 Found 2800 hits with Last Name = 'lam' and Initial = 'k'
Found 2800 hits with Last Name = 'lam' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178
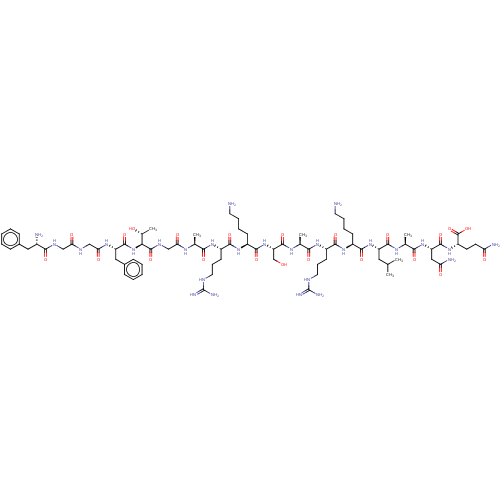
(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM50453675
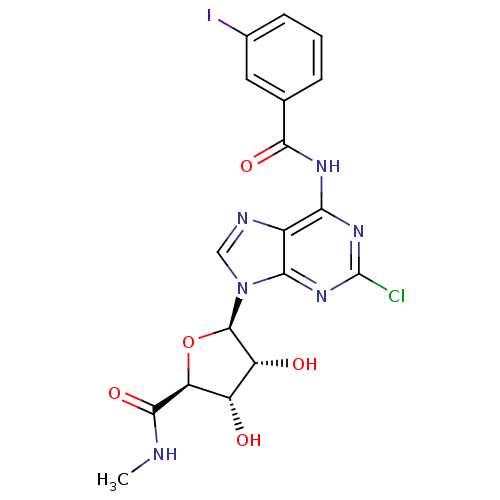
(CHEMBL2113400)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC(=O)c3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H16ClIN6O5/c1-21-16(30)12-10(27)11(28)17(31-12)26-6-22-9-13(24-18(19)25-14(9)26)23-15(29)7-3-2-4-8(20)5-7/h2-6,10-12,17,27-28H,1H3,(H,21,30)(H,23,24,25,29)/t10-,11+,12-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A3 receptor from rat brain. |
J Med Chem 38: 1720-35 (1995)
BindingDB Entry DOI: 10.7270/Q21Z453H |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50474902
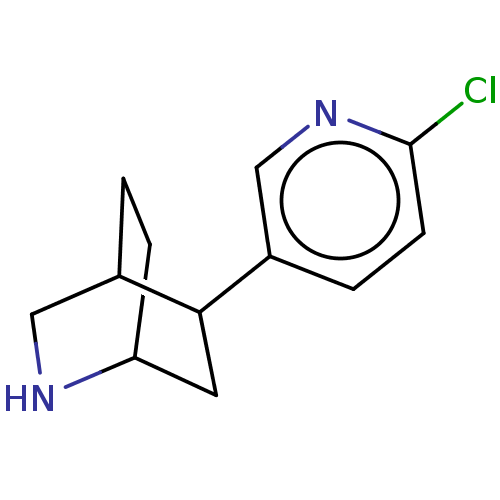
(CHEMBL187309)Show SMILES Clc1ccc(cn1)C1CC2CCC1CN2 |THB:4:7:14.13:11.10| Show InChI InChI=1S/C12H15ClN2/c13-12-4-2-9(7-15-12)11-5-10-3-1-8(11)6-14-10/h2,4,7-8,10-11,14H,1,3,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- cytisine binding to Nicotinic acetylcholine receptor alpha4-beta2 of rat cortical membranes |
Bioorg Med Chem Lett 14: 5573-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.058
BindingDB Entry DOI: 10.7270/Q2XG9TWT |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617
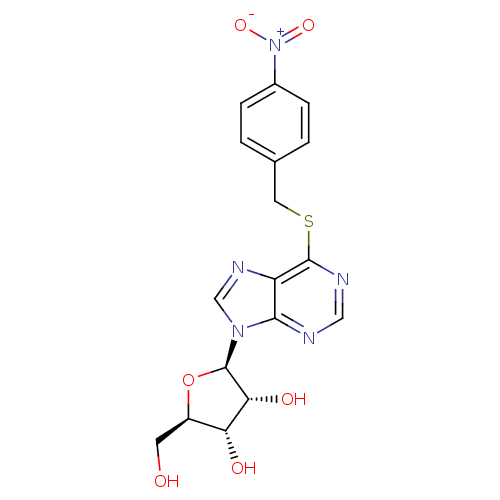
((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | -52.9 | 7.60 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50370291
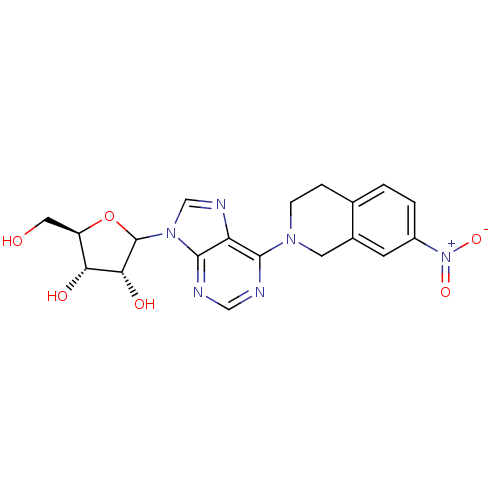
(CHEMBL608208)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(ncnc12)N1CCc2ccc(cc2C1)[N+]([O-])=O |r| Show InChI InChI=1S/C19H20N6O6/c26-7-13-15(27)16(28)19(31-13)24-9-22-14-17(20-8-21-18(14)24)23-4-3-10-1-2-12(25(29)30)5-11(10)6-23/h1-2,5,8-9,13,15-16,19,26-28H,3-4,6-7H2/t13-,15-,16-,19?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... |
J Med Chem 46: 831-7 (2003)
Article DOI: 10.1021/jm020405p
BindingDB Entry DOI: 10.7270/Q21R6R8G |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50376975

(CHEMBL260570)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(ncnc12)N1CCc2ccc(cc2C1)[N+]([O-])=O Show InChI InChI=1S/C19H20N6O6/c26-7-13-15(27)16(28)19(31-13)24-9-22-14-17(20-8-21-18(14)24)23-4-3-10-1-2-12(25(29)30)5-11(10)6-23/h1-2,5,8-9,13,15-16,19,26-28H,3-4,6-7H2/t13-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from human ENT1 transporter in human K562 cells |
Bioorg Med Chem 16: 3848-65 (2008)
Article DOI: 10.1016/j.bmc.2008.01.044
BindingDB Entry DOI: 10.7270/Q2GM8866 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23631

(2-{[4,8-bis(azocan-1-yl)-6-[bis(2-hydroxyethyl)ami...)Show SMILES OCCN(CCO)c1nc(N2CCCCCCC2)c2nc(nc(N3CCCCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C28H48N8O4/c37-19-15-35(16-20-38)27-30-24-23(25(31-27)33-11-7-3-1-4-8-12-33)29-28(36(17-21-39)18-22-40)32-26(24)34-13-9-5-2-6-10-14-34/h37-40H,1-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.490 | -52.6 | 8.67 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from human ENT1 transporter in human K562 cells |
Bioorg Med Chem 16: 3848-65 (2008)
Article DOI: 10.1016/j.bmc.2008.01.044
BindingDB Entry DOI: 10.7270/Q2GM8866 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Nucleoside transporter es-type by facile competitive binding flow cytometric assay using the human K562 chronic ... |
J Med Chem 46: 831-7 (2003)
Article DOI: 10.1021/jm020405p
BindingDB Entry DOI: 10.7270/Q21R6R8G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Binding affinity to human ENT1 assessed as [3H]uridine uptake by flow cytometry |
Bioorg Med Chem Lett 19: 917-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.112
BindingDB Entry DOI: 10.7270/Q2K07440 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23617

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophe...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-1-3-10(4-2-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50526852
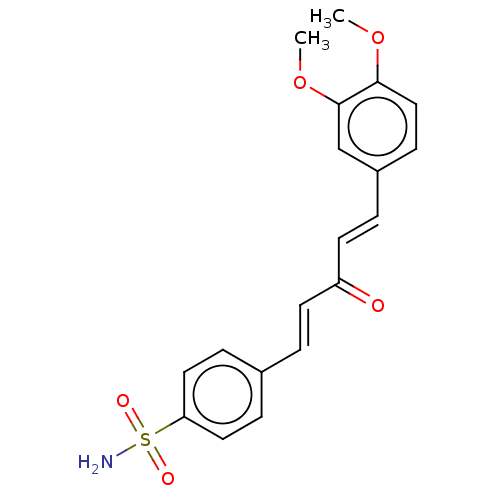
(CHEMBL4458429)Show SMILES COc1ccc(\C=C\C(=O)\C=C\c2ccc(cc2)S(N)(=O)=O)cc1OC Show InChI InChI=1S/C19H19NO5S/c1-24-18-12-7-15(13-19(18)25-2)4-9-16(21)8-3-14-5-10-17(11-6-14)26(20,22)23/h3-13H,1-2H3,(H2,20,22,23)/b8-3+,9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kuala Lumpur
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 incubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111704
BindingDB Entry DOI: 10.7270/Q2RN3C9Q |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23633
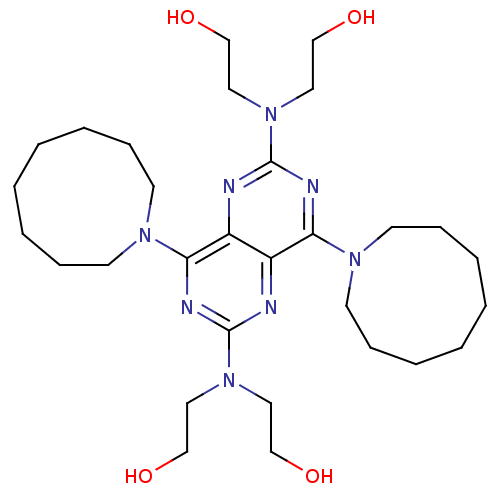
(2-{[4,8-bis(azonan-1-yl)-6-[bis(2-hydroxyethyl)ami...)Show SMILES OCCN(CCO)c1nc(N2CCCCCCCC2)c2nc(nc(N3CCCCCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C30H52N8O4/c39-21-17-37(18-22-40)29-32-26-25(27(33-29)35-13-9-5-1-2-6-10-14-35)31-30(38(19-23-41)20-24-42)34-28(26)36-15-11-7-3-4-8-12-16-36/h39-42H,1-24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | -51.5 | 13.6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23629

(2-{[4,8-bis(azepan-1-yl)-6-[bis(2-hydroxyethyl)ami...)Show SMILES OCCN(CCO)c1nc(N2CCCCCC2)c2nc(nc(N3CCCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C26H44N8O4/c35-17-13-33(14-18-36)25-28-22-21(23(29-25)31-9-5-1-2-6-10-31)27-26(34(15-19-37)16-20-38)30-24(22)32-11-7-3-4-8-12-32/h35-38H,1-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | -51.2 | 15.2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50526850
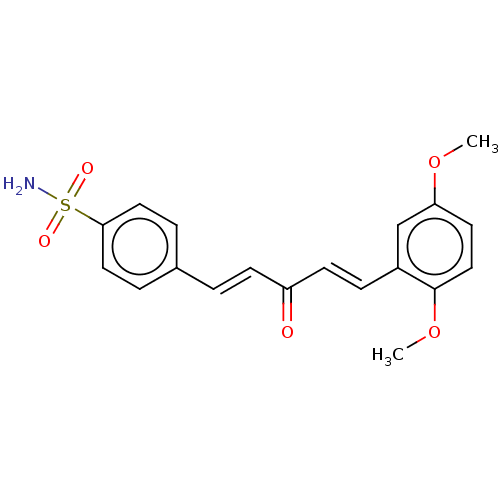
(CHEMBL4588478)Show SMILES COc1ccc(OC)c(\C=C\C(=O)\C=C\c2ccc(cc2)S(N)(=O)=O)c1 Show InChI InChI=1S/C19H19NO5S/c1-24-17-9-12-19(25-2)15(13-17)6-8-16(21)7-3-14-4-10-18(11-5-14)26(20,22)23/h3-13H,1-2H3,(H2,20,22,23)/b7-3+,8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kuala Lumpur
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 incubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111704
BindingDB Entry DOI: 10.7270/Q2RN3C9Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A3 receptor from rat brain. |
J Med Chem 38: 1720-35 (1995)
BindingDB Entry DOI: 10.7270/Q21Z453H |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50222744

((2R,3R,4S,5R)-2-(6-(4-nitrobenzylthio)-2-amino-9H-...)Show SMILES Nc1nc(SCc2ccc(cc2)[N+]([O-])=O)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C17H18N6O6S/c18-17-20-14-11(19-7-22(14)16-13(26)12(25)10(5-24)29-16)15(21-17)30-6-8-1-3-9(4-2-8)23(27)28/h1-4,7,10,12-13,16,24-26H,5-6H2,(H2,18,20,21)/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50413751

(CHEMBL61773)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3cccc(c3)[N+]([O-])=O)ncnc12 |r| Show InChI InChI=1S/C17H17N5O6S/c23-5-11-13(24)14(25)17(28-11)21-8-20-12-15(21)18-7-19-16(12)29-6-9-2-1-3-10(4-9)22(26)27/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50222749
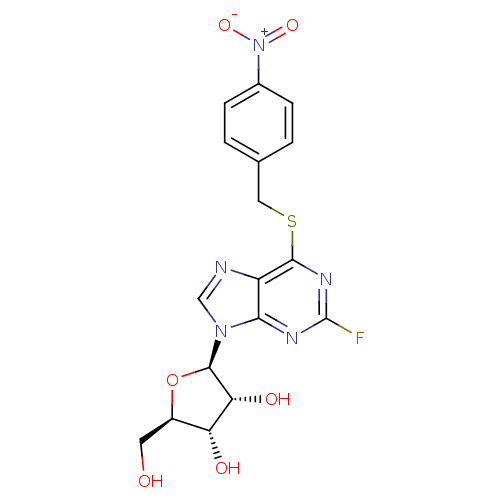
(2-fluoro-6-(4-nitrobenzylthio)-9-beta-D-ribofurano...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)nc(F)nc12 Show InChI InChI=1S/C17H16FN5O6S/c18-17-20-14-11(19-7-22(14)16-13(26)12(25)10(5-24)29-16)15(21-17)30-6-8-1-3-9(4-2-8)23(27)28/h1-4,7,10,12-13,16,24-26H,5-6H2/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366947
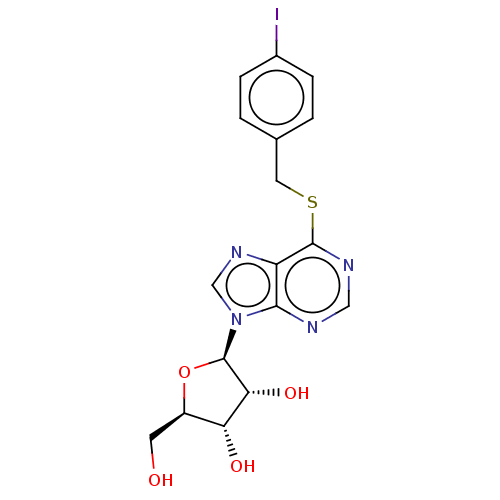
(BDBM50413737 | CHEMBL512162 | CHEMBL610941)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(I)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17IN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153127
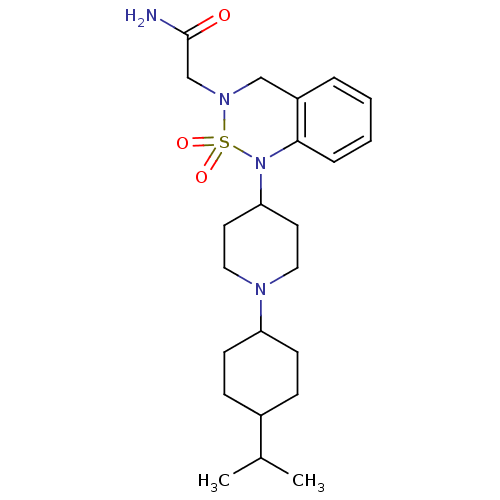
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CC(N)=O)S1(=O)=O |(-1.46,-9.19,;-.14,-8.41,;1.2,-9.18,;-.16,-6.87,;-1.5,-6.08,;-1.5,-4.56,;-.16,-3.77,;1.17,-4.56,;1.17,-6.08,;-.16,-2.23,;-1.51,-1.52,;-1.54,.05,;-.23,.84,;1.12,.11,;1.15,-1.45,;-.27,2.4,;-1.61,3.1,;-2.92,2.29,;-4.25,3.04,;-4.3,4.6,;-2.99,5.39,;-1.64,4.64,;-.34,5.44,;1,4.71,;2.31,5.51,;3.67,4.77,;4.98,5.58,;3.7,3.22,;1.04,3.18,;2.53,3.59,;1.45,1.68,)| Show InChI InChI=1S/C23H36N4O3S/c1-17(2)18-7-9-20(10-8-18)25-13-11-21(12-14-25)27-22-6-4-3-5-19(22)15-26(16-23(24)28)31(27,29)30/h3-6,17-18,20-21H,7-16H2,1-2H3,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366947

(BDBM50413737 | CHEMBL512162 | CHEMBL610941)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(I)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17IN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23697
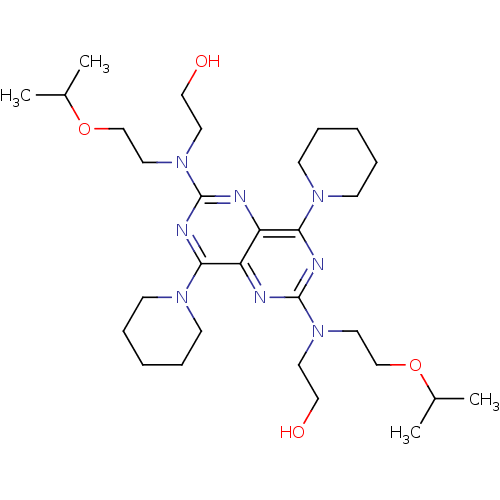
(2-({6-[(2-hydroxyethyl)[2-(propan-2-yloxy)ethyl]am...)Show SMILES CC(C)OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCOC(C)C Show InChI InChI=1S/C30H52N8O4/c1-23(2)41-21-17-37(15-19-39)29-31-25-26(27(33-29)35-11-7-5-8-12-35)32-30(34-28(25)36-13-9-6-10-14-36)38(16-20-40)18-22-42-24(3)4/h23-24,39-40H,5-22H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50413747

(CHEMBL61741)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(Cl)cc3Cl)ncnc12 |r| Show InChI InChI=1S/C17H16Cl2N4O4S/c18-9-2-1-8(10(19)3-9)5-28-16-12-15(20-6-21-16)23(7-22-12)17-14(26)13(25)11(4-24)27-17/h1-3,6-7,11,13-14,17,24-26H,4-5H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50413740

(CHEMBL61915)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)C#N)ncnc12 |r| Show InChI InChI=1S/C18H17N5O4S/c19-5-10-1-3-11(4-2-10)7-28-17-13-16(20-8-21-17)23(9-22-13)18-15(26)14(25)12(6-24)27-18/h1-4,8-9,12,14-15,18,24-26H,6-7H2/t12-,14-,15-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153124
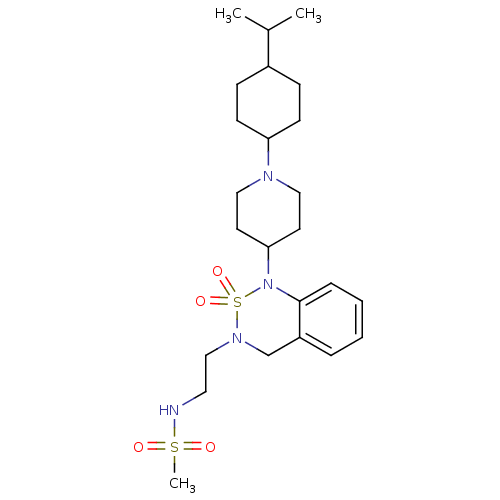
(CHEMBL182967 | N-(2-{1-[1-(4-Isopropyl-cyclohexyl)...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCNS(C)(=O)=O)S1(=O)=O |(.52,-9.66,;-.82,-8.88,;-2.15,-9.66,;-.84,-7.34,;.49,-6.56,;.49,-5.02,;-.84,-4.25,;-2.18,-5.02,;-2.18,-6.56,;-.84,-2.69,;-2.2,-1.98,;-2.23,-.41,;-.91,.39,;.44,-.35,;.48,-1.91,;-.94,1.94,;-2.29,2.65,;-3.6,1.84,;-4.94,2.59,;-4.99,4.14,;-3.69,4.93,;-2.32,4.18,;-1.02,4.99,;.34,4.26,;1.65,5.07,;3,4.33,;4.31,5.12,;5.67,4.4,;7.03,3.67,;6.78,5.47,;4.57,3.3,;.37,2.74,;1.86,3.13,;.77,1.24,)| Show InChI InChI=1S/C24H40N4O4S2/c1-19(2)20-8-10-22(11-9-20)26-15-12-23(13-16-26)28-24-7-5-4-6-21(24)18-27(34(28,31)32)17-14-25-33(3,29)30/h4-7,19-20,22-23,25H,8-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153121
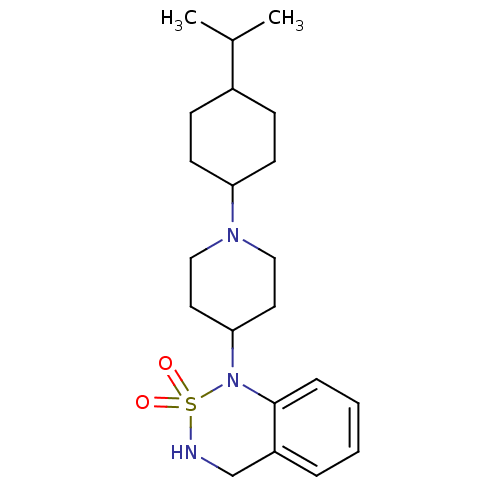
(1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-3,4-...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CNS1(=O)=O |(7.68,-3.52,;7.37,-2.01,;8.54,-.99,;5.92,-1.52,;4.78,-2.53,;3.31,-2.06,;3,-.55,;4.15,.48,;5.61,-.01,;1.55,-.05,;1.24,1.44,;-.23,1.92,;-1.37,.89,;-1.05,-.62,;.41,-1.1,;-2.82,1.37,;-3.81,.18,;-3.27,-1.27,;-4.23,-2.46,;-5.77,-2.21,;-6.31,-.77,;-5.35,.44,;-5.89,1.87,;-4.9,3.06,;-3.37,2.81,;-1.84,2.96,;-3.52,4.37,)| Show InChI InChI=1S/C21H33N3O2S/c1-16(2)17-7-9-19(10-8-17)23-13-11-20(12-14-23)24-21-6-4-3-5-18(21)15-22-27(24,25)26/h3-6,16-17,19-20,22H,7-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50413744

(CHEMBL432688)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(OC(F)(F)F)cc3)ncnc12 |r| Show InChI InChI=1S/C18H17F3N4O5S/c19-18(20,21)30-10-3-1-9(2-4-10)6-31-16-12-15(22-7-23-16)25(8-24-12)17-14(28)13(27)11(5-26)29-17/h1-4,7-8,11,13-14,17,26-28H,5-6H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50153132
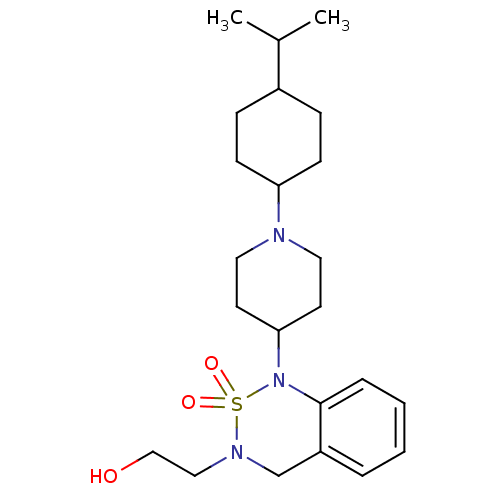
(2-{1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-2...)Show SMILES CC(C)C1CCC(CC1)N1CCC(CC1)N1c2ccccc2CN(CCO)S1(=O)=O |(1.33,-9.05,;-.02,-8.29,;-1.35,-9.08,;-.04,-6.75,;-1.38,-5.98,;-1.38,-4.43,;-.04,-3.66,;1.31,-4.43,;1.31,-5.98,;-.04,-2.12,;-1.39,-1.4,;-1.42,.16,;-.11,.96,;1.24,.21,;1.27,-1.33,;-.14,2.49,;-1.49,3.2,;-2.79,2.4,;-4.13,3.14,;-4.17,4.68,;-2.86,5.48,;-1.52,4.74,;-.23,5.53,;1.12,4.82,;2.43,5.6,;3.78,4.87,;5.09,5.69,;1.17,3.28,;2.64,3.69,;1.56,1.8,)| Show InChI InChI=1S/C23H37N3O3S/c1-18(2)19-7-9-21(10-8-19)24-13-11-22(12-14-24)26-23-6-4-3-5-20(23)17-25(15-16-27)30(26,28)29/h3-6,18-19,21-22,27H,7-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Staphylococcus aureus) | BDBM18071
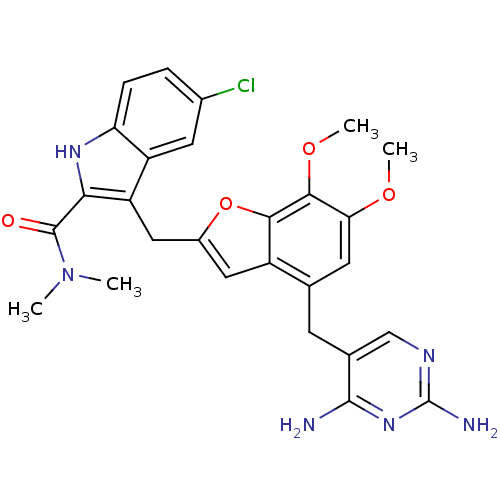
(5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...)Show SMILES COc1cc(Cc2cnc(N)nc2N)c2cc(Cc3c([nH]c4ccc(Cl)cc34)C(=O)N(C)C)oc2c1OC Show InChI InChI=1S/C27H27ClN6O4/c1-34(2)26(35)22-19(18-9-15(28)5-6-20(18)32-22)11-16-10-17-13(7-14-12-31-27(30)33-25(14)29)8-21(36-3)24(37-4)23(17)38-16/h5-6,8-10,12,32H,7,11H2,1-4H3,(H4,29,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| | 8 | -47.0 | 50 | n/a | n/a | n/a | n/a | 7.0 | 30 |
ARPIDA AG
| Assay Description
Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... |
Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006)
BindingDB Entry DOI: 10.7270/Q2959FTP |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23620
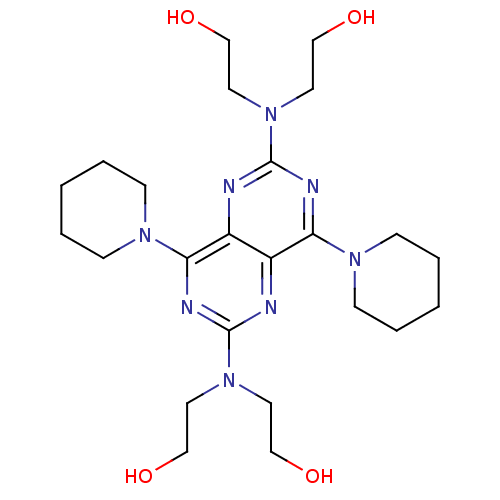
(2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin...)Show SMILES OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.18 | -45.7 | 145 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23692
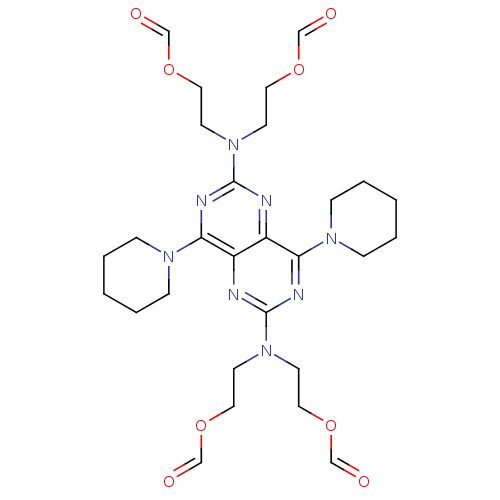
(Dipyridamole Analogue, 74 | {2-[(6-{bis[2-(formylo...)Show SMILES O=COCCN(CCOC=O)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCOC=O)CCOC=O Show InChI InChI=1S/C28H40N8O8/c37-19-41-15-11-35(12-16-42-20-38)27-30-24-23(25(31-27)33-7-3-1-4-8-33)29-28(32-26(24)34-9-5-2-6-10-34)36(13-17-43-21-39)14-18-44-22-40/h19-22H,1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
| Assay Description
The compounds were tested to determine their ENT1 nucleoside transporter binding ability by a flow cytometric assay using human leukemia K562 cells i... |
J Med Chem 50: 3906-20 (2007)
Article DOI: 10.1021/jm070311l
BindingDB Entry DOI: 10.7270/Q2XK8CVN |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM23620

(2-({6-[bis(2-hydroxyethyl)amino]-4,8-bis(piperidin...)Show SMILES OCCN(CCO)c1nc(N2CCCCC2)c2nc(nc(N3CCCCC3)c2n1)N(CCO)CCO Show InChI InChI=1S/C24H40N8O4/c33-15-11-31(12-16-34)23-26-20-19(21(27-23)29-7-3-1-4-8-29)25-24(32(13-17-35)14-18-36)28-22(20)30-9-5-2-6-10-30/h33-36H,1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Streptococcus pneumoniae (ATCC49619)) | BDBM18071

(5-chloro-3-({4-[(2,4-diaminopyrimidin-5-yl)methyl]...)Show SMILES COc1cc(Cc2cnc(N)nc2N)c2cc(Cc3c([nH]c4ccc(Cl)cc34)C(=O)N(C)C)oc2c1OC Show InChI InChI=1S/C27H27ClN6O4/c1-34(2)26(35)22-19(18-9-15(28)5-6-20(18)32-22)11-16-10-17-13(7-14-12-31-27(30)33-25(14)29)8-21(36-3)24(37-4)23(17)38-16/h5-6,8-10,12,32H,7,11H2,1-4H3,(H4,29,30,31,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| | 9 | -46.7 | 90 | n/a | n/a | n/a | n/a | 7.0 | 30 |
ARPIDA AG
| Assay Description
Activity was measured as a change in absorbance over time at a wavelength of 340 nm (A340), so as to monitor the disappearance of NADPH. After incuba... |
Interscience Conference on Antimicrobial Agents in Chemotherapy 1-1 (2006)
BindingDB Entry DOI: 10.7270/Q2959FTP |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366949

(BDBM50413728 | CHEMBL418496 | CHEMBL611841)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(F)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17FN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366949

(BDBM50413728 | CHEMBL418496 | CHEMBL611841)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(F)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17FN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366954

(BDBM50413734 | CHEMBL430946 | CHEMBL608592)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(Br)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17BrN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50222756

(2-chloro-6-(4-nitrobenzylthio)-9-beta-D-ribofurano...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)nc(Cl)nc12 Show InChI InChI=1S/C17H16ClN5O6S/c18-17-20-14-11(19-7-22(14)16-13(26)12(25)10(5-24)29-16)15(21-17)30-6-8-1-3-9(4-2-8)23(27)28/h1-4,7,10,12-13,16,24-26H,5-6H2/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50222755
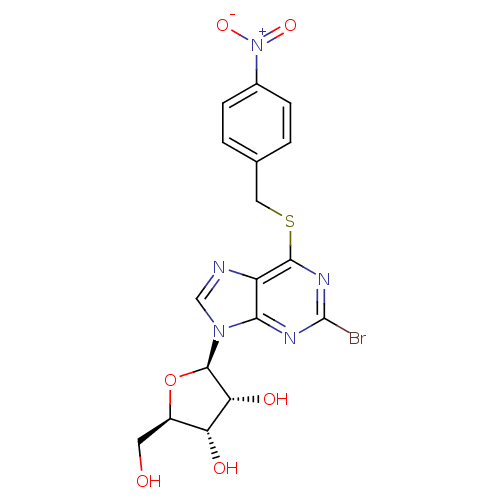
(2-bromo-6-(4-nitrobenzylthio)-9-beta-D-ribofuranos...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)nc(Br)nc12 Show InChI InChI=1S/C17H16BrN5O6S/c18-17-20-14-11(19-7-22(14)16-13(26)12(25)10(5-24)29-16)15(21-17)30-6-8-1-3-9(4-2-8)23(27)28/h1-4,7,10,12-13,16,24-26H,5-6H2/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366948
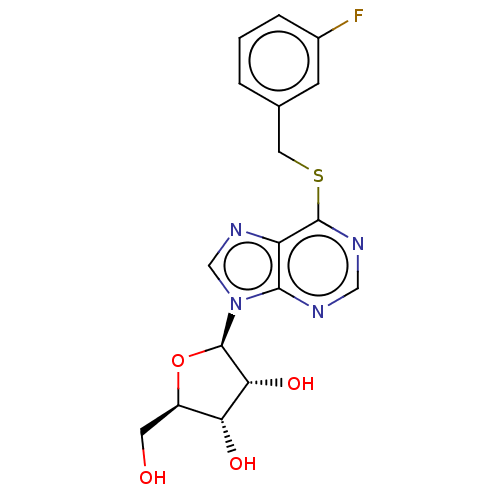
(BDBM50413727 | CHEMBL517945 | CHEMBL611842)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3cccc(F)c3)ncnc12 |r| Show InChI InChI=1S/C17H17FN4O4S/c18-10-3-1-2-9(4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50222745
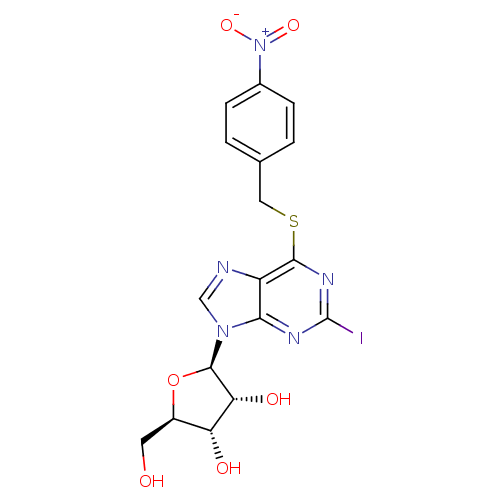
(2-iodo-6-(4-nitrobenzylthio)-9-beta-D-ribofuranosy...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)nc(I)nc12 Show InChI InChI=1S/C17H16IN5O6S/c18-17-20-14-11(19-7-22(14)16-13(26)12(25)10(5-24)29-16)15(21-17)30-6-8-1-3-9(4-2-8)23(27)28/h1-4,7,10,12-13,16,24-26H,5-6H2/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of SAENTA-fluorescein from human ENT1 in K562 cells after 45 mins by flow cytometry |
Bioorg Med Chem 15: 7726-37 (2007)
Article DOI: 10.1016/j.bmc.2007.08.058
BindingDB Entry DOI: 10.7270/Q26Q1WZD |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366954

(BDBM50413734 | CHEMBL430946 | CHEMBL608592)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(Br)cc3)ncnc12 |r| Show InChI InChI=1S/C17H17BrN4O4S/c18-10-3-1-9(2-4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Rattus norvegicus) | BDBM50453671
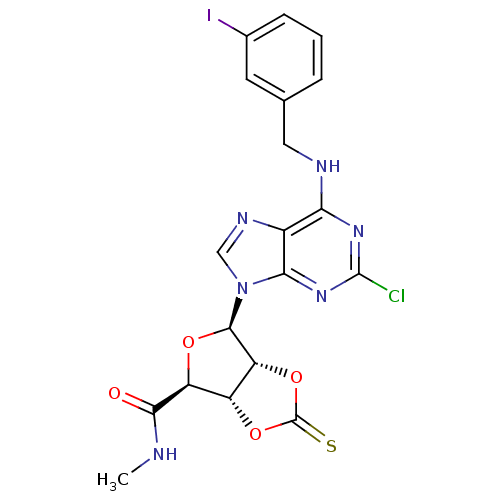
(CHEMBL2113701)Show SMILES [H][C@]12OC(=S)O[C@@]1([H])[C@@H](O[C@@H]2C(=O)NC)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C19H16ClIN6O4S/c1-22-16(28)12-11-13(31-19(32)30-11)17(29-12)27-7-24-10-14(25-18(20)26-15(10)27)23-6-8-3-2-4-9(21)5-8/h2-5,7,11-13,17H,6H2,1H3,(H,22,28)(H,23,25,26)/t11-,12+,13-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A3 receptor from rat brain. |
J Med Chem 38: 1720-35 (1995)
BindingDB Entry DOI: 10.7270/Q21Z453H |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50413748

(CHEMBL58072)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(Cl)c(Cl)c3)ncnc12 |r| Show InChI InChI=1S/C17H16Cl2N4O4S/c18-9-2-1-8(3-10(9)19)5-28-16-12-15(20-6-21-16)23(7-22-12)17-14(26)13(25)11(4-24)27-17/h1-3,6-7,11,13-14,17,24-26H,4-5H2/t11-,13-,14-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366948

(BDBM50413727 | CHEMBL517945 | CHEMBL611842)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3cccc(F)c3)ncnc12 |r| Show InChI InChI=1S/C17H17FN4O4S/c18-10-3-1-2-9(4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153135
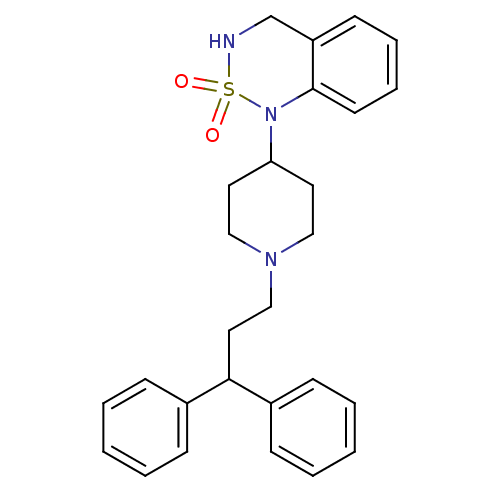
(1-[1-(3,3-Diphenyl-propyl)-piperidin-4-yl]-3,4-dih...)Show SMILES O=S1(=O)NCc2ccccc2N1C1CCN(CCC(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2S/c31-33(32)28-21-24-13-7-8-14-27(24)30(33)25-15-18-29(19-16-25)20-17-26(22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-14,25-26,28H,15-21H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]diprenorphine binding to human Opioid receptor mu 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366946

(BDBM50413736 | CHEMBL473492 | CHEMBL610938)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C17H17IN4O4S/c18-10-3-1-2-9(4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Inhibition of human ENT1 |
Bioorg Med Chem Lett 19: 314-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.092
BindingDB Entry DOI: 10.7270/Q2H996F3 |
More data for this
Ligand-Target Pair | |
Equilibrative nucleoside transporter 1
(Homo sapiens (Human)) | BDBM50366946

(BDBM50413736 | CHEMBL473492 | CHEMBL610938)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C17H17IN4O4S/c18-10-3-1-2-9(4-10)6-27-16-12-15(19-7-20-16)22(8-21-12)17-14(25)13(24)11(5-23)26-17/h1-4,7-8,11,13-14,17,23-25H,5-6H2/t11-,13-,14-,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Sciences Center
Curated by ChEMBL
| Assay Description
Displacement of 5-(SAENTA)-X8-fluorescein from K562 cell nucleoside transporter |
Bioorg Med Chem Lett 14: 2257-60 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.016
BindingDB Entry DOI: 10.7270/Q2NK3FKH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data