 Found 1648 hits with Last Name = 'langen' and Initial = 'b'
Found 1648 hits with Last Name = 'langen' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357868
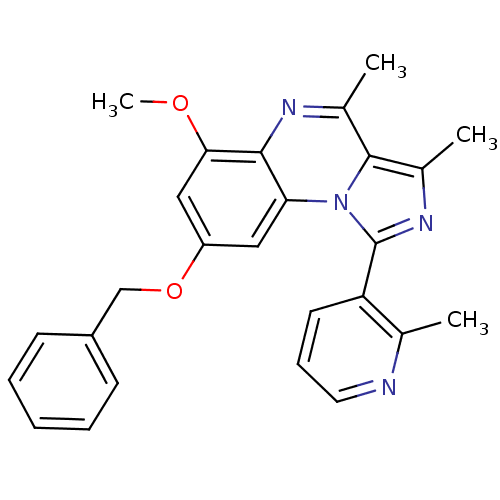
(CHEMBL1916128)Show SMILES COc1cc(OCc2ccccc2)cc2c1nc(C)c1c(C)nc(-c3cccnc3C)n21 Show InChI InChI=1S/C26H24N4O2/c1-16-21(11-8-12-27-16)26-29-18(3)25-17(2)28-24-22(30(25)26)13-20(14-23(24)31-4)32-15-19-9-6-5-7-10-19/h5-14H,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357869

(CHEMBL1916129)Show SMILES COc1cc(OC(F)F)cc2c1nc(C)c1c(C)nc(-c3cccnc3C)n21 Show InChI InChI=1S/C20H18F2N4O2/c1-10-14(6-5-7-23-10)19-25-12(3)18-11(2)24-17-15(26(18)19)8-13(28-20(21)22)9-16(17)27-4/h5-9,20H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357870

(CHEMBL1916130)Show SMILES COc1cc(cc2c1nc(C)c1c(C)nc(-c3ccncc3C)n21)C(F)(F)F Show InChI InChI=1S/C20H17F3N4O/c1-10-9-24-6-5-14(10)19-26-12(3)18-11(2)25-17-15(27(18)19)7-13(20(21,22)23)8-16(17)28-4/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357867
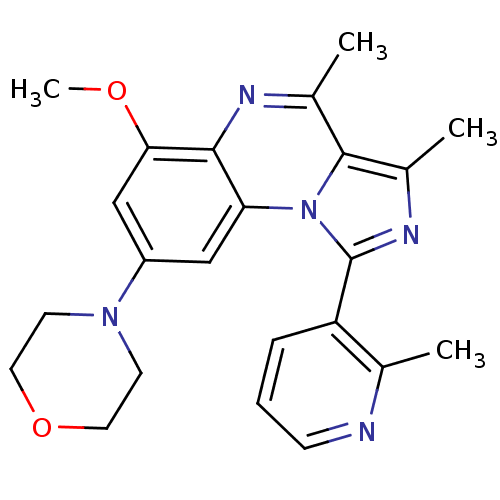
(CHEMBL1916127)Show SMILES COc1cc(cc2c1nc(C)c1c(C)nc(-c3cccnc3C)n21)N1CCOCC1 Show InChI InChI=1S/C23H25N5O2/c1-14-18(6-5-7-24-14)23-26-16(3)22-15(2)25-21-19(28(22)23)12-17(13-20(21)29-4)27-8-10-30-11-9-27/h5-7,12-13H,8-11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357865
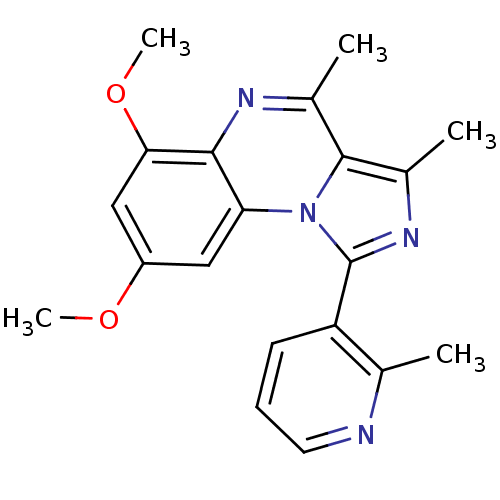
(CHEMBL1916125)Show SMILES COc1cc(OC)c2nc(C)c3c(C)nc(-c4cccnc4C)n3c2c1 Show InChI InChI=1S/C20H20N4O2/c1-11-15(7-6-8-21-11)20-23-13(3)19-12(2)22-18-16(24(19)20)9-14(25-4)10-17(18)26-5/h6-10H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM171943
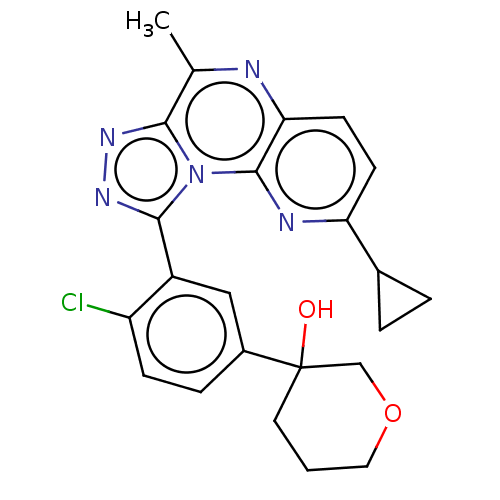
(US9085584, 113)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(ccc1Cl)C1(O)CCCOC1)C1CC1 Show InChI InChI=1S/C23H22ClN5O2/c1-13-20-27-28-21(29(20)22-19(25-13)8-7-18(26-22)14-3-4-14)16-11-15(5-6-17(16)24)23(30)9-2-10-31-12-23/h5-8,11,14,30H,2-4,9-10,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357864

(CHEMBL1916124)Show SMILES COc1cc(OC)c2nc(C)c3c(C)nc(-c4ccncc4C)n3c2c1 Show InChI InChI=1S/C20H20N4O2/c1-11-10-21-7-6-15(11)20-23-13(3)19-12(2)22-18-16(24(19)20)8-14(25-4)9-17(18)26-5/h6-10H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50390812
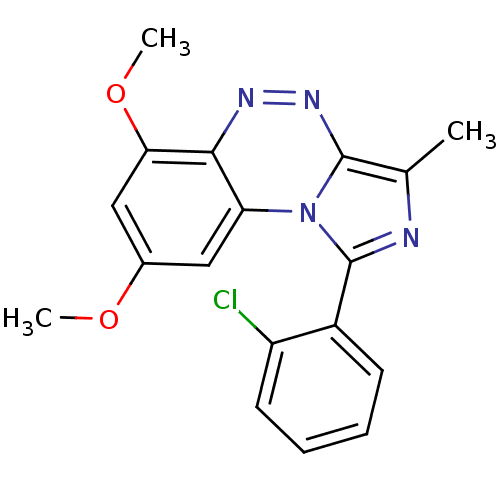
(CHEMBL2069321)Show InChI InChI=1S/C18H15ClN4O2/c1-10-17-22-21-16-14(8-11(24-2)9-15(16)25-3)23(17)18(20-10)12-6-4-5-7-13(12)19/h4-9H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A-catalyzed cAMP hydrolysis |
Bioorg Med Chem Lett 22: 5876-84 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.076
BindingDB Entry DOI: 10.7270/Q2FB5413 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357857
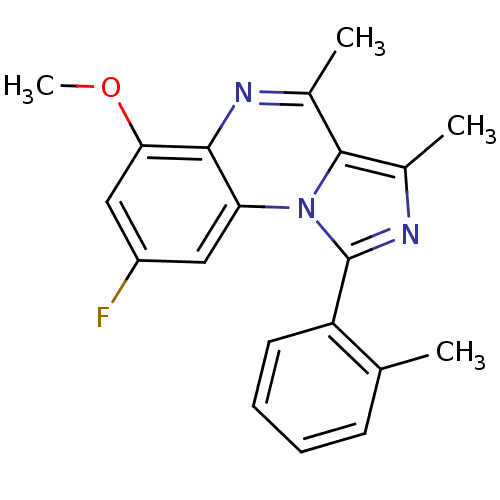
(CHEMBL1916117)Show InChI InChI=1S/C20H18FN3O/c1-11-7-5-6-8-15(11)20-23-13(3)19-12(2)22-18-16(24(19)20)9-14(21)10-17(18)25-4/h5-10H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396904
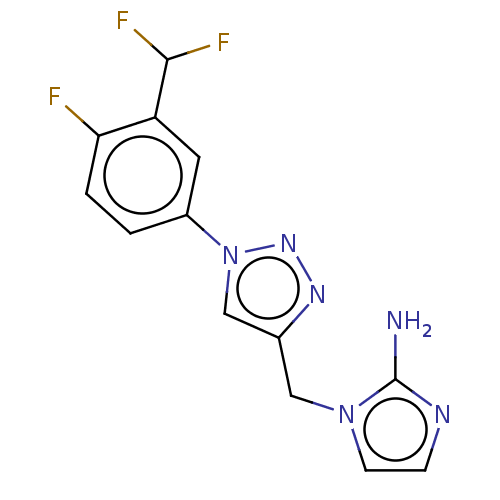
(US9981950, Example 113B)Show InChI InChI=1S/C13H11F3N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM396904

(US9981950, Example 113B)Show InChI InChI=1S/C13H11F3N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396904

(US9981950, Example 113B)Show InChI InChI=1S/C13H11F3N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357863
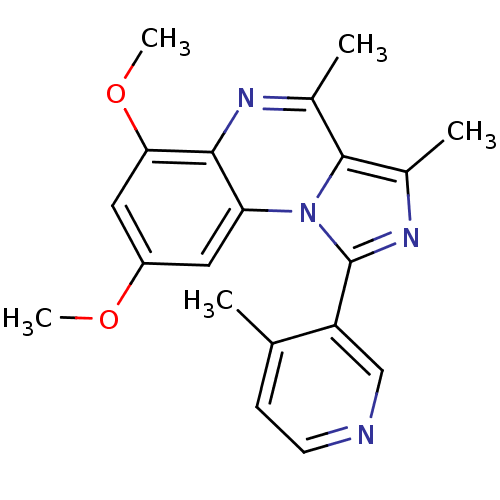
(CHEMBL1916123)Show SMILES COc1cc(OC)c2nc(C)c3c(C)nc(-c4cnccc4C)n3c2c1 Show InChI InChI=1S/C20H20N4O2/c1-11-6-7-21-10-15(11)20-23-13(3)19-12(2)22-18-16(24(19)20)8-14(25-4)9-17(18)26-5/h6-10H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396905

(US9981950, Example 114)Show InChI InChI=1S/C14H13F3N6/c1-14(16,17)11-6-10(2-3-12(11)15)23-8-9(20-21-23)7-22-5-4-19-13(22)18/h2-6,8H,7H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM398608
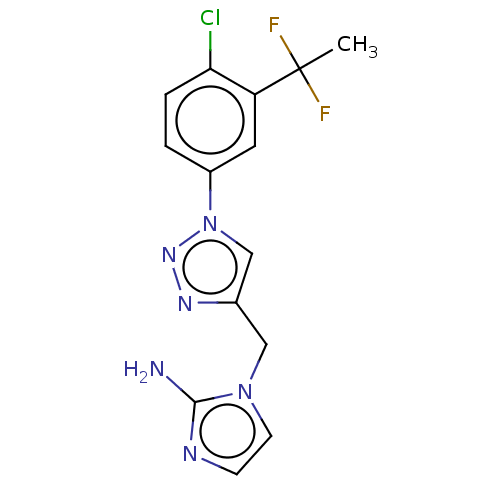
(US10323021, Example 114)Show InChI InChI=1S/C14H13ClF2N6/c1-14(16,17)11-6-10(2-3-12(11)15)23-8-9(20-21-23)7-22-5-4-19-13(22)18/h2-6,8H,7H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM398608

(US10323021, Example 114)Show InChI InChI=1S/C14H13ClF2N6/c1-14(16,17)11-6-10(2-3-12(11)15)23-8-9(20-21-23)7-22-5-4-19-13(22)18/h2-6,8H,7H2,1H3,(H2,18,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM396901

(US9981950, Example 110)Show InChI InChI=1S/C13H11ClF2N6O/c14-10-2-1-9(5-11(10)23-12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396901

(US9981950, Example 110)Show InChI InChI=1S/C13H11ClF2N6O/c14-10-2-1-9(5-11(10)23-12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396901

(US9981950, Example 110)Show InChI InChI=1S/C13H11ClF2N6O/c14-10-2-1-9(5-11(10)23-12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357854

(CHEMBL1916114)Show InChI InChI=1S/C19H17FN4O/c1-10-14(6-5-7-21-10)19-23-12(3)18-11(2)22-17-15(24(18)19)8-13(20)9-16(17)25-4/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM396903
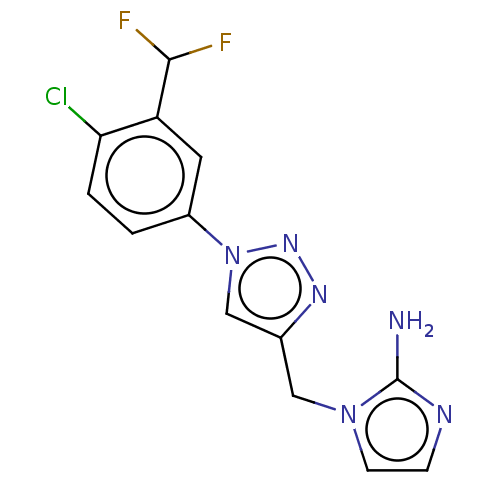
(US9981950, Example 113A)Show InChI InChI=1S/C13H11ClF2N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396903

(US9981950, Example 113A)Show InChI InChI=1S/C13H11ClF2N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396903

(US9981950, Example 113A)Show InChI InChI=1S/C13H11ClF2N6/c14-11-2-1-9(5-10(11)12(15)16)22-7-8(19-20-22)6-21-4-3-18-13(21)17/h1-5,7,12H,6H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357872
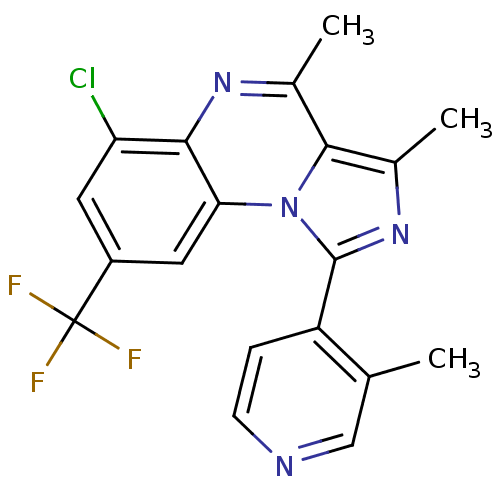
(CHEMBL1916132)Show SMILES Cc1nc(-c2ccncc2C)n2c1c(C)nc1c(Cl)cc(cc21)C(F)(F)F Show InChI InChI=1S/C19H14ClF3N4/c1-9-8-24-5-4-13(9)18-26-11(3)17-10(2)25-16-14(20)6-12(19(21,22)23)7-15(16)27(17)18/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357803

(CHEMBL1916113)Show InChI InChI=1S/C19H17FN4O/c1-10-9-21-6-5-14(10)19-23-12(3)18-11(2)22-17-15(24(18)19)7-13(20)8-16(17)25-4/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357871
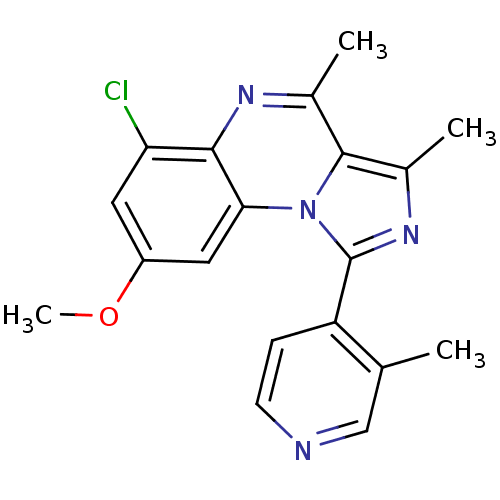
(CHEMBL1916131)Show SMILES COc1cc(Cl)c2nc(C)c3c(C)nc(-c4ccncc4C)n3c2c1 Show InChI InChI=1S/C19H17ClN4O/c1-10-9-21-6-5-14(10)19-23-12(3)18-11(2)22-17-15(20)7-13(25-4)8-16(17)24(18)19/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357856
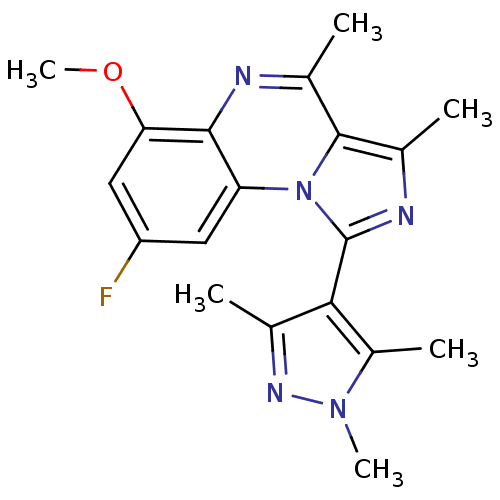
(CHEMBL1916116)Show SMILES COc1cc(F)cc2c1nc(C)c1c(C)nc(-c3c(C)nn(C)c3C)n21 |(13.56,-14.42,;12.23,-15.2,;12.23,-16.74,;10.9,-17.51,;10.9,-19.05,;9.57,-19.82,;12.23,-19.82,;13.56,-19.05,;13.57,-17.5,;14.9,-16.73,;16.24,-17.5,;17.57,-16.74,;16.24,-19.05,;17.38,-20.09,;18.89,-19.78,;16.75,-21.5,;15.21,-21.33,;14.29,-22.56,;14.74,-24.04,;16.2,-24.54,;13.48,-24.92,;12.25,-23.99,;10.78,-24.44,;12.75,-22.54,;11.87,-21.28,;14.9,-19.82,)| Show InChI InChI=1S/C19H20FN5O/c1-9-16(12(4)24(5)23-9)19-22-11(3)18-10(2)21-17-14(25(18)19)7-13(20)8-15(17)26-6/h7-8H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50390818

(CHEMBL2070655)Show SMILES COc1cc(cc2c1nnc1c(C)nc(-c3ccccc3Cl)n21)N1CCOCC1 Show InChI InChI=1S/C21H20ClN5O2/c1-13-20-25-24-19-17(27(20)21(23-13)15-5-3-4-6-16(15)22)11-14(12-18(19)28-2)26-7-9-29-10-8-26/h3-6,11-12H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A-catalyzed cAMP hydrolysis |
Bioorg Med Chem Lett 22: 5876-84 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.076
BindingDB Entry DOI: 10.7270/Q2FB5413 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM171839
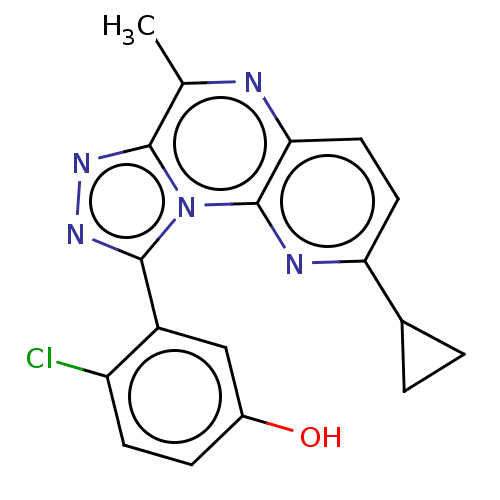
(US9085584, 9)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(O)ccc1Cl)C1CC1 Show InChI InChI=1S/C18H14ClN5O/c1-9-16-22-23-17(12-8-11(25)4-5-13(12)19)24(16)18-15(20-9)7-6-14(21-18)10-2-3-10/h4-8,10,25H,2-3H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM171910
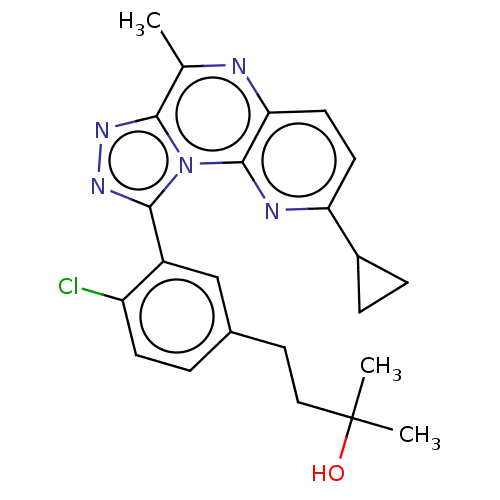
(US9085584, 69)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(CCC(C)(C)O)ccc1Cl)C1CC1 Show InChI InChI=1S/C23H24ClN5O/c1-13-20-27-28-21(16-12-14(4-7-17(16)24)10-11-23(2,3)30)29(20)22-19(25-13)9-8-18(26-22)15-5-6-15/h4,7-9,12,15,30H,5-6,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM204680
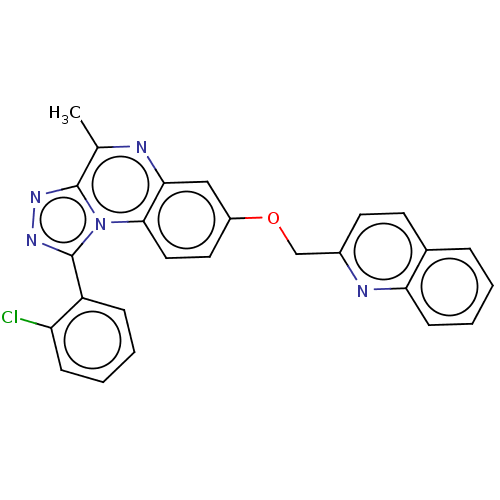
(1-(2-chloro-phenyl)-4-methyl-7-(quinolin-2-ylmetho...)Show SMILES Cc1nc2cc(OCc3ccc4ccccc4n3)ccc2n2c(nnc12)-c1ccccc1Cl Show InChI InChI=1S/C26H18ClN5O/c1-16-25-30-31-26(20-7-3-4-8-21(20)27)32(25)24-13-12-19(14-23(24)28-16)33-15-18-11-10-17-6-2-5-9-22(17)29-18/h2-14H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The DNA of PDE10A1 (AB 020593, 2340 bp) was synthesized and cloned into the vector pCR4. TOPO (Entelechon GmbH, Regensburg, Germany). The gene was th... |
US Patent US9540379 (2017)
BindingDB Entry DOI: 10.7270/Q2RB72SP |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM171932
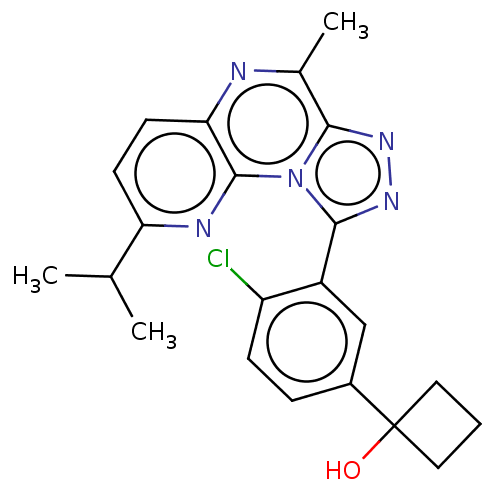
(US9085584, 91)Show SMILES CC(C)c1ccc2nc(C)c3nnc(-c4cc(ccc4Cl)C4(O)CCC4)n3c2n1 Show InChI InChI=1S/C22H22ClN5O/c1-12(2)17-7-8-18-21(25-17)28-19(13(3)24-18)26-27-20(28)15-11-14(5-6-16(15)23)22(29)9-4-10-22/h5-8,11-12,29H,4,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
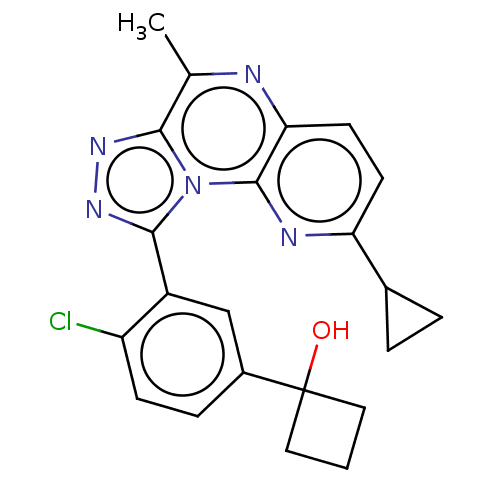
(Homo sapiens (Human)) | BDBM171929
(US9085584, 88)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(ccc1Cl)C1(O)CCC1)C1CC1 Show InChI InChI=1S/C22H20ClN5O/c1-12-19-26-27-20(15-11-14(5-6-16(15)23)22(29)9-2-10-22)28(19)21-18(24-12)8-7-17(25-21)13-3-4-13/h5-8,11,13,29H,2-4,9-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
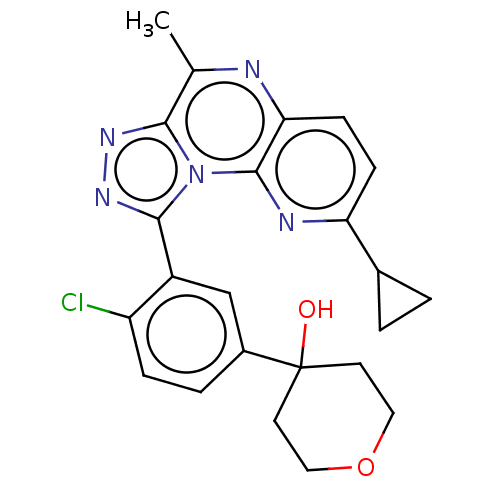
(Homo sapiens (Human)) | BDBM171935
(US9085584, 94)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(ccc1Cl)C1(O)CCOCC1)C1CC1 Show InChI InChI=1S/C23H22ClN5O2/c1-13-20-27-28-21(29(20)22-19(25-13)7-6-18(26-22)14-2-3-14)16-12-15(4-5-17(16)24)23(30)8-10-31-11-9-23/h4-7,12,14,30H,2-3,8-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM171890
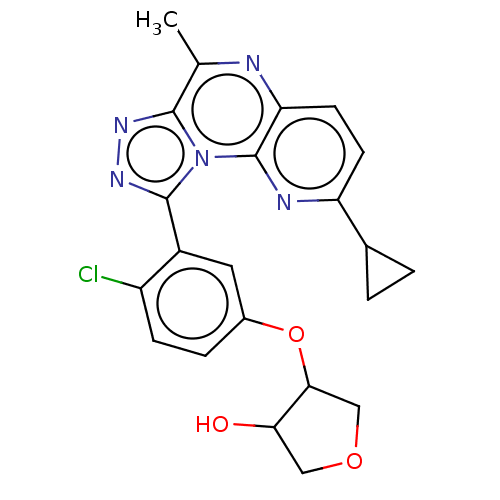
(US9085584, 56 | US9085584, 56a | US9085584, 56b | ...)Show SMILES Cc1nc2ccc(nc2n2c(nnc12)-c1cc(OC2COCC2O)ccc1Cl)C1CC1 Show InChI InChI=1S/C22H20ClN5O3/c1-11-20-26-27-21(28(20)22-17(24-11)7-6-16(25-22)12-2-3-12)14-8-13(4-5-15(14)23)31-19-10-30-9-18(19)29/h4-8,12,18-19,29H,2-3,9-10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
The inhibition of PDE 2A or 10 enzyme activity was assessed using IMAP-Phosphodiesterase-cAMP fluorescence labeled substrate (Molecular Devices, Orde... |
US Patent US9085584 (2015)
BindingDB Entry DOI: 10.7270/Q27P8X54 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396886
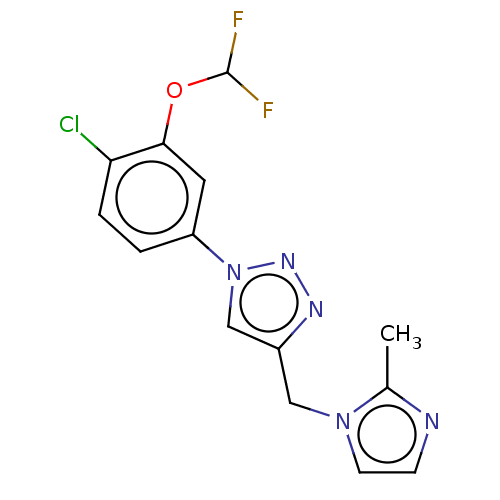
(US9981950, Example 93)Show InChI InChI=1S/C14H12ClF2N5O/c1-9-18-4-5-21(9)7-10-8-22(20-19-10)11-2-3-12(15)13(6-11)23-14(16)17/h2-6,8,14H,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
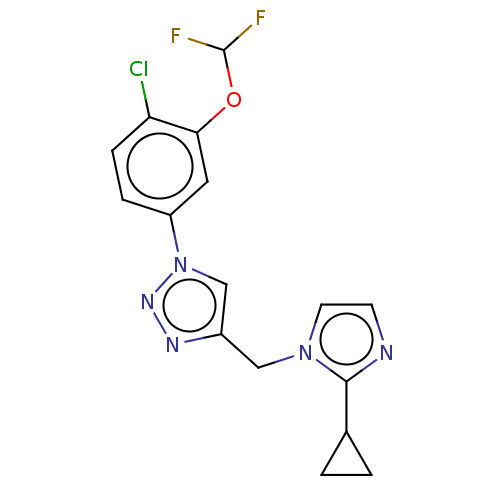
(Homo sapiens (Human)) | BDBM396887
(US9981950, Example 94)Show InChI InChI=1S/C16H14ClF2N5O/c17-13-4-3-12(7-14(13)25-16(18)19)24-9-11(21-22-24)8-23-6-5-20-15(23)10-1-2-10/h3-7,9-10,16H,1-2,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
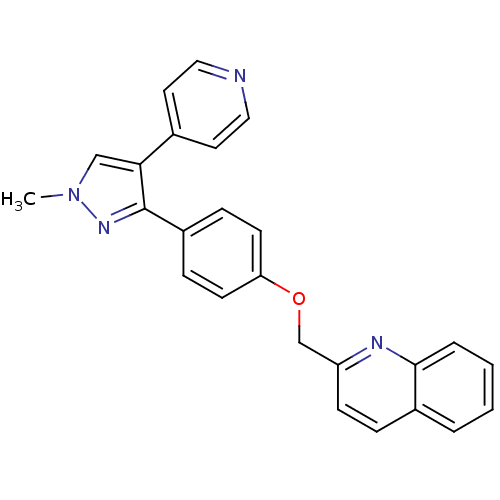
(Homo sapiens (Human)) | BDBM31592
(PF-2545920 | US9138494, MP-10 | substituted pyraz...)Show SMILES Cn1cc(c(n1)-c1ccc(OCc2ccc3ccccc3n2)cc1)-c1ccncc1 Show InChI InChI=1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biotie Therapies GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A expressed in baculovirus-SF21 cell system assessed as hydrolysis of [3H]cAMP after 1 hr by liquid scintillatio... |
J Med Chem 53: 4399-411 (2010)
Article DOI: 10.1021/jm1002793
BindingDB Entry DOI: 10.7270/Q2MK6D3N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
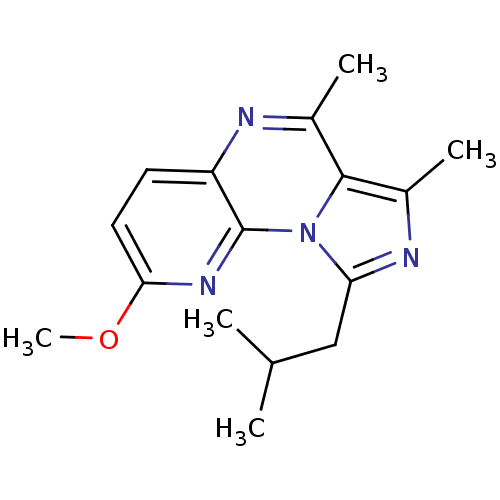
(Homo sapiens (Human)) | BDBM50319169
(3,4-Dimethoxy-1-isobutyl-8-methoxy-imidazo[1,5-a]p...)Show InChI InChI=1S/C16H20N4O/c1-9(2)8-13-18-11(4)15-10(3)17-12-6-7-14(21-5)19-16(12)20(13)15/h6-7,9H,8H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biotie Therapies GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A expressed in baculovirus-SF21 cell system assessed as hydrolysis of [3H]cAMP after 1 hr by liquid scintillatio... |
J Med Chem 53: 4399-411 (2010)
Article DOI: 10.1021/jm1002793
BindingDB Entry DOI: 10.7270/Q2MK6D3N |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50390829

(CHEMBL2070666)Show SMILES COc1cc(cc2c1nnc1c(C)nc(-c3ccncc3C)n21)C(F)(F)F Show InChI InChI=1S/C18H14F3N5O/c1-9-8-22-5-4-12(9)17-23-10(2)16-25-24-15-13(26(16)17)6-11(18(19,20)21)7-14(15)27-3/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A-catalyzed cAMP hydrolysis |
Bioorg Med Chem Lett 22: 5876-84 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.076
BindingDB Entry DOI: 10.7270/Q2FB5413 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50390834
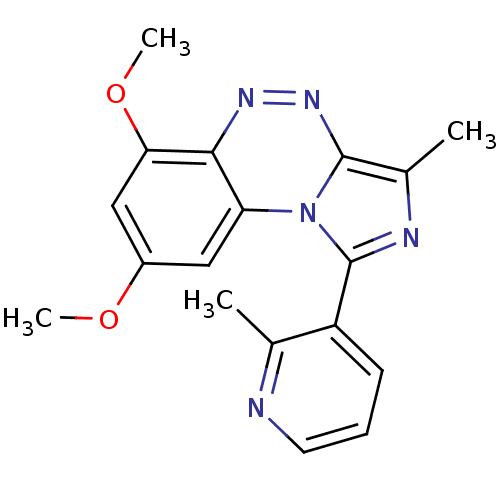
(CHEMBL2070651)Show InChI InChI=1S/C18H17N5O2/c1-10-13(6-5-7-19-10)18-20-11(2)17-22-21-16-14(23(17)18)8-12(24-3)9-15(16)25-4/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A-catalyzed cAMP hydrolysis |
Bioorg Med Chem Lett 22: 5876-84 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.076
BindingDB Entry DOI: 10.7270/Q2FB5413 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357855

(CHEMBL1916115)Show SMILES COc1cc(F)cc2c1nc(C)c1c(C)nc(-c3sc(C)nc3C)n21 Show InChI InChI=1S/C18H17FN4OS/c1-8-16-9(2)22-18(17-10(3)20-11(4)25-17)23(16)13-6-12(19)7-14(24-5)15(13)21-8/h6-7H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50357875

(CHEMBL1916135)Show InChI InChI=1S/C19H17FN4O/c1-10-14(6-5-7-21-10)19-23-12(3)18-11(2)22-17-15(20)8-13(25-4)9-16(17)24(18)19/h5-9H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PDE10A expressed in Escherichia coli using [3H]cAMP after 1 hr by scintillation proximity assay |
J Med Chem 54: 7621-38 (2011)
Article DOI: 10.1021/jm2009138
BindingDB Entry DOI: 10.7270/Q2125T2B |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50390817
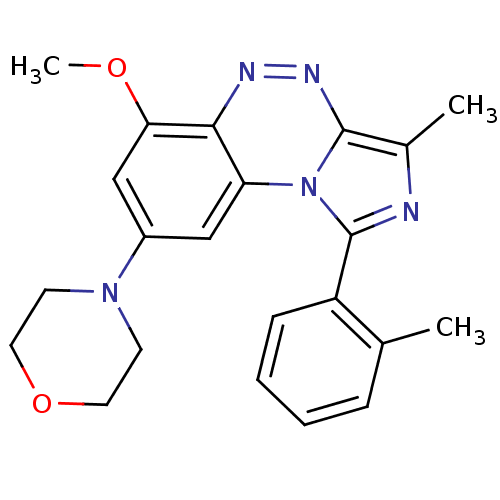
(CHEMBL2070654)Show SMILES COc1cc(cc2c1nnc1c(C)nc(-c3ccccc3C)n21)N1CCOCC1 Show InChI InChI=1S/C22H23N5O2/c1-14-6-4-5-7-17(14)22-23-15(2)21-25-24-20-18(27(21)22)12-16(13-19(20)28-3)26-8-10-29-11-9-26/h4-7,12-13H,8-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A-catalyzed cAMP hydrolysis |
Bioorg Med Chem Lett 22: 5876-84 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.076
BindingDB Entry DOI: 10.7270/Q2FB5413 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396889
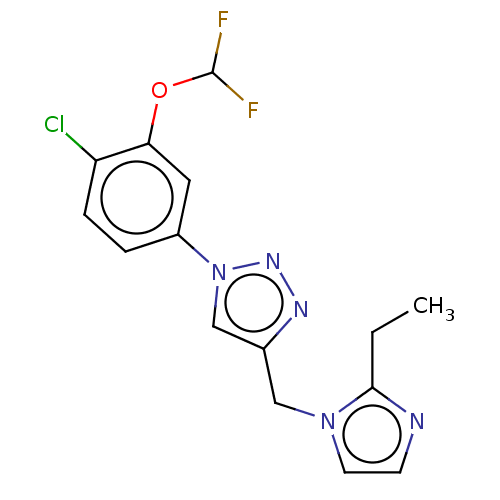
(US9981950, Example 96)Show InChI InChI=1S/C15H14ClF2N5O/c1-2-14-19-5-6-22(14)8-10-9-23(21-20-10)11-3-4-12(16)13(7-11)24-15(17)18/h3-7,9,15H,2,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396889

(US9981950, Example 96)Show InChI InChI=1S/C15H14ClF2N5O/c1-2-14-19-5-6-22(14)8-10-9-23(21-20-10)11-3-4-12(16)13(7-11)24-15(17)18/h3-7,9,15H,2,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM396889

(US9981950, Example 96)Show InChI InChI=1S/C15H14ClF2N5O/c1-2-14-19-5-6-22(14)8-10-9-23(21-20-10)11-3-4-12(16)13(7-11)24-15(17)18/h3-7,9,15H,2,8H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM396848

(US9981950, Example 55)Show InChI InChI=1S/C14H14ClN5/c1-10-7-13(3-4-14(10)15)20-9-12(17-18-20)8-19-6-5-16-11(19)2/h3-7,9H,8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
Male Wistar rats (180 to 200 g) were killed by suffocation in a CO2 chamber for two minutes. Whole brains without cerebellum were removed and dissect... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396848

(US9981950, Example 55)Show InChI InChI=1S/C14H14ClN5/c1-10-7-13(3-4-14(10)15)20-9-12(17-18-20)8-19-6-5-16-11(19)2/h3-7,9H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
| Assay Description
TBD |
Bioorg Med Chem Lett 18: 3168-72 (2008)
BindingDB Entry DOI: 10.7270/Q2JW8H7X |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM396848

(US9981950, Example 55)Show InChI InChI=1S/C14H14ClN5/c1-10-7-13(3-4-14(10)15)20-9-12(17-18-20)8-19-6-5-16-11(19)2/h3-7,9H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadys Pharmaceuticals
| Assay Description
Cells were split 2-3 times weekly between 1:3 and 1:4. For binding assays and membrane preparations the cell culture medium was removed, cells were w... |
Bioorg Med Chem Lett 19: 451-8 (2009)
BindingDB Entry DOI: 10.7270/Q2TH8Q1D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data