 Found 448 hits with Last Name = 'laufersweiler' and Initial = 'mc'
Found 448 hits with Last Name = 'laufersweiler' and Initial = 'mc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Collagenase 3
(Homo sapiens (Human)) | BDBM30359
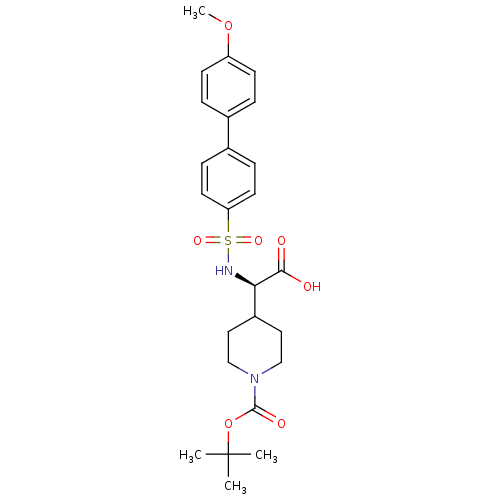
(BOC-piperidinyl glycine derivative, 34)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O |r| Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102477

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O8S/c1-32-15-16-34-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(33-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102479

(4-[Carboxy-(4'-methylsulfanyl-biphenyl-4-sulfonyla...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(SC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O7S2/c1-32-15-16-33-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(34-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102477

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O8S/c1-32-15-16-34-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(33-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102468
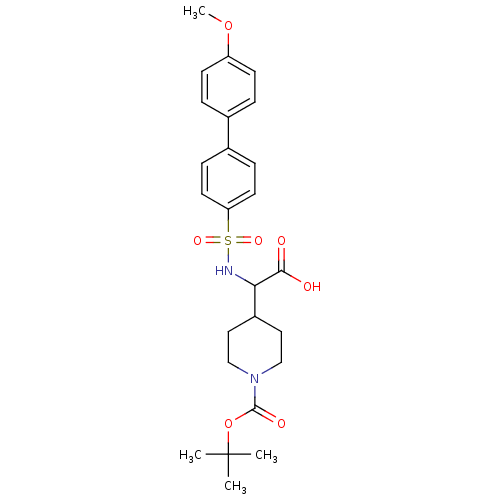
(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102478

(4-{Carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-(2-me...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(CCOC)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C27H36N2O9S/c1-35-17-16-29(25(26(30)31)22-12-14-28(15-13-22)27(32)38-19-18-36-2)39(33,34)24-10-6-21(7-11-24)20-4-8-23(37-3)9-5-20/h4-11,22,25H,12-19H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102468

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102477

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O8S/c1-32-15-16-34-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(33-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102474
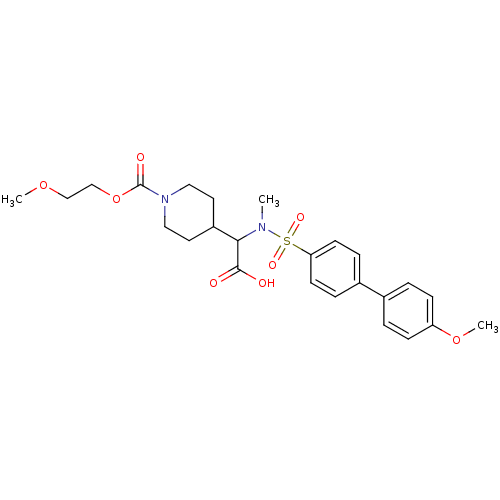
(4-{carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-methy...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(C)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C25H32N2O8S/c1-26(23(24(28)29)20-12-14-27(15-13-20)25(30)35-17-16-33-2)36(31,32)22-10-6-19(7-11-22)18-4-8-21(34-3)9-5-18/h4-11,20,23H,12-17H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102479

(4-[Carboxy-(4'-methylsulfanyl-biphenyl-4-sulfonyla...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(SC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O7S2/c1-32-15-16-33-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(34-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193734
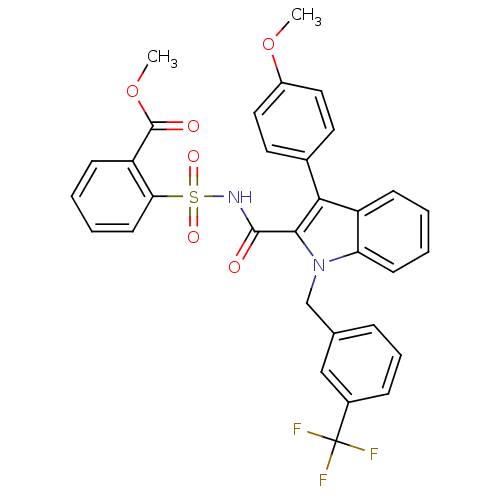
(2-{[3-(4-methoxy-phenyl)-1-(3-trifluoromethyl-benz...)Show SMILES COC(=O)c1ccccc1S(=O)(=O)NC(=O)c1c(-c2ccc(OC)cc2)c2ccccc2n1Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C32H25F3N2O6S/c1-42-23-16-14-21(15-17-23)28-24-10-3-5-12-26(24)37(19-20-8-7-9-22(18-20)32(33,34)35)29(28)30(38)36-44(40,41)27-13-6-4-11-25(27)31(39)43-2/h3-18H,19H2,1-2H3,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199749
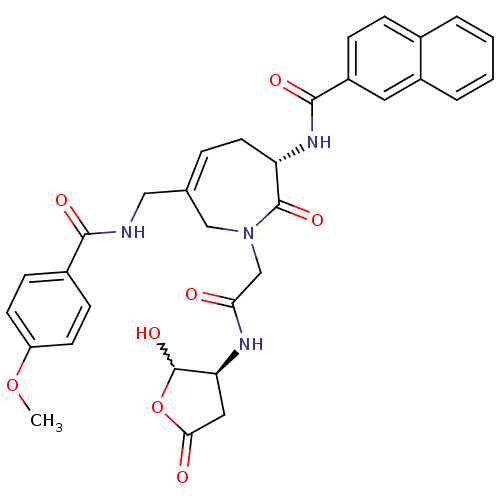
(CHEMBL268772 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1ccc(cc1)C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-24-11-9-21(10-12-24)29(39)33-16-19-6-13-25(35-30(40)23-8-7-20-4-2-3-5-22(20)14-23)31(41)36(17-19)18-27(37)34-26-15-28(38)44-32(26)42/h2-12,14,25-26,32,42H,13,15-18H2,1H3,(H,33,39)(H,34,37)(H,35,40)/t25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199766
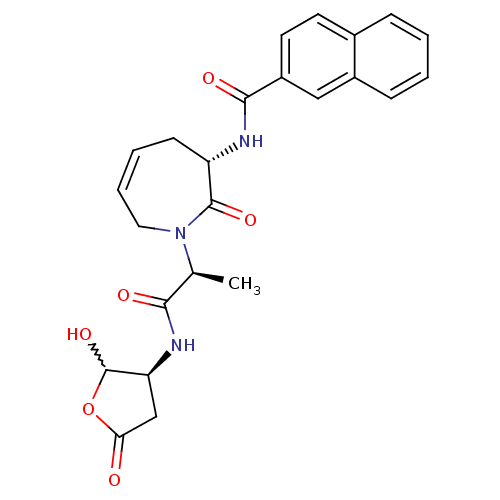
(CHEMBL394351 | N-((S,Z)-1-((S)-1-((3S)-2-hydroxy-5...)Show SMILES C[C@H](N1CC=CC[C@H](NC(=O)c2ccc3ccccc3c2)C1=O)C(=O)N[C@H]1CC(=O)OC1O |w:31.35,c:4| Show InChI InChI=1S/C24H25N3O6/c1-14(21(29)26-19-13-20(28)33-24(19)32)27-11-5-4-8-18(23(27)31)25-22(30)17-10-9-15-6-2-3-7-16(15)12-17/h2-7,9-10,12,14,18-19,24,32H,8,11,13H2,1H3,(H,25,30)(H,26,29)/t14-,18-,19-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102469
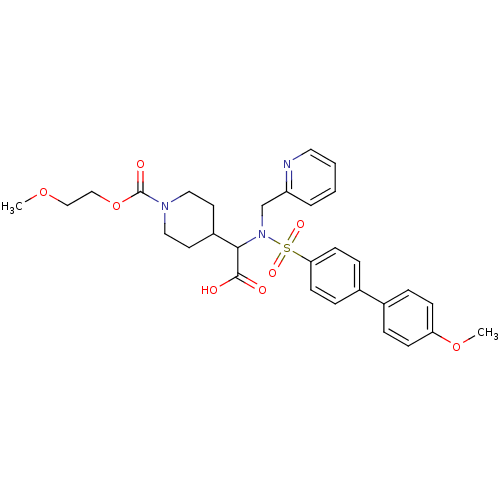
(4-{Carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-pyrid...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(Cc1ccccn1)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C30H35N3O8S/c1-39-19-20-41-30(36)32-17-14-24(15-18-32)28(29(34)35)33(21-25-5-3-4-16-31-25)42(37,38)27-12-8-23(9-13-27)22-6-10-26(40-2)11-7-22/h3-13,16,24,28H,14-15,17-21H2,1-2H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199762
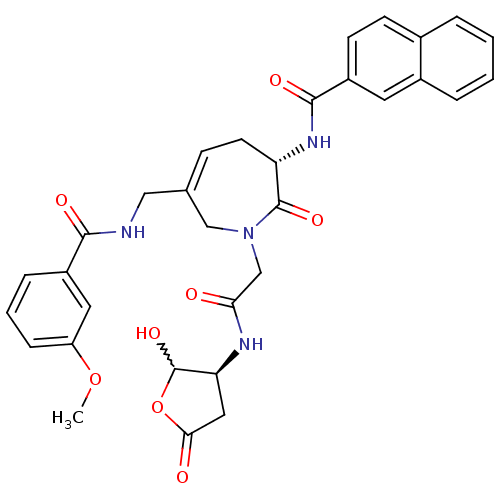
(CHEMBL411178 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1cccc(c1)C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-24-8-4-7-22(14-24)29(39)33-16-19-9-12-25(35-30(40)23-11-10-20-5-2-3-6-21(20)13-23)31(41)36(17-19)18-27(37)34-26-15-28(38)44-32(26)42/h2-11,13-14,25-26,32,42H,12,15-18H2,1H3,(H,33,39)(H,34,37)(H,35,40)/t25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175348

((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C32H34N4O8S/c37-28-17-26(32(41)44-28)34-30(39)27-19-35(45(42,43)24-11-2-1-3-12-24)18-23-10-6-7-13-25(31(40)36(23)27)33-29(38)22-15-14-20-8-4-5-9-21(20)16-22/h1-5,8-9,11-12,14-16,23,25-27,32,41H,6-7,10,13,17-19H2,(H,33,38)(H,34,39)/t23-,25-,26-,27-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175326
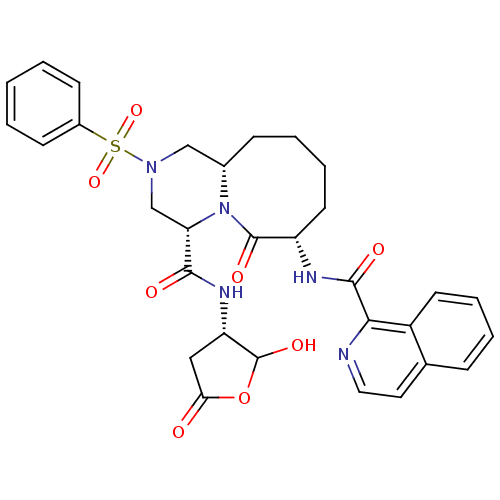
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C31H33N5O8S/c37-26-16-24(31(41)44-26)34-28(38)25-18-35(45(42,43)21-10-2-1-3-11-21)17-20-9-5-7-13-23(30(40)36(20)25)33-29(39)27-22-12-6-4-8-19(22)14-15-32-27/h1-4,6,8,10-12,14-15,20,23-25,31,41H,5,7,9,13,16-18H2,(H,33,39)(H,34,38)/t20-,23-,24-,25-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175327
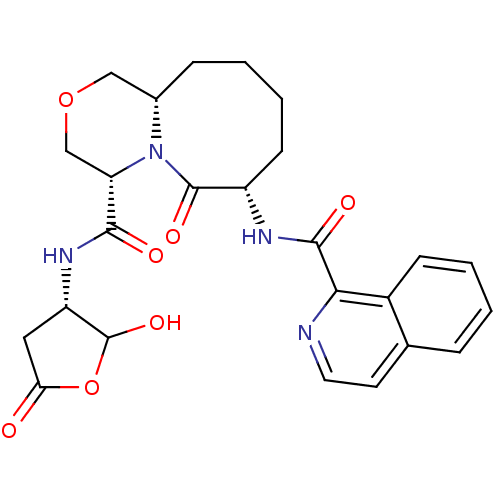
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 Show InChI InChI=1S/C25H28N4O7/c30-20-11-18(25(34)36-20)28-22(31)19-13-35-12-15-6-2-4-8-17(24(33)29(15)19)27-23(32)21-16-7-3-1-5-14(16)9-10-26-21/h1,3,5,7,9-10,15,17-19,25,34H,2,4,6,8,11-13H2,(H,27,32)(H,28,31)/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175340
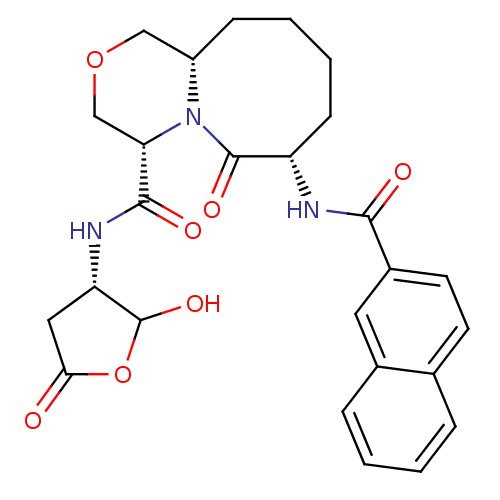
((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12 Show InChI InChI=1S/C26H29N3O7/c30-22-12-20(26(34)36-22)28-24(32)21-14-35-13-18-7-3-4-8-19(25(33)29(18)21)27-23(31)17-10-9-15-5-1-2-6-16(15)11-17/h1-2,5-6,9-11,18-21,26,34H,3-4,7-8,12-14H2,(H,27,31)(H,28,32)/t18-,19-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175323
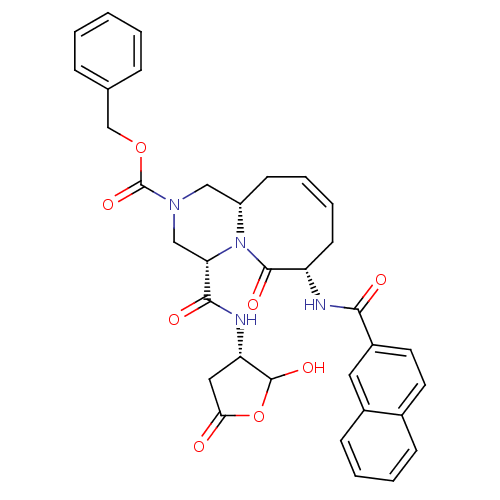
((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C34H34N4O8/c39-29-17-27(33(43)46-29)36-31(41)28-19-37(34(44)45-20-21-8-2-1-3-9-21)18-25-12-6-7-13-26(32(42)38(25)28)35-30(40)24-15-14-22-10-4-5-11-23(22)16-24/h1-11,14-16,25-28,33,43H,12-13,17-20H2,(H,35,40)(H,36,41)/b7-6-/t25-,26-,27-,28-,33?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102478

(4-{Carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-(2-me...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(CCOC)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C27H36N2O9S/c1-35-17-16-29(25(26(30)31)22-12-14-28(15-13-22)27(32)38-19-18-36-2)39(33,34)24-10-6-21(7-11-24)20-4-8-23(37-3)9-5-20/h4-11,22,25H,12-19H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102482

(4-[carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES CCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C23H28N2O7S/c1-3-32-23(28)25-14-12-18(13-15-25)21(22(26)27)24-33(29,30)20-10-6-17(7-11-20)16-4-8-19(31-2)9-5-16/h4-11,18,21,24H,3,12-15H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102476
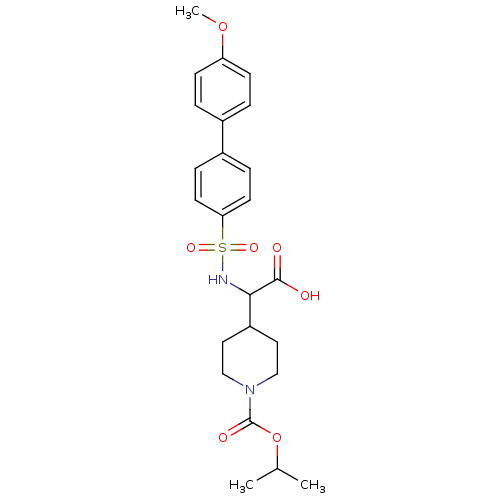
(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)OC(C)C)C(O)=O Show InChI InChI=1S/C24H30N2O7S/c1-16(2)33-24(29)26-14-12-19(13-15-26)22(23(27)28)25-34(30,31)21-10-6-18(7-11-21)17-4-8-20(32-3)9-5-17/h4-11,16,19,22,25H,12-15H2,1-3H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175328

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3ccccc3)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C30H32N4O8/c35-25-15-23(29(39)42-25)32-27(37)24-17-33(30(40)41-18-19-9-3-1-4-10-19)16-21-13-7-8-14-22(28(38)34(21)24)31-26(36)20-11-5-2-6-12-20/h1-12,21-24,29,39H,13-18H2,(H,31,36)(H,32,37)/b8-7-/t21-,22-,23-,24-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175329
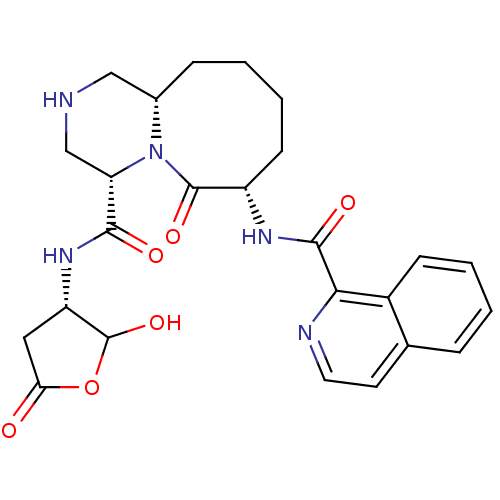
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CNC[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 Show InChI InChI=1S/C25H29N5O6/c31-20-11-18(25(35)36-20)29-22(32)19-13-26-12-15-6-2-4-8-17(24(34)30(15)19)28-23(33)21-16-7-3-1-5-14(16)9-10-27-21/h1,3,5,7,9-10,15,17-19,25-26,35H,2,4,6,8,11-13H2,(H,28,33)(H,29,32)/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175338

((4S,7S,11aS)-7-(benzo[b]thiophene-2-carboxamido)-N...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2CCCC[C@H](NC(=O)c3cc4ccccc4s3)C(=O)N12 Show InChI InChI=1S/C24H27N3O7S/c28-20-10-16(24(32)34-20)26-21(29)17-12-33-11-14-6-2-3-7-15(23(31)27(14)17)25-22(30)19-9-13-5-1-4-8-18(13)35-19/h1,4-5,8-9,14-17,24,32H,2-3,6-7,10-12H2,(H,25,30)(H,26,29)/t14-,15-,16-,17-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175337

((4S,7S,11aS)-benzyl 4-(((3S)-2-hydroxy-5-oxo-tetra...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12)C(=O)OCc1ccccc1 |c:17| Show InChI InChI=1S/C33H33N5O8/c39-27-16-25(32(43)46-27)36-29(40)26-18-37(33(44)45-19-20-8-2-1-3-9-20)17-22-11-5-7-13-24(31(42)38(22)26)35-30(41)28-23-12-6-4-10-21(23)14-15-34-28/h1-10,12,14-15,22,24-26,32,43H,11,13,16-19H2,(H,35,41)(H,36,40)/b7-5-/t22-,24-,25-,26-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175335

((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CNC[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N12 Show InChI InChI=1S/C26H30N4O6/c31-22-12-20(26(35)36-22)29-24(33)21-14-27-13-18-7-3-4-8-19(25(34)30(18)21)28-23(32)17-10-9-15-5-1-2-6-16(15)11-17/h1-2,5-6,9-11,18-21,26-27,35H,3-4,7-8,12-14H2,(H,28,32)(H,29,33)/t18-,19-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175347
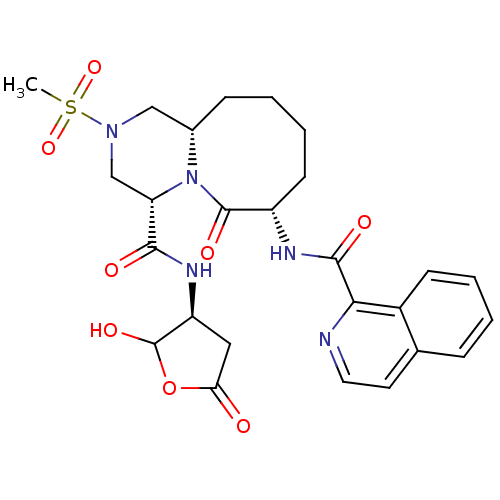
((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES CS(=O)(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3nccc4ccccc34)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C26H31N5O8S/c1-40(37,38)30-13-16-7-3-5-9-18(28-24(34)22-17-8-4-2-6-15(17)10-11-27-22)25(35)31(16)20(14-30)23(33)29-19-12-21(32)39-26(19)36/h2,4,6,8,10-11,16,18-20,26,36H,3,5,7,9,12-14H2,1H3,(H,28,34)(H,29,33)/t16-,18-,19-,20-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175346
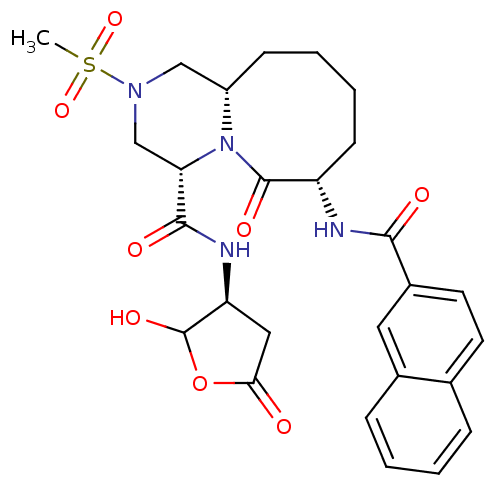
((4S,7S,11aS)-7-(2-naphthamido)-N-((3S)-2-hydroxy-5...)Show SMILES CS(=O)(=O)N1C[C@@H]2CCCC[C@H](NC(=O)c3ccc4ccccc4c3)C(=O)N2[C@@H](C1)C(=O)N[C@H]1CC(=O)OC1O Show InChI InChI=1S/C27H32N4O8S/c1-40(37,38)30-14-19-8-4-5-9-20(28-24(33)18-11-10-16-6-2-3-7-17(16)12-18)26(35)31(19)22(15-30)25(34)29-21-13-23(32)39-27(21)36/h2-3,6-7,10-12,19-22,27,36H,4-5,8-9,13-15H2,1H3,(H,28,33)(H,29,34)/t19-,20-,21-,22-,27?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102473
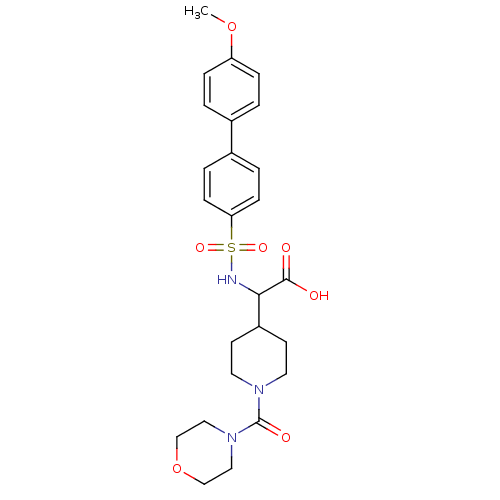
((4'-methoxy-biphenyl-4-sulfonylamino)-[1-(morpholi...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)N1CCOCC1)C(O)=O Show InChI InChI=1S/C25H31N3O7S/c1-34-21-6-2-18(3-7-21)19-4-8-22(9-5-19)36(32,33)26-23(24(29)30)20-10-12-27(13-11-20)25(31)28-14-16-35-17-15-28/h2-9,20,23,26H,10-17H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102468

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM30359

(BOC-piperidinyl glycine derivative, 34)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O |r| Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102476

(4-[Carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)OC(C)C)C(O)=O Show InChI InChI=1S/C24H30N2O7S/c1-16(2)33-24(29)26-14-12-19(13-15-26)22(23(27)28)25-34(30,31)21-10-6-18(7-11-21)17-4-8-20(32-3)9-5-17/h4-11,16,19,22,25H,12-15H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102479

(4-[Carboxy-(4'-methylsulfanyl-biphenyl-4-sulfonyla...)Show SMILES COCCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(SC)cc1)C(O)=O Show InChI InChI=1S/C24H30N2O7S2/c1-32-15-16-33-24(29)26-13-11-19(12-14-26)22(23(27)28)25-35(30,31)21-9-5-18(6-10-21)17-3-7-20(34-2)8-4-17/h3-10,19,22,25H,11-16H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102473

((4'-methoxy-biphenyl-4-sulfonylamino)-[1-(morpholi...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)N1CCOCC1)C(O)=O Show InChI InChI=1S/C25H31N3O7S/c1-34-21-6-2-18(3-7-21)19-4-8-22(9-5-19)36(32,33)26-23(24(29)30)20-10-12-27(13-11-20)25(31)28-14-16-35-17-15-28/h2-9,20,23,26H,10-17H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50189360

((1S,10S)-9-[(Isoquinoline-1-carbonyl)-amino]-6,10-...)Show SMILES CCO[C@@H]1OC(=O)C[C@@H]1NC(=O)[C@@H]1CCCN2N1C(=O)[C@H](CCC2=O)NC(=O)c1nccc2ccccc12 Show InChI InChI=1S/C26H29N5O7/c1-2-37-26-18(14-21(33)38-26)29-23(34)19-8-5-13-30-20(32)10-9-17(25(36)31(19)30)28-24(35)22-16-7-4-3-6-15(16)11-12-27-22/h3-4,6-7,11-12,17-19,26H,2,5,8-10,13-14H2,1H3,(H,28,35)(H,29,34)/t17-,18-,19-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter& Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of caspase-1 using fluorogenic substrate and BMG Fluostar plate reader for 30 min at 37 degree C |
Bioorg Med Chem Lett 15: 4291-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.050
BindingDB Entry DOI: 10.7270/Q2J103ZQ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199763

(CHEMBL397184 | N-((S,Z)-6-(benzamidomethyl)-1-(2-(...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)CN1CC(CNC(=O)c2ccccc2)=CC[C@H](NC(=O)c2ccc3ccccc3c2)C1=O |w:1.0,c:25| Show InChI InChI=1S/C31H30N4O7/c36-26(33-25-15-27(37)42-31(25)41)18-35-17-19(16-32-28(38)21-7-2-1-3-8-21)10-13-24(30(35)40)34-29(39)23-12-11-20-6-4-5-9-22(20)14-23/h1-12,14,24-25,31,41H,13,15-18H2,(H,32,38)(H,33,36)(H,34,39)/t24-,25-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193736
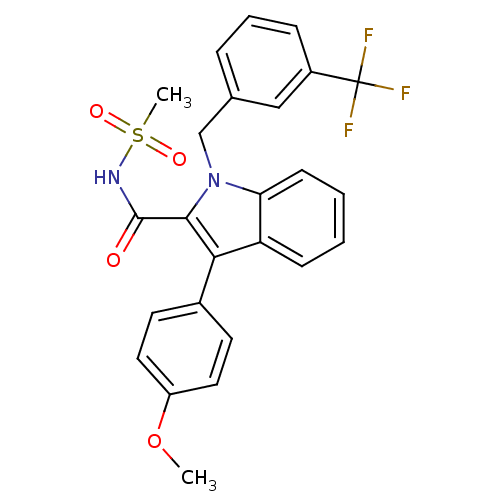
(1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(C)(=O)=O)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C25H21F3N2O4S/c1-34-19-12-10-17(11-13-19)22-20-8-3-4-9-21(20)30(23(22)24(31)29-35(2,32)33)15-16-6-5-7-18(14-16)25(26,27)28/h3-14H,15H2,1-2H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175345

((4S,7S,11aS)-7-benzamido-N-((3S)-2-hydroxy-5-oxo-t...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1CN(C[C@@H]2CCCC[C@H](NC(=O)c3ccccc3)C(=O)N12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H32N4O8S/c33-24-15-22(28(37)40-24)30-26(35)23-17-31(41(38,39)20-12-5-2-6-13-20)16-19-11-7-8-14-21(27(36)32(19)23)29-25(34)18-9-3-1-4-10-18/h1-6,9-10,12-13,19,21-23,28,37H,7-8,11,14-17H2,(H,29,34)(H,30,35)/t19-,21-,22-,23-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50199750

(CHEMBL243823 | N-((S,Z)-1-(2-((3S)-2-hydroxy-5-oxo...)Show SMILES COc1ccccc1C(=O)NCC1=CC[C@H](NC(=O)c2ccc3ccccc3c2)C(=O)N(CC(=O)N[C@H]2CC(=O)OC2O)C1 |w:41.45,t:13| Show InChI InChI=1S/C32H32N4O8/c1-43-26-9-5-4-8-23(26)30(40)33-16-19-10-13-24(35-29(39)22-12-11-20-6-2-3-7-21(20)14-22)31(41)36(17-19)18-27(37)34-25-15-28(38)44-32(25)42/h2-12,14,24-25,32,42H,13,15-18H2,1H3,(H,33,40)(H,34,37)(H,35,39)/t24-,25-,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ICE |
Bioorg Med Chem 15: 1311-22 (2007)
Article DOI: 10.1016/j.bmc.2006.11.011
BindingDB Entry DOI: 10.7270/Q2GF0T5M |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102474

(4-{carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-methy...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(C)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C25H32N2O8S/c1-26(23(24(28)29)20-12-14-27(15-13-20)25(30)35-17-16-33-2)36(31,32)22-10-6-19(7-11-22)18-4-8-21(34-3)9-5-18/h4-11,20,23H,12-17H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102478

(4-{Carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-(2-me...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(CCOC)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C27H36N2O9S/c1-35-17-16-29(25(26(30)31)22-12-14-28(15-13-22)27(32)38-19-18-36-2)39(33,34)24-10-6-21(7-11-24)20-4-8-23(37-3)9-5-20/h4-11,22,25H,12-19H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102482

(4-[carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES CCOC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C23H28N2O7S/c1-3-32-23(28)25-14-12-18(13-15-25)21(22(26)27)24-33(29,30)20-10-6-17(7-11-20)16-4-8-19(31-2)9-5-16/h4-11,18,21,24H,3,12-15H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50102475
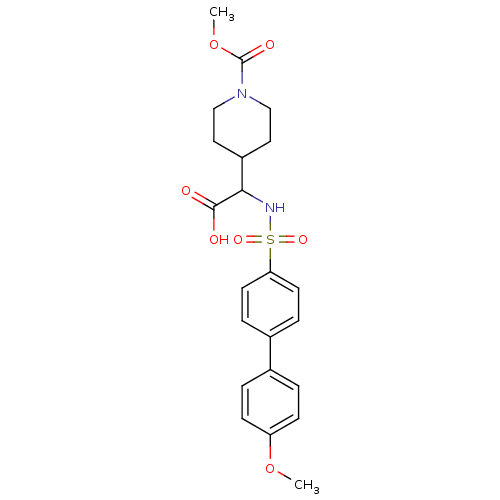
(4-[carboxy-(4'-methoxy-biphenyl-4-sulfonylamino)-m...)Show SMILES COC(=O)N1CCC(CC1)C(NS(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H26N2O7S/c1-30-18-7-3-15(4-8-18)16-5-9-19(10-6-16)32(28,29)23-20(21(25)26)17-11-13-24(14-12-17)22(27)31-2/h3-10,17,20,23H,11-14H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-2 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50102473

((4'-methoxy-biphenyl-4-sulfonylamino)-[1-(morpholi...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NC(C1CCN(CC1)C(=O)N1CCOCC1)C(O)=O Show InChI InChI=1S/C25H31N3O7S/c1-34-21-6-2-18(3-7-21)19-4-8-22(9-5-19)36(32,33)26-23(24(29)30)20-10-12-27(13-11-20)25(31)28-14-16-35-17-15-28/h2-9,20,23,26H,10-17H2,1H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193748

(1-(3-(trifluoromethyl)benzyl)-3-(4-methoxyphenyl)-...)Show SMILES COc1ccc(cc1)-c1c(C(=O)NS(=O)(=O)c2ccccc2)n(Cc2cccc(c2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C30H23F3N2O4S/c1-39-23-16-14-21(15-17-23)27-25-12-5-6-13-26(25)35(19-20-8-7-9-22(18-20)30(31,32)33)28(27)29(36)34-40(37,38)24-10-3-2-4-11-24/h2-18H,19H2,1H3,(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50102469

(4-{Carboxy-[(4'-methoxy-biphenyl-4-sulfonyl)-pyrid...)Show SMILES COCCOC(=O)N1CCC(CC1)C(N(Cc1ccccn1)S(=O)(=O)c1ccc(cc1)-c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C30H35N3O8S/c1-39-19-20-41-30(36)32-17-14-24(15-18-32)28(29(34)35)33(21-25-5-3-4-16-31-25)42(37,38)27-12-8-23(9-13-27)22-6-10-26(40-2)11-7-22/h3-13,16,24,28H,14-15,17-21H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-13 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50175342

((4S,7S,11aS)-N-((3S)-2-hydroxy-5-oxo-tetrahydrofur...)Show SMILES OC1OC(=O)C[C@@H]1NC(=O)[C@@H]1COC[C@@H]2C\C=C/C[C@H](NC(=O)c3nccc4ccccc34)C(=O)N12 |c:17| Show InChI InChI=1S/C25H26N4O7/c30-20-11-18(25(34)36-20)28-22(31)19-13-35-12-15-6-2-4-8-17(24(33)29(15)19)27-23(32)21-16-7-3-1-5-14(16)9-10-26-21/h1-5,7,9-10,15,17-19,25,34H,6,8,11-13H2,(H,27,32)(H,28,31)/b4-2-/t15-,17-,18-,19-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Caspase 1 |
Bioorg Med Chem Lett 15: 5434-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.109
BindingDB Entry DOI: 10.7270/Q21G0KTJ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50369834
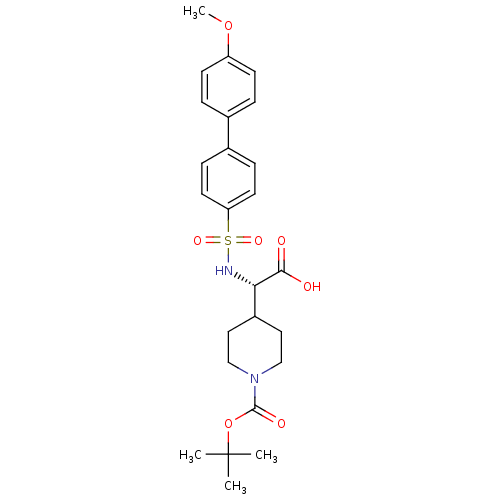
(CHEMBL402745)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C1CCN(CC1)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C25H32N2O7S/c1-25(2,3)34-24(30)27-15-13-19(14-16-27)22(23(28)29)26-35(31,32)21-11-7-18(8-12-21)17-5-9-20(33-4)10-6-17/h5-12,19,22,26H,13-16H2,1-4H3,(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 2499-502 (2001)
BindingDB Entry DOI: 10.7270/Q2Z89D47 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data