H3,(H2,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM24991

(4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@@H](N)C3)c12)-c1nonc1N |r| Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM24990
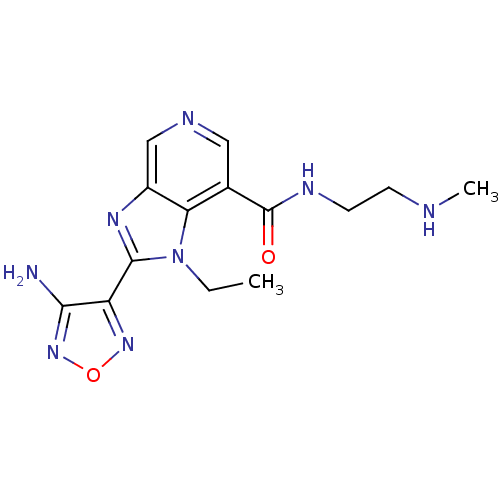
(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-[2-(met...)Show InChI InChI=1S/C14H18N8O2/c1-3-22-11-8(14(23)18-5-4-16-2)6-17-7-9(11)19-13(22)10-12(15)21-24-20-10/h6-7,16H,3-5H2,1-2H3,(H2,15,21)(H,18,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25016

((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...)Show SMILES CCn1c(nc2c(ncc(OCCCNC[C@H](O)CO)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H29N7O5/c1-4-28-18-15(32-9-5-8-23-10-13(30)12-29)11-24-14(6-7-21(2,3)31)16(18)25-20(28)17-19(22)27-33-26-17/h11,13,23,29-31H,4-5,8-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data