

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577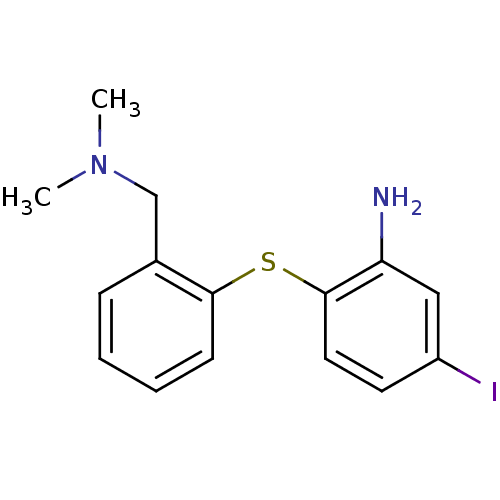 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110578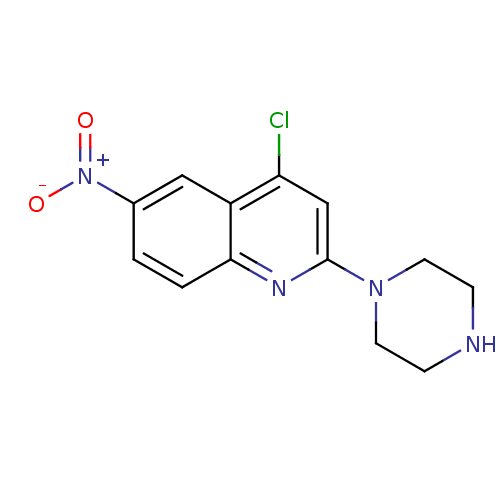 (4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266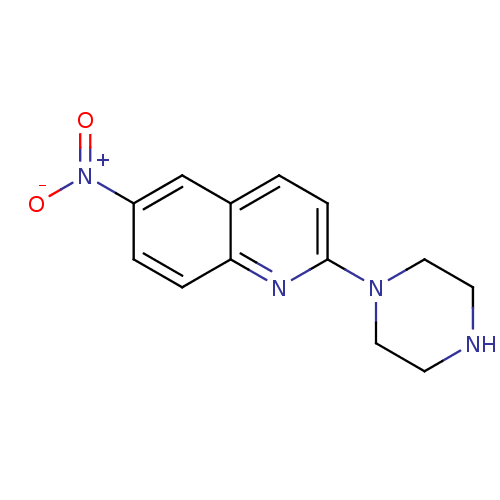 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110584 (3-(3-Fluoro-propyl)-6-nitro-2-piperazin-1-yl-quino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110574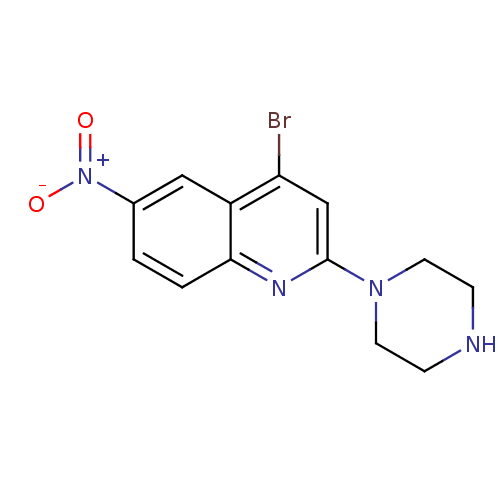 (4-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208770 (2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208769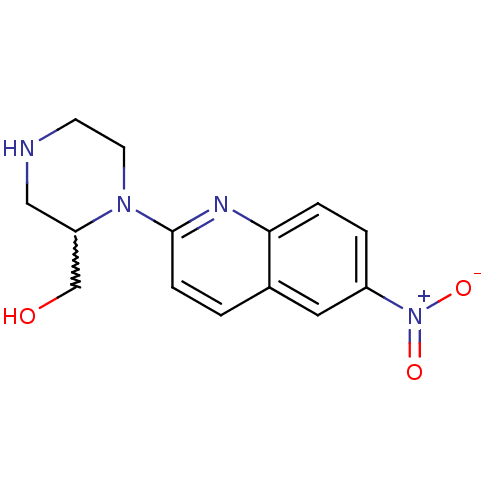 (2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208771 (2-[(2-methoxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090213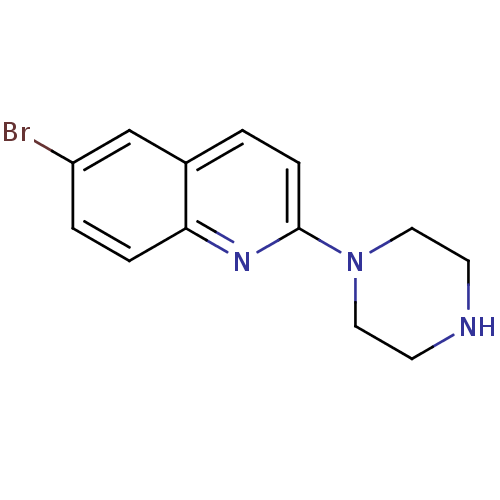 (6-Bromo-2-piperazin-1-yl-quinoline | CHEMBL39164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM25870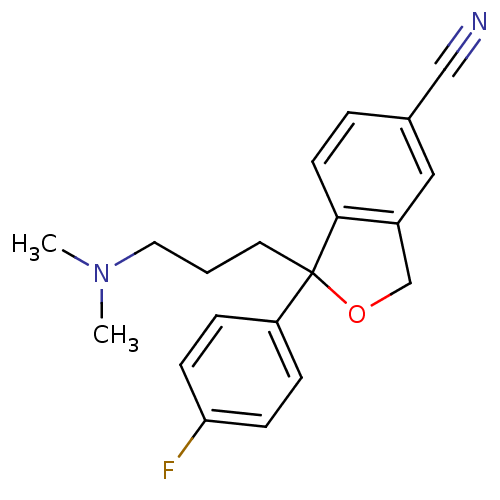 (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110579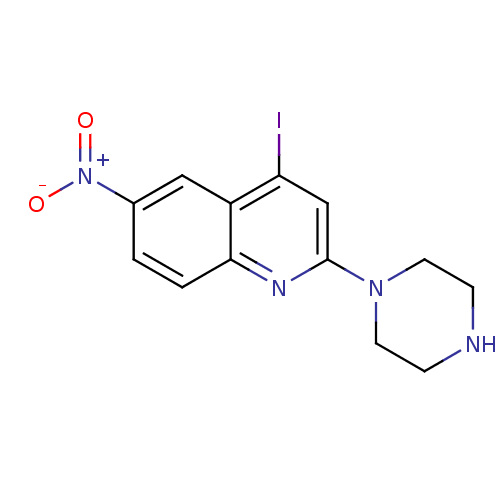 (4-Iodo-6-nitro-2-piperazin-1-yl-quinoline | CHEMBL...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110585 (4-(4-Bromo-6-nitro-quinolin-2-yl)-piperazine-1-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]citalopram binding to the rat cortical Serotonin transporter | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110587 (4-Allyl-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090216 (6-Chloro-2-piperazin-1-yl-quinoline | CHEMBL290537) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110583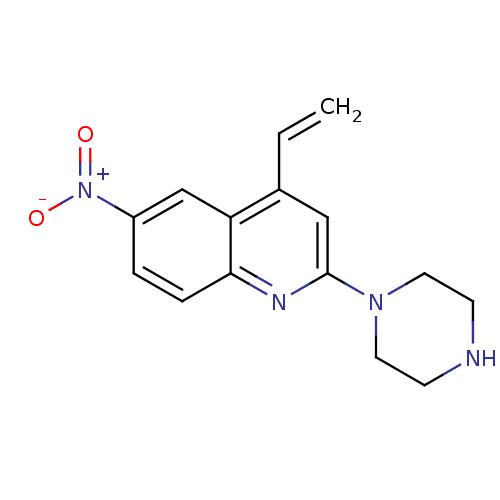 (6-Nitro-2-piperazin-1-yl-4-vinyl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090215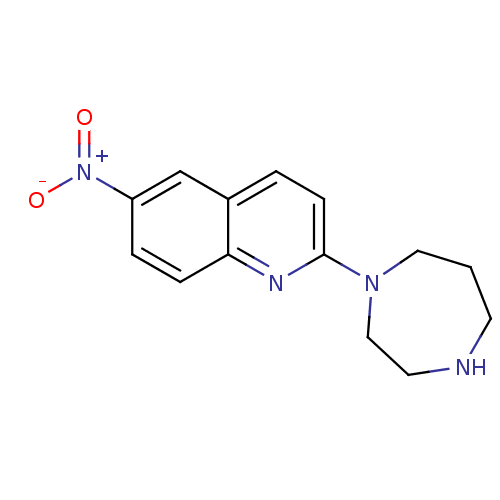 (2-[1,4]Diazepan-1-yl-6-nitro-quinoline | CHEMBL431...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070476 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,6-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110576 (4-Furan-2-yl-6-nitro-2-piperazin-1-yl-quinoline | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208772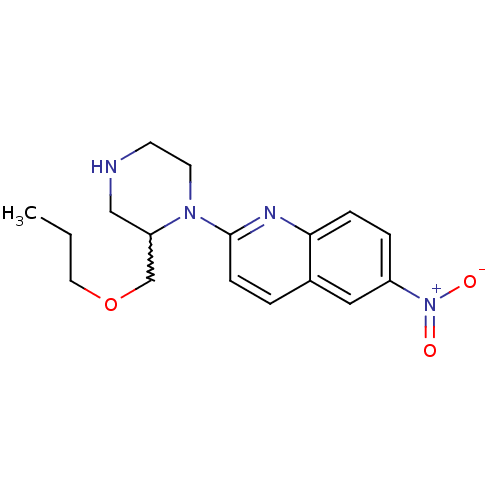 (6-nitro-2-(2-propoxymethylpiperazin-1-yl)quinoline...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090210 (6-Iodo-2-piperazin-1-yl-quinoline | CHEMBL38466) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090217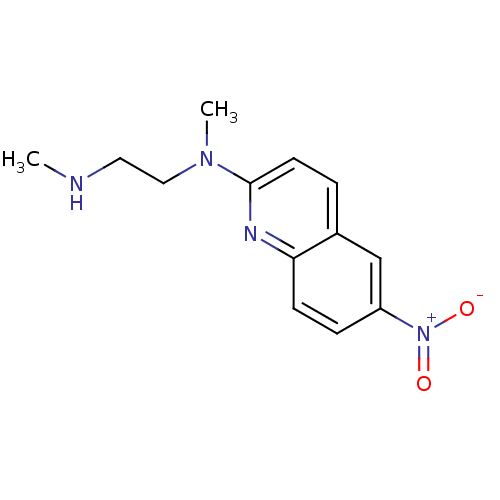 (CHEMBL39265 | N,N'-Dimethyl-N-(6-nitro-quinolin-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070473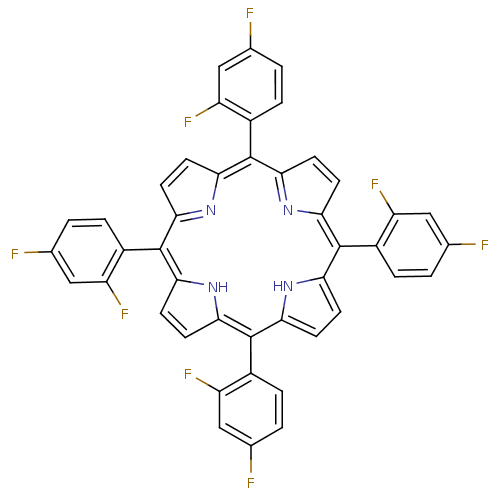 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,4-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090214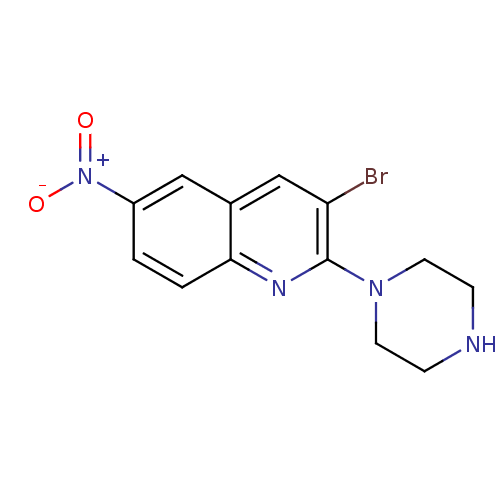 (3-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208768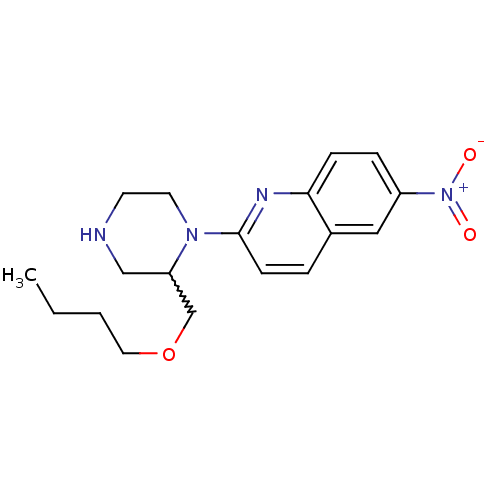 (2-[(2-butoxymethyl)piperazin-1-yl]-6-nitroquinolin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090211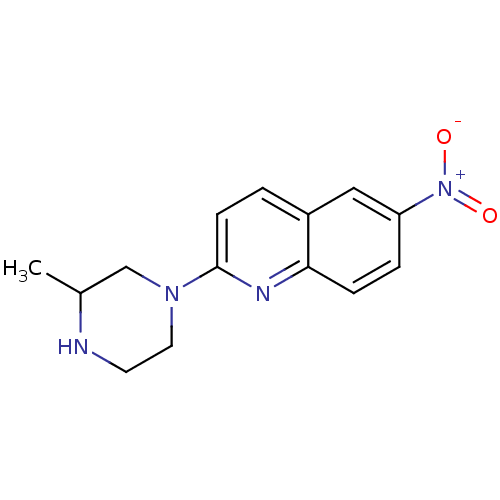 (2-(3-Methyl-piperazin-1-yl)-6-nitro-quinoline | CH...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110575 (6-Nitro-4-phenyl-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110586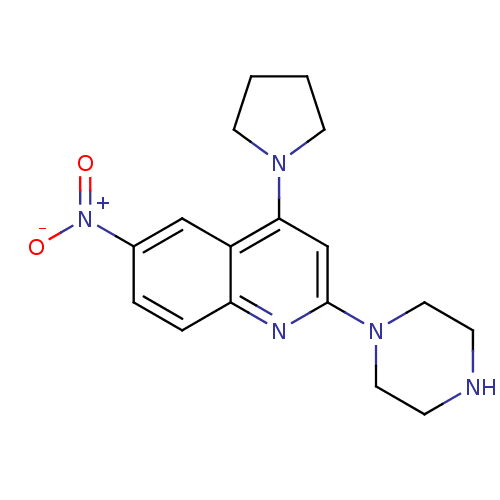 (6-Nitro-2-piperazin-1-yl-4-pyrrolidin-1-yl-quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50014407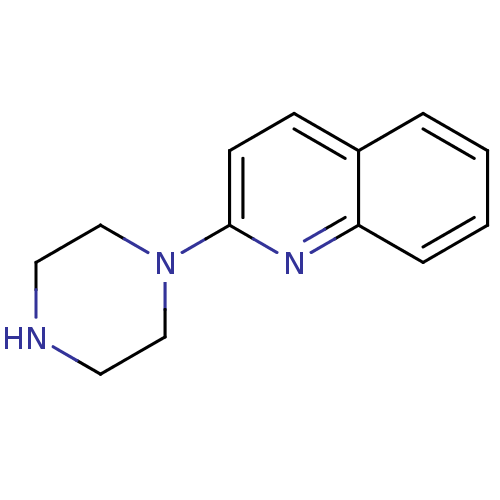 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110582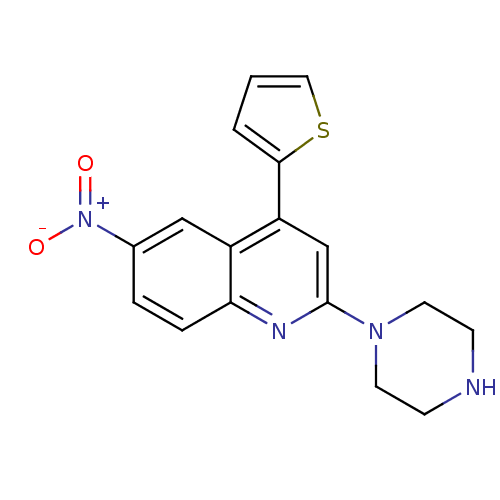 (6-Nitro-2-piperazin-1-yl-4-thiophen-2-yl-quinoline...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110580 (4-Benzyl-6-nitro-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090212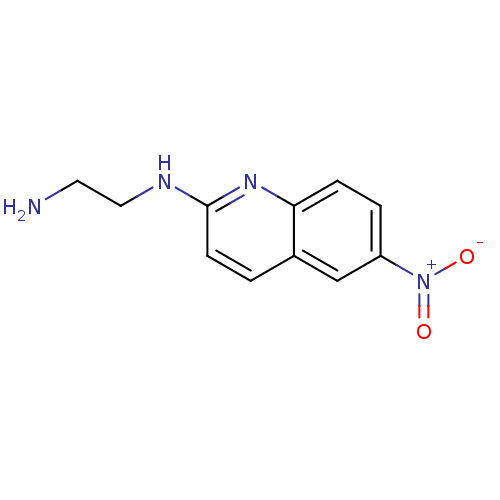 (CHEMBL38754 | N*1*-(6-Nitro-quinolin-2-yl)-ethane-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50090218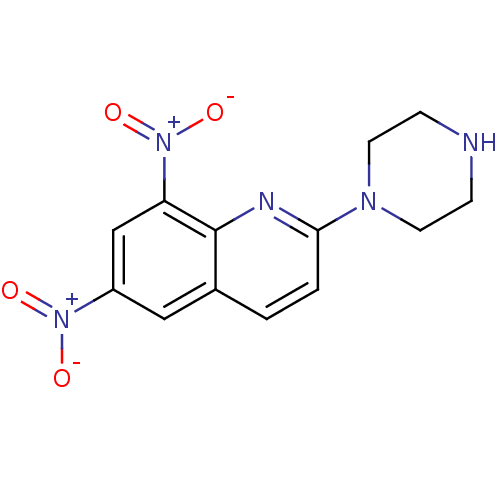 (6,8-Dinitro-2-piperazin-1-yl-quinoline | CHEMBL377...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089503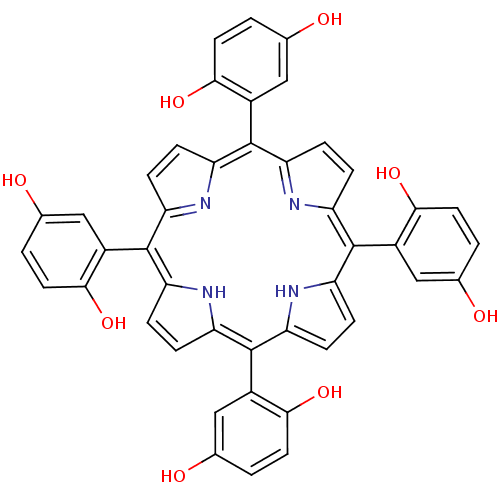 (2-[7,12,17-tri(2,5-hydroxyphenyl)-21,22,23,24-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089504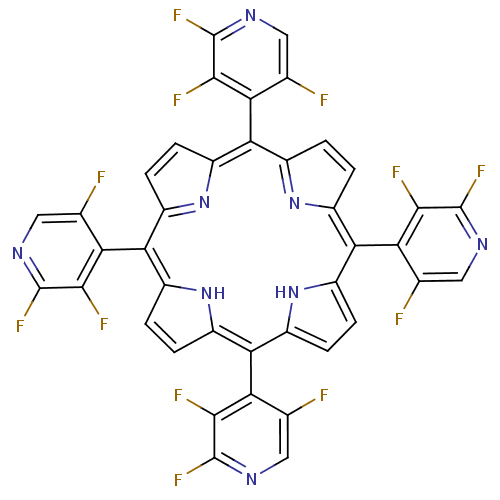 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,3,5-trifluor...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089505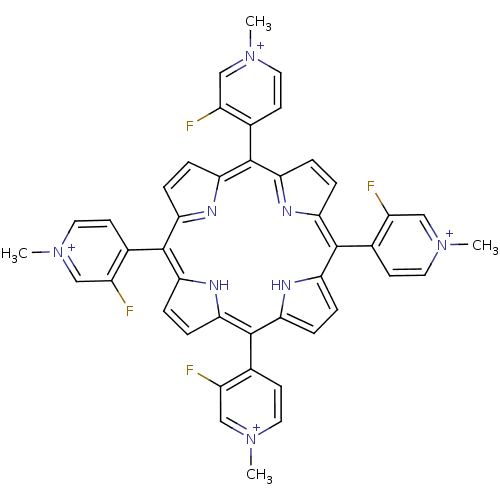 (2,7,12,17-tetra(3-fluoro-1-methyl-4-pyridiniumyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089507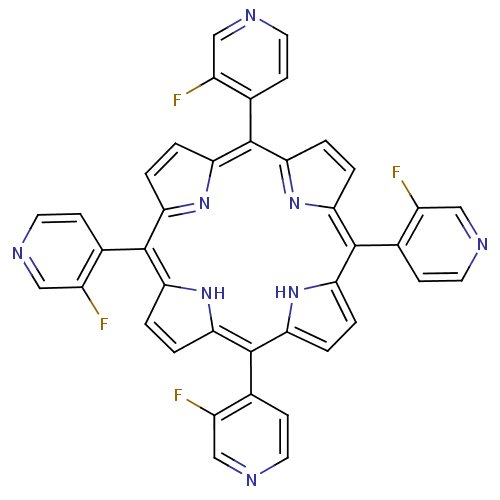 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(3-fluoro-pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089502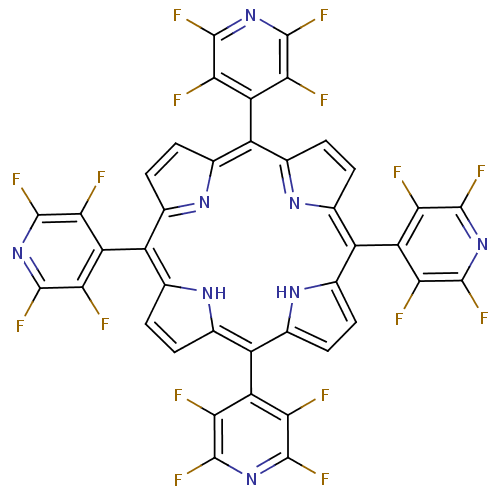 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,3,5,6-tetraf...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089510 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(3-fluoro-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070472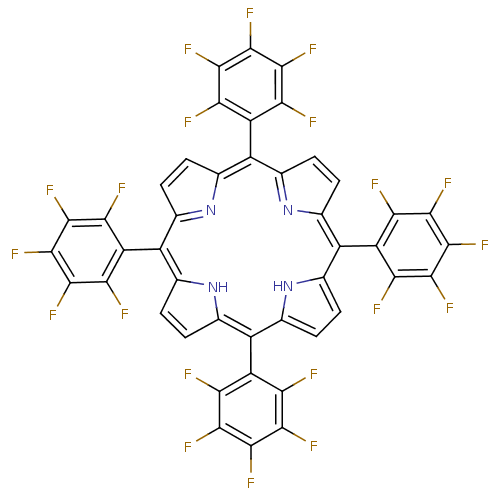 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-pentafluorophen...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089509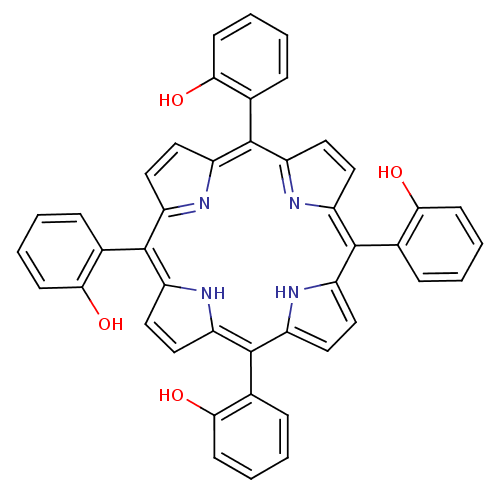 (2-[7,12,17-tri(2-hydroxyphenyl)-21,22,23,24-tetraa...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089508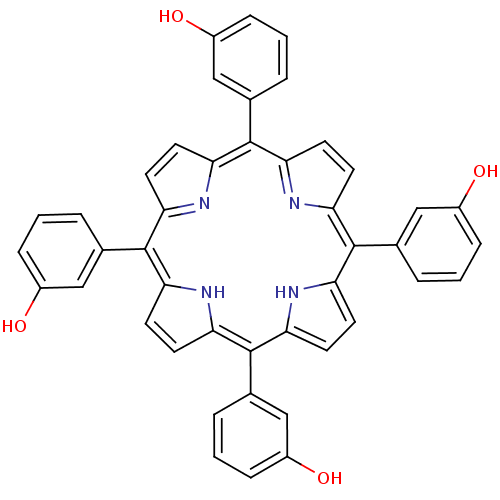 (2-[7,12,17-tri(3-hydroxyphenyl)-21,22,23,24-tetraa...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50089506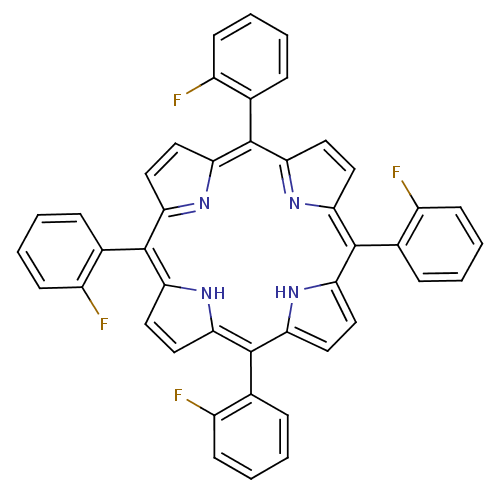 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2-fluoro-pheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585092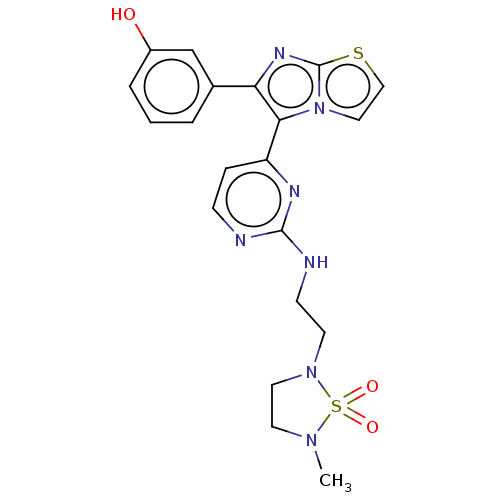 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 245 total ) | Next | Last >> |