

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Homo sapiens (Human)) | BDBM50518241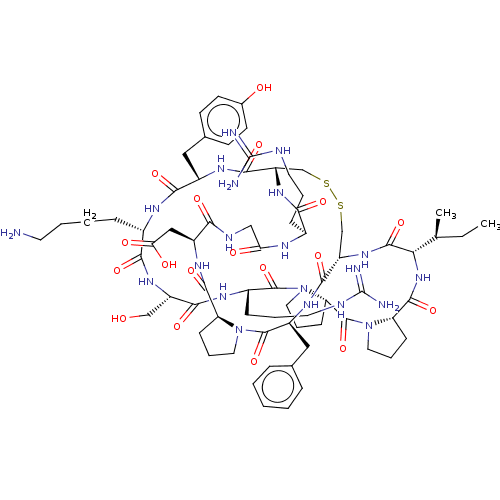 (CHEMBL4569923) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518240 (CHEMBL4439523) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038677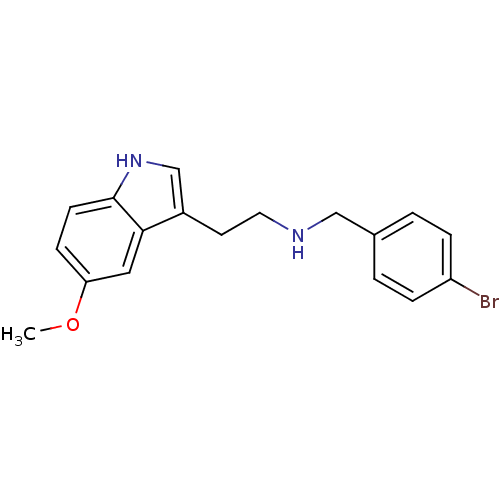 ((4-Bromo-benzyl)-[2-(5-methoxy-1H-indol-3-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518249 (CHEMBL4588827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518247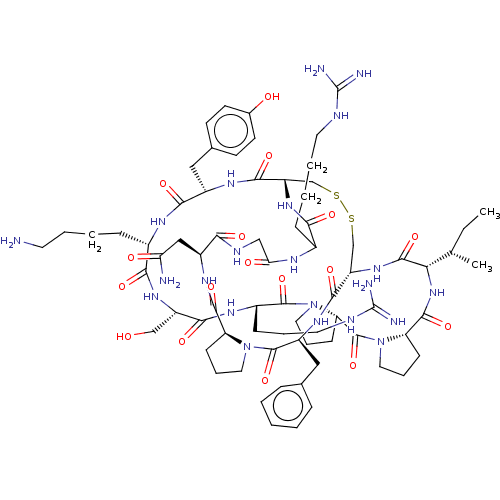 (CHEMBL4454304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM30712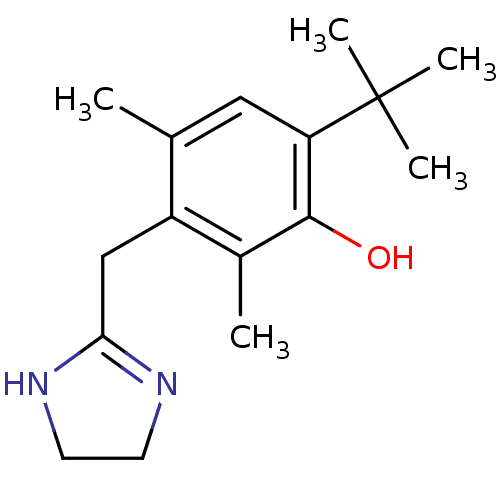 (6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1B receptor using [3H]-5-HT trifluoroacetate as radioligand | J Med Chem 41: 2243-51 (1998) Article DOI: 10.1021/jm970513p BindingDB Entry DOI: 10.7270/Q28K7872 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50151705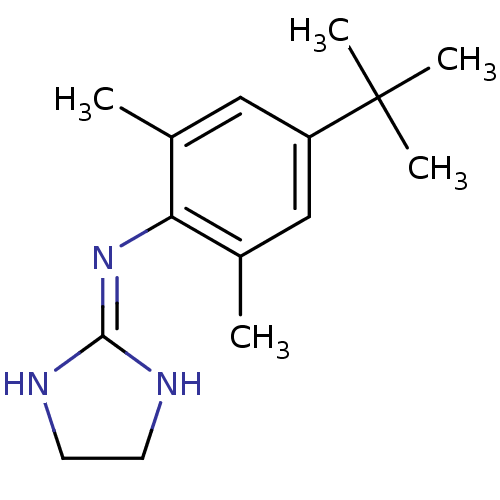 ((4-tert-Butyl-2,6-dimethyl-phenyl)-(4,5-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor | Bioorg Med Chem Lett 14: 4697-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.085 BindingDB Entry DOI: 10.7270/Q2FJ2G7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038672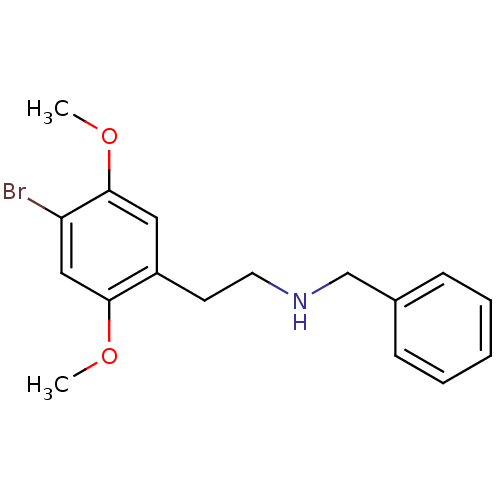 (Benzyl-[2-(4-bromo-2,5-dimethoxy-phenyl)-ethyl]-am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50421256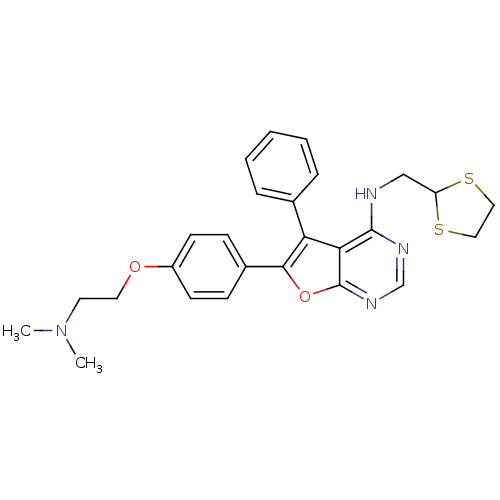 (CHEMBL2087874) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Inhibition of ACK1 kinase (unknown origin) | J Med Chem 58: 2746-63 (2015) Article DOI: 10.1021/jm501929n BindingDB Entry DOI: 10.7270/Q2H996XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM30712 (6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand | J Med Chem 41: 2243-51 (1998) Article DOI: 10.1021/jm970513p BindingDB Entry DOI: 10.7270/Q28K7872 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038665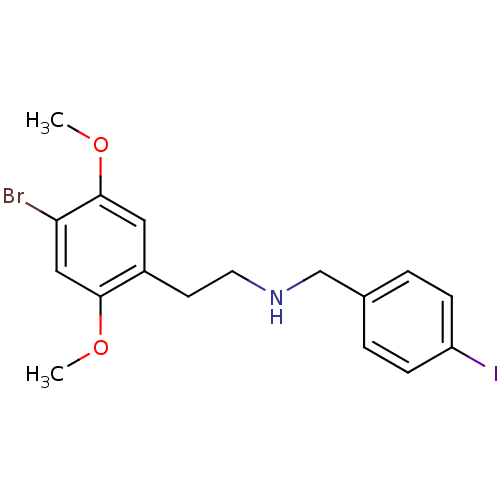 (CHEMBL57939 | [2-(4-Bromo-2,5-dimethoxy-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038675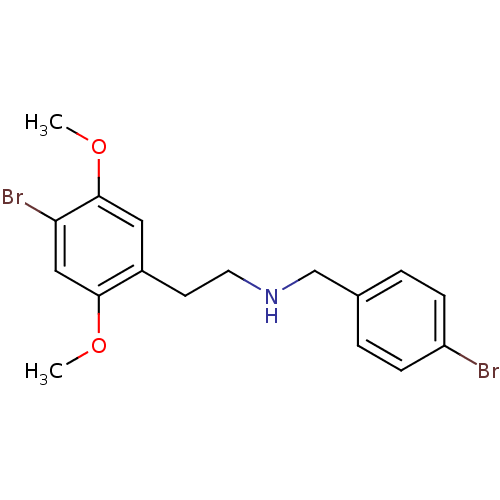 ((4-Bromo-benzyl)-[2-(4-bromo-2,5-dimethoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518253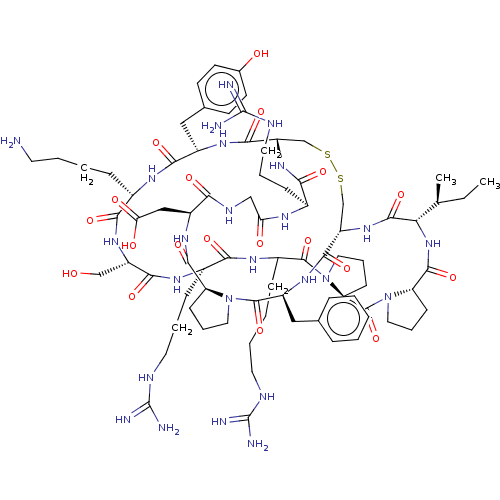 (CHEMBL4579797) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518252 (CHEMBL4592533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518255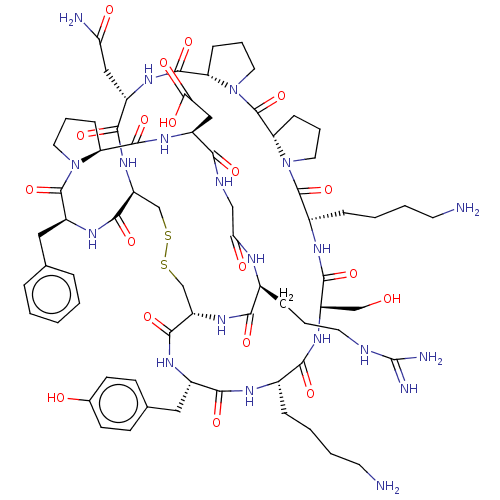 (CHEMBL4443353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518243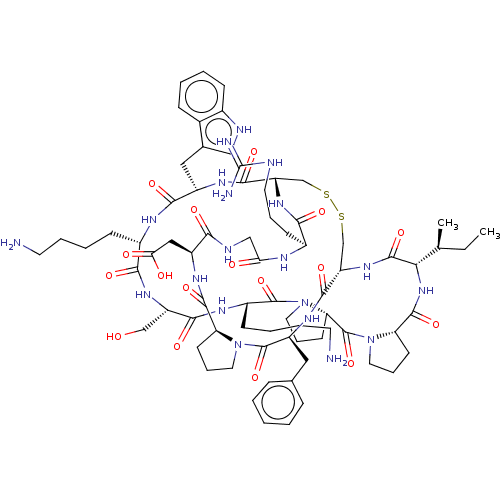 (CHEMBL4435567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM30703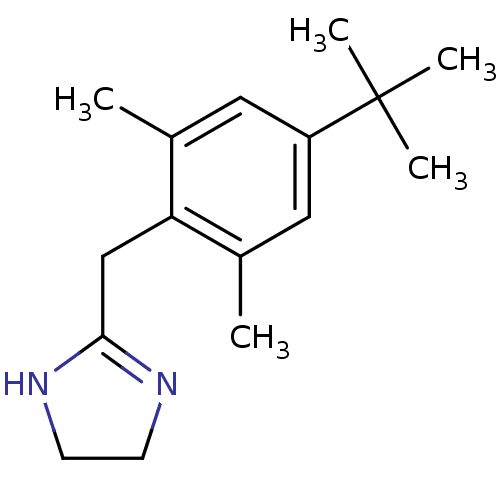 (2-(4-tert-butyl-2,6-dimethyl-benzyl)-2-imidazoline...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor | Bioorg Med Chem Lett 14: 4697-9 (2004) Article DOI: 10.1016/j.bmcl.2004.06.085 BindingDB Entry DOI: 10.7270/Q2FJ2G7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM30703 (2-(4-tert-butyl-2,6-dimethyl-benzyl)-2-imidazoline...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand | J Med Chem 41: 2243-51 (1998) Article DOI: 10.1021/jm970513p BindingDB Entry DOI: 10.7270/Q28K7872 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049091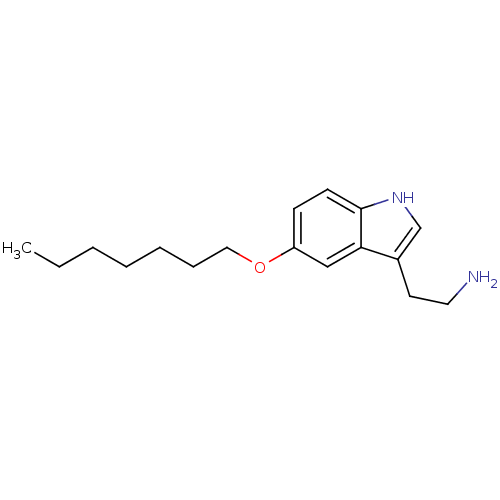 (2-(5-Heptyloxy-1H-indol-3-yl)-ethylamine | CHEMBL3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50005267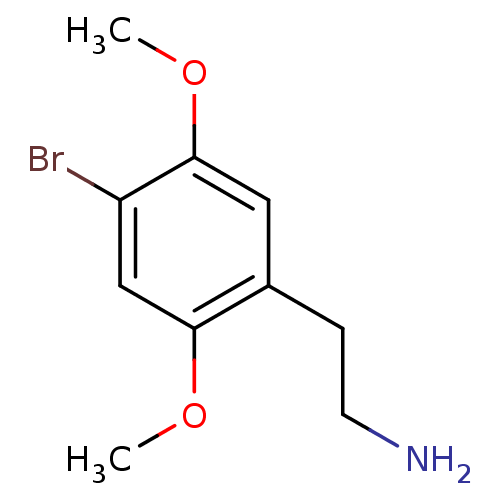 (2,5-dimethoxy-4-bromophenethylamine | 2-(4-Bromo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049093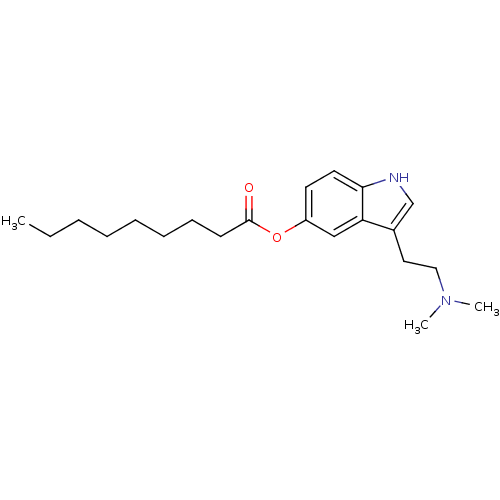 (CHEMBL321190 | Nonanoic acid 3-(2-dimethylamino-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50064806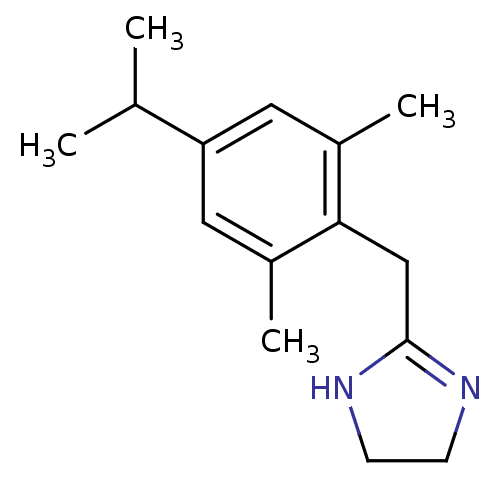 (2-(4-Isopropyl-2,6-dimethyl-benzyl)-4,5-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor using [3H]-5-HT trifluoroacetate as radioligand | J Med Chem 41: 2243-51 (1998) Article DOI: 10.1021/jm970513p BindingDB Entry DOI: 10.7270/Q28K7872 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518239 (CHEMBL4453437) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518245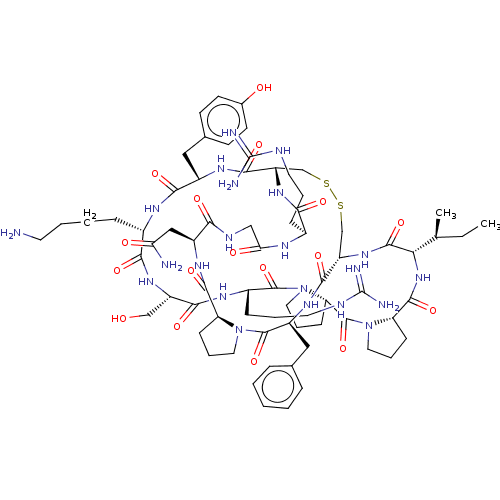 (CHEMBL4471930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50049085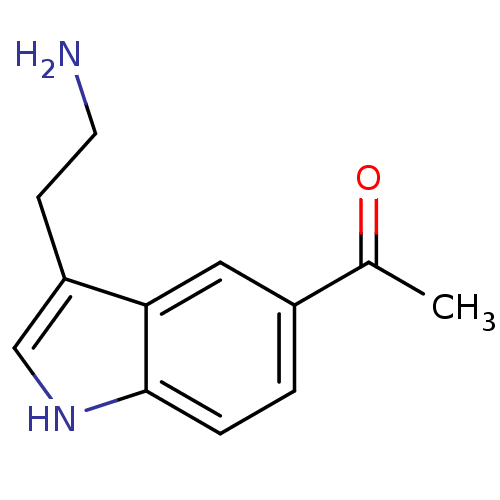 (1-[3-(2-Amino-ethyl)-1H-indol-5-yl]-ethanone | Ace...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049077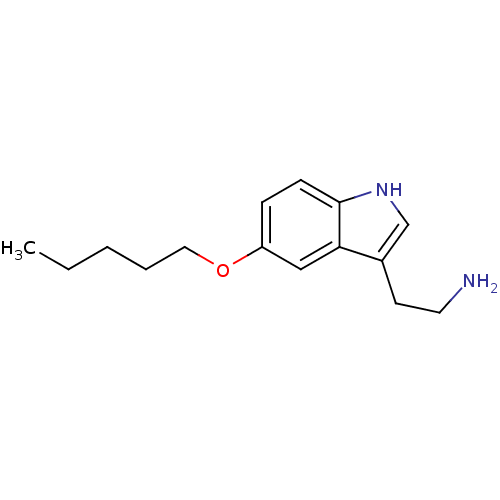 (2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049077 (2-(5-Pentyloxy-1H-indol-3-yl)-ethylamine | CHEMBL1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038675 ((4-Bromo-benzyl)-[2-(4-bromo-2,5-dimethoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (K) labeled with [3H]-ketanserin. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049082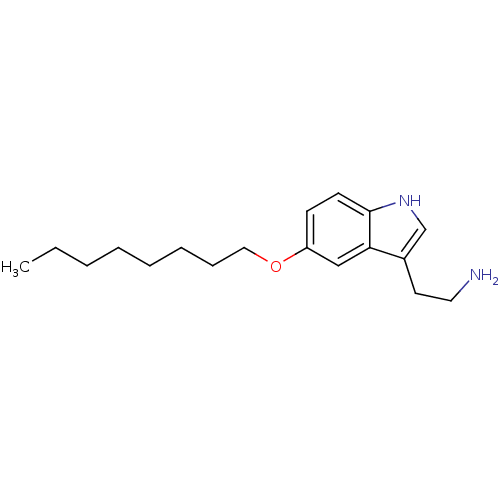 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518251 (CHEMBL4515559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518244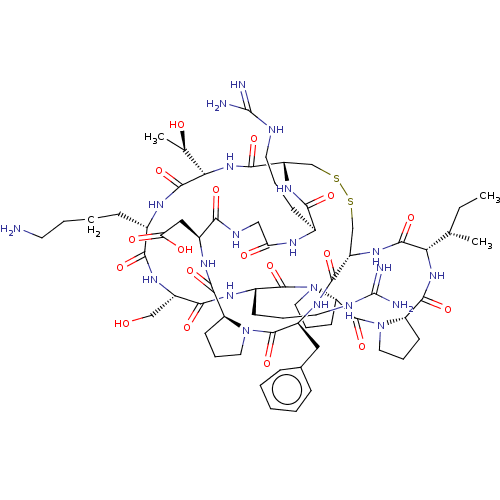 (CHEMBL4550948) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049081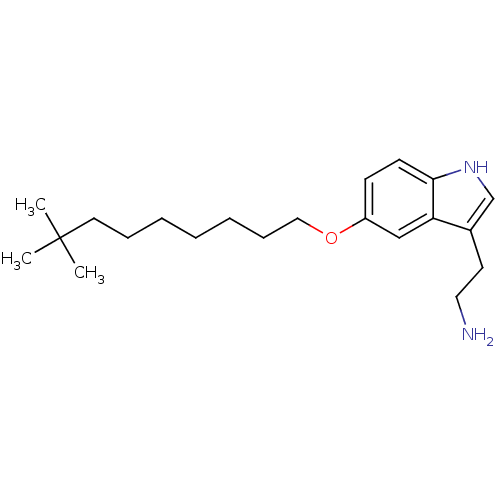 (2-[5-(8,8-Dimethyl-nonyloxy)-1H-indol-3-yl]-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049084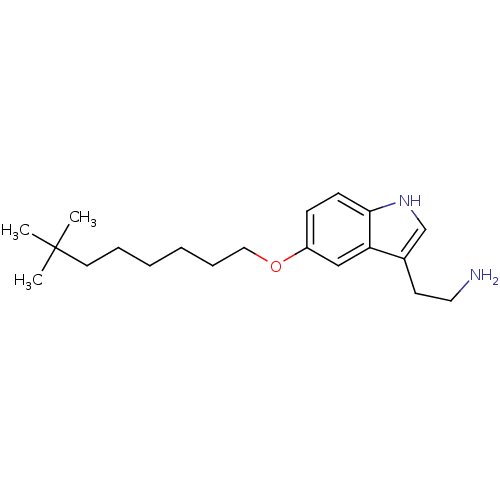 (2-[5-(7,7-Dimethyl-octyloxy)-1H-indol-3-yl]-ethyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518246 (CHEMBL4563203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038678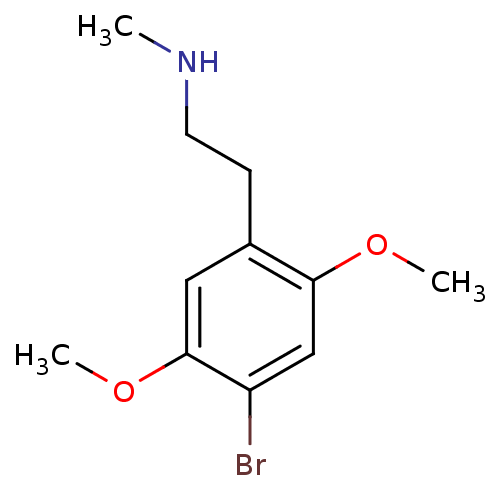 (CHEMBL57000 | [2-(4-Bromo-2,5-dimethoxy-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049082 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049082 (2-(5-Octyloxy-1H-indol-3-yl)-ethylamine | CHEMBL32...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM82087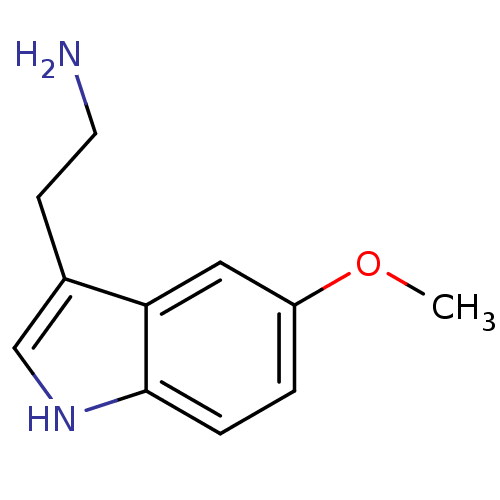 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affinity at human cloned 5-hydroxytryptamine 1A receptor by [3H]-8-OH-DPAT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038676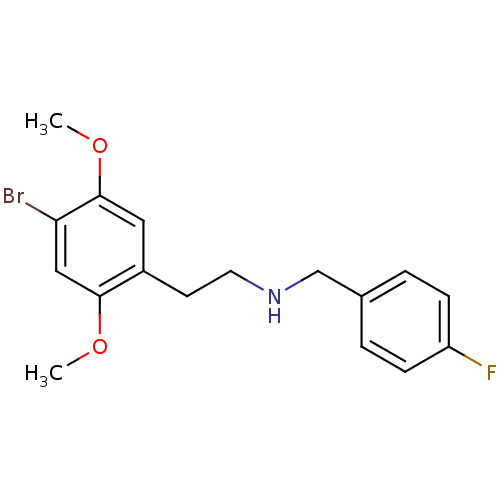 (CHEMBL58136 | [2-(4-Bromo-2,5-dimethoxy-phenyl)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of the compound towards cloned human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-8-OH-DPAT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038691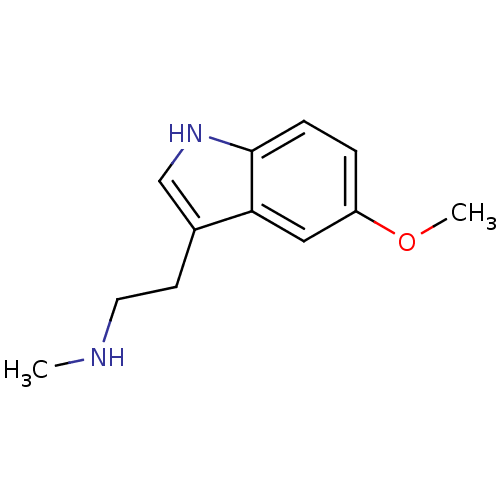 (CHEMBL58579 | [2-(5-Methoxy-1H-indol-3-yl)-ethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of the compound towards 5-hydroxytryptamine 1D receptor was determined in calf striatum homogenate | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50049089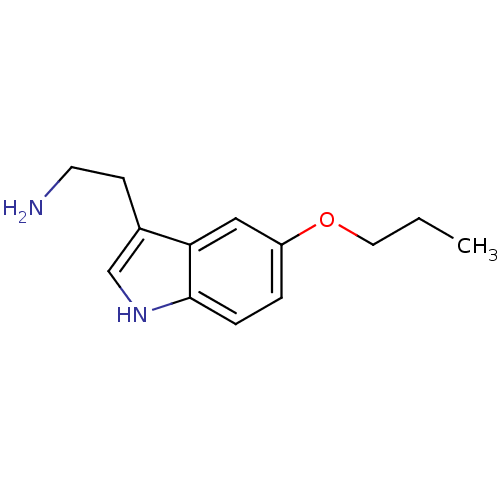 (2-(5-Propoxy-1H-indol-3-yl)-ethylamine | CHEMBL109...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity for recombinant human 5-hydroxytryptamine 1D receptor beta was determined using [3H]-5-HT as radioligand | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50518254 (CHEMBL4585149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human plasmin using Ac-RM(O2)YR-pNA as substrate after 30 mins | J Med Chem 62: 552-560 (2019) Article DOI: 10.1021/acs.jmedchem.8b01139 BindingDB Entry DOI: 10.7270/Q2MS3X3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM82087 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50049091 (2-(5-Heptyloxy-1H-indol-3-yl)-ethylamine | CHEMBL3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description In vitro affitnity human cloned 5-hydroxytryptamine 1D receptor alpha by [3H]-5-HT displacement. | J Med Chem 39: 314-22 (1996) Article DOI: 10.1021/jm950498t BindingDB Entry DOI: 10.7270/Q2DJ5DRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038677 ((4-Bromo-benzyl)-[2-(5-methoxy-1H-indol-3-yl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (K) labeled with [3H]-ketanserin. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50038679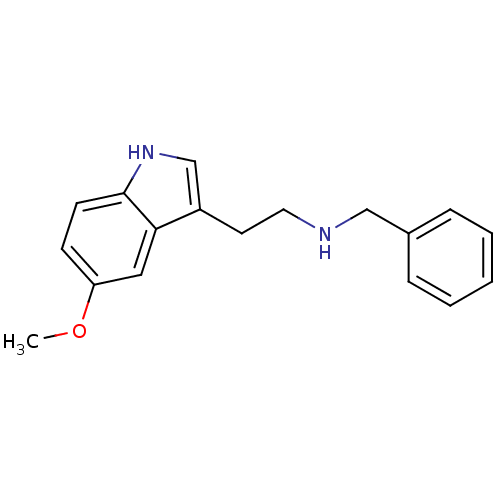 (Benzyl-[2-(5-methoxy-1H-indol-3-yl)-ethyl]-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. | J Med Chem 37: 1929-35 (1994) BindingDB Entry DOI: 10.7270/Q2GQ6WT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1353 total ) | Next | Last >> |