

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin-like growth factor 1 receptor (Rattus norvegicus) | BDBM50318112 ((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of rat IGF1R | Bioorg Med Chem Lett 20: 3182-5 (2010) Article DOI: 10.1016/j.bmcl.2010.03.057 BindingDB Entry DOI: 10.7270/Q2GX4BQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048982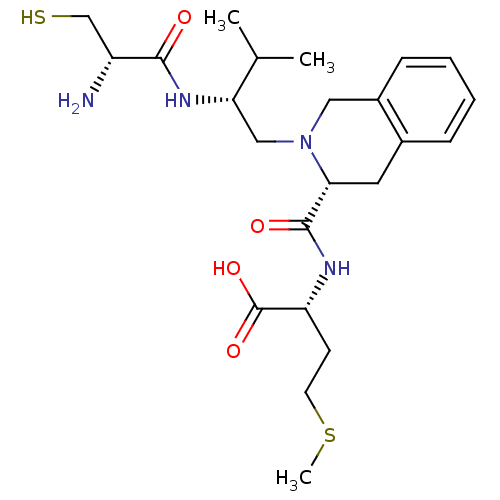 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of lck inase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048970 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of yes kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of src kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048963 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048972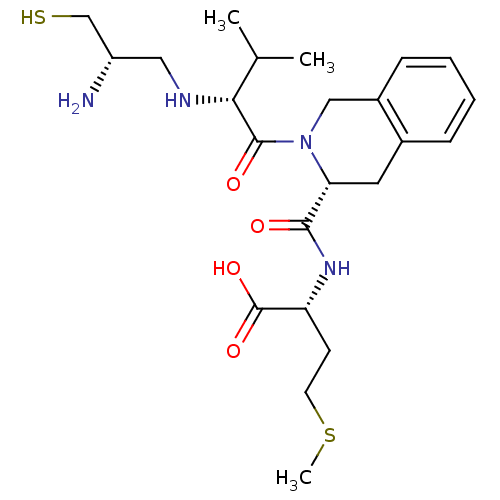 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048967 ((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048964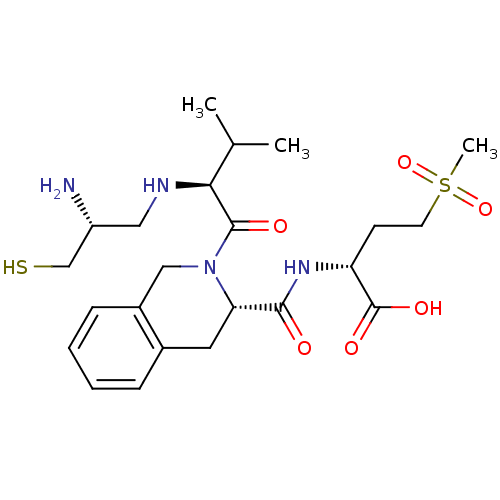 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 2 (Homo sapiens (Human)) | BDBM50074694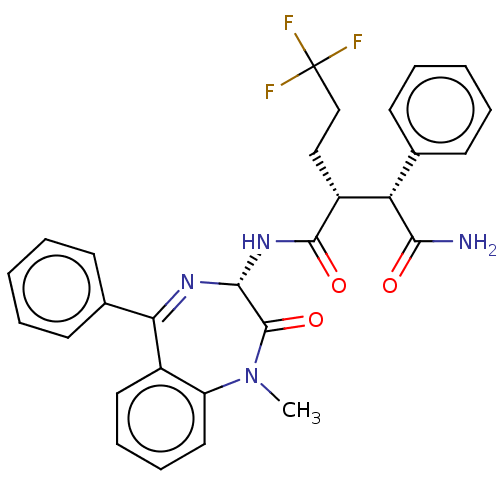 (CHEMBL3410161) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch2 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM50074796 (CHEMBL3410169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM50074799 (CHEMBL3410167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074771 (CHEMBL3410153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074818 (CHEMBL3410163) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50586606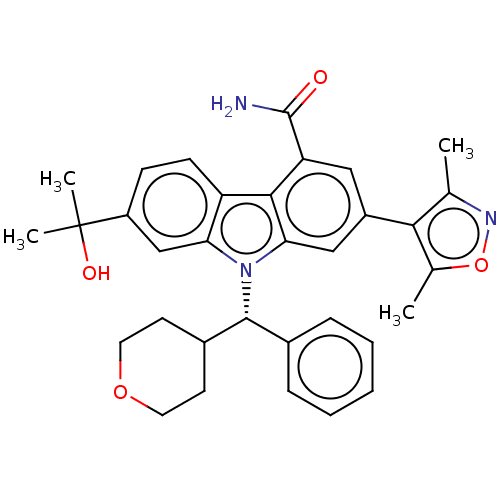 (CHEMBL5080354) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074796 (CHEMBL3410169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM50074771 (CHEMBL3410153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5725 (2-aminothiazole 9 | 5-{[(5-tert-butyl-1,3-oxazol-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | Bioorg Med Chem Lett 14: 2973-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.105 BindingDB Entry DOI: 10.7270/Q27942WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM50074693 (CHEMBL3410162) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch3 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breakpoint cluster region protein/Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Bcr-Abl kinase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048981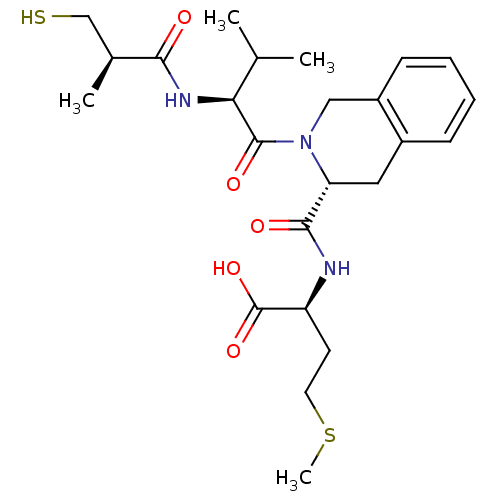 ((S)-2-({(R)-2-[(S)-2-((R)-3-Mercapto-2-methyl-prop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM297146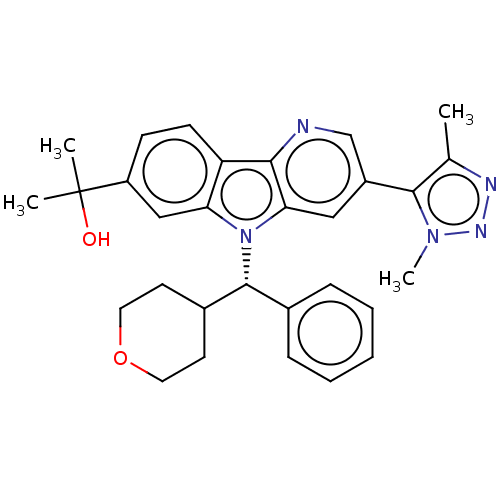 (2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD3 measured by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM297146 (2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50048966 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Geranylgeranyl transferase type I | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092365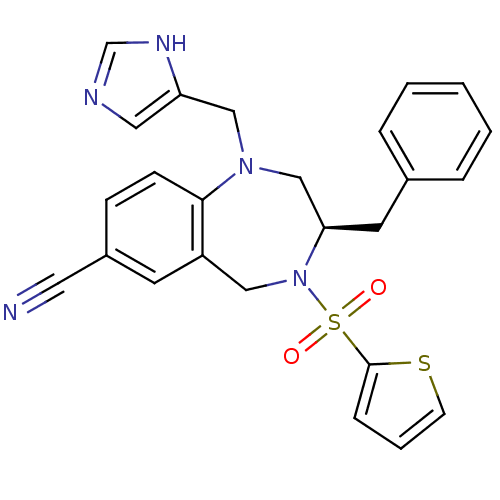 ((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM209895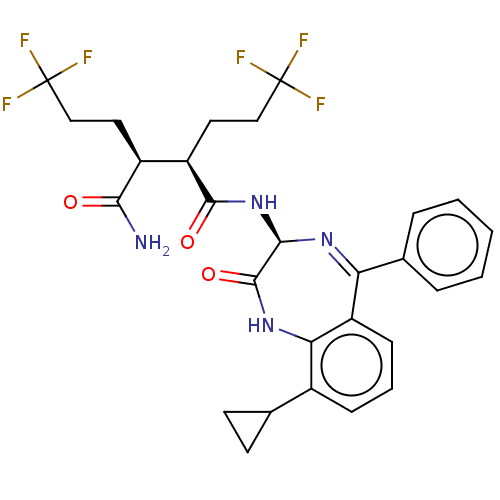 (US9273014, 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048968 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM297146 (2-{3-[4-(2H3)Methyl-1-methyl-1H-1,2,3-triazol-5-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD2 measured by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM209891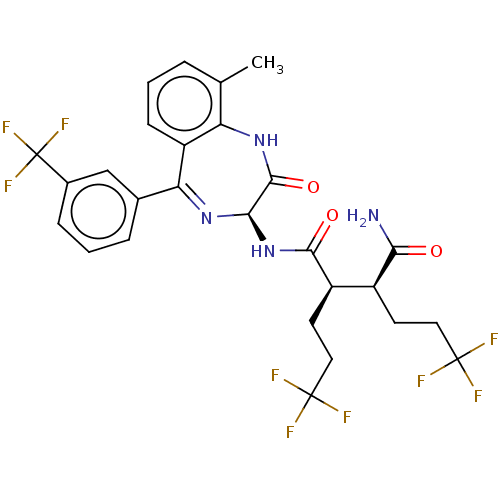 (US9273014, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50586604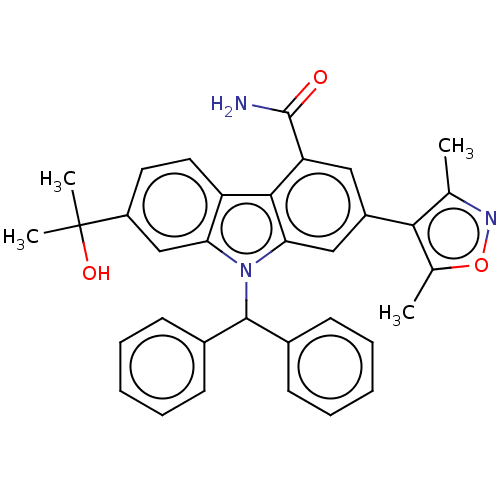 (CHEMBL5093440) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092366 (3-Benzyl-4-(2-dimethylamino-ethanesulfonyl)-1-(3H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM209891 (US9273014, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM209896 (US9273014, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM296886 (2-[3-(Dimethyl-1,2-oxazol-4-yl)-5-[oxan-4-yl(pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD2 measured by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092363 (3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(propane-1-s...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092377 (4-Benzenesulfonyl-3-benzyl-1-(3H-imidazol-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50048969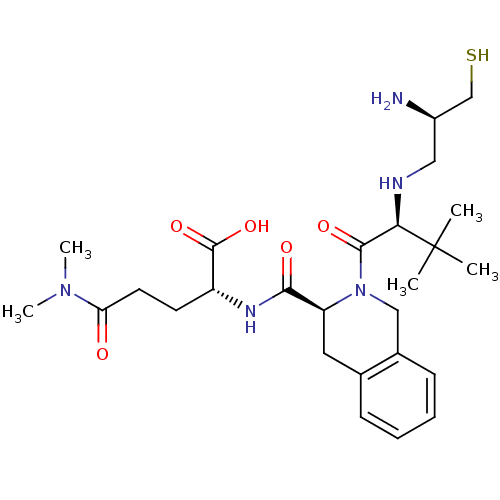 ((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Farnesyltransferase | J Med Chem 39: 224-36 (1996) Article DOI: 10.1021/jm950642a BindingDB Entry DOI: 10.7270/Q2610ZDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM209874 (US9273014, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50586607 (CHEMBL5082474) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 3 (Homo sapiens (Human)) | BDBM209874 (US9273014, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The pharmacological properties of the compounds of this invention may be confirmed by a number of biological assays. | US Patent US9273014 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM296886 (2-[3-(Dimethyl-1,2-oxazol-4-yl)-5-[oxan-4-yl(pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD4 (1 to 477 residues) measured after 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM296886 (2-[3-(Dimethyl-1,2-oxazol-4-yl)-5-[oxan-4-yl(pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRD3 measured by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00625 BindingDB Entry DOI: 10.7270/Q2HT2T7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5723 (2-aminothiazole 7 | 5-{[(5-tert-butyl-1,3-oxazol-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | Bioorg Med Chem Lett 14: 2973-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.105 BindingDB Entry DOI: 10.7270/Q27942WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5727 (2-aminothiazole 23 | N-{4-[(5-{[(5-tert-butyl-1,3-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Bristol-Myers Squibb Company | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | Bioorg Med Chem Lett 14: 2973-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.105 BindingDB Entry DOI: 10.7270/Q27942WW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM5666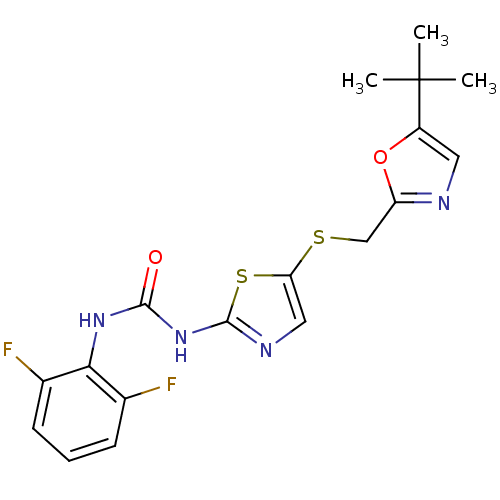 (2-amino-5-thio-substituted thiazole 40 | 3-(5-{[(5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description The enzyme was assayed with substrate in the presence of 25 uM ATP/[gamma-33P] ATP and test compound. Dose response curves were generated to determ... | J Med Chem 45: 3905-27 (2002) Article DOI: 10.1021/jm0201520 BindingDB Entry DOI: 10.7270/Q2GT5KC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074798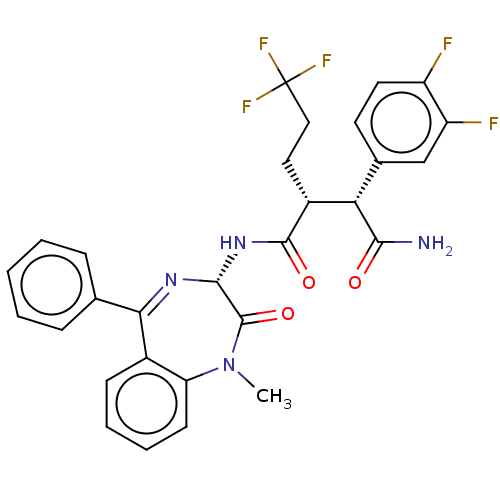 (CHEMBL3410168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074799 (CHEMBL3410167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2 (Homo sapiens (Human)) | BDBM50299138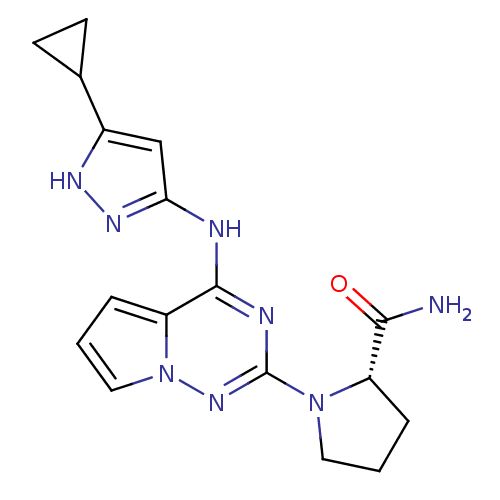 ((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin E after 60 mins by fluorescence electrophoresis | J Med Chem 52: 7360-3 (2009) Article DOI: 10.1021/jm900786r BindingDB Entry DOI: 10.7270/Q2P27022 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurogenic locus notch homolog protein 1 (Homo sapiens (Human)) | BDBM50074795 (CHEMBL3410170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of Notch1 intracellular domain fragment transfected in human HeLa cells co-transfected with CBF1-PGL3 luciferase reporter vector by transa... | Bioorg Med Chem Lett 25: 1905-9 (2015) Article DOI: 10.1016/j.bmcl.2015.03.038 BindingDB Entry DOI: 10.7270/Q2X92D18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1280 total ) | Next | Last >> |