 Found 40 hits with Last Name = 'lee' and Initial = 'tr'
Found 40 hits with Last Name = 'lee' and Initial = 'tr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50153015

((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50153015

((-)-Epicatechin-3-gallate | (-)-epicatechin 3-O-ga...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50236531
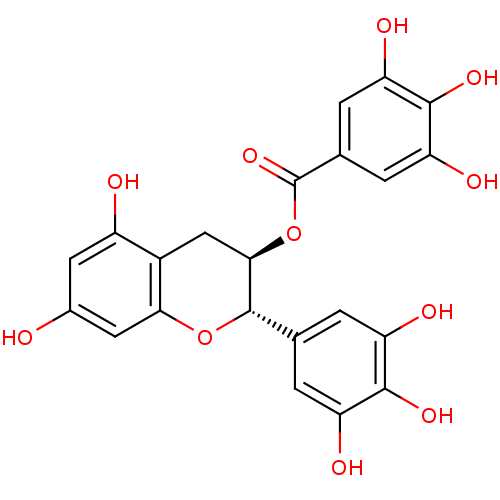
((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50135169
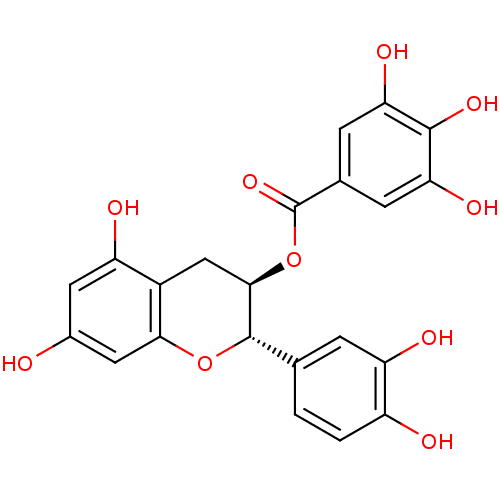
((-)-Catechin gallate | (2S,3R)-2-(3,4-dihydroxyphe...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1ccc(O)c(O)c1 Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50369796
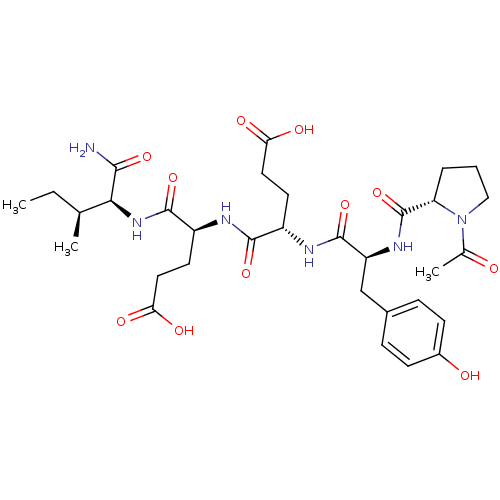
(CHEMBL1793996)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(N)=O Show InChI InChI=1S/C32H46N6O11/c1-4-17(2)27(28(33)45)37-30(47)22(12-14-26(43)44)34-29(46)21(11-13-25(41)42)35-31(48)23(16-19-7-9-20(40)10-8-19)36-32(49)24-6-5-15-38(24)18(3)39/h7-10,17,21-24,27,40H,4-6,11-16H2,1-3H3,(H2,33,45)(H,34,46)(H,35,48)(H,36,49)(H,37,47)(H,41,42)(H,43,44)/t17-,21-,22-,23-,24-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck SH2-domain in ELISA |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50135169

((-)-Catechin gallate | (2S,3R)-2-(3,4-dihydroxyphe...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1ccc(O)c(O)c1 Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50086192
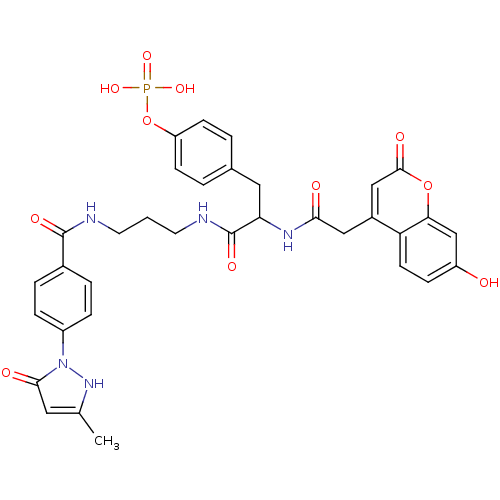
(CHEMBL277350 | Phosphoric acid mono-[4-(2-[2-(7-hy...)Show SMILES Cc1cc(=O)n([nH]1)-c1ccc(cc1)C(=O)NCCCNC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)Cc1cc(=O)oc2cc(O)ccc12 Show InChI InChI=1S/C34H34N5O11P/c1-20-15-31(42)39(38-20)24-7-5-22(6-8-24)33(44)35-13-2-14-36-34(45)28(16-21-3-10-26(11-4-21)50-51(46,47)48)37-30(41)17-23-18-32(43)49-29-19-25(40)9-12-27(23)29/h3-12,15,18-19,28,38,40H,2,13-14,16-17H2,1H3,(H,35,44)(H,36,45)(H,37,41)(H2,46,47,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck SH2-domain in ELISA |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50236531

((-)-gallocatechin gallate | (2R,3S)-5,7-dihydroxy-...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50086194
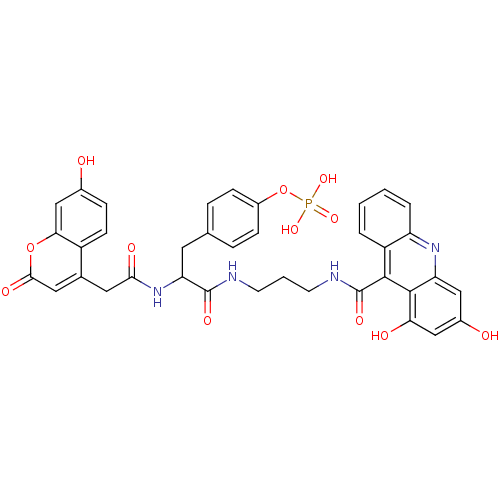
(CHEMBL279990 | Phosphoric acid mono-(4-{2-{3-[(1,3...)Show SMILES Oc1ccc2c(CC(=O)NC(Cc3ccc(OP(O)(O)=O)cc3)C(=O)NCCCNC(=O)c3c4ccccc4nc4cc(O)cc(O)c34)cc(=O)oc2c1 Show InChI InChI=1S/C37H33N4O12P/c42-22-8-11-25-21(16-33(46)52-31(25)19-22)15-32(45)41-29(14-20-6-9-24(10-7-20)53-54(49,50)51)36(47)38-12-3-13-39-37(48)34-26-4-1-2-5-27(26)40-28-17-23(43)18-30(44)35(28)34/h1-2,4-11,16-19,29,42-44H,3,12-15H2,(H,38,47)(H,39,48)(H,41,45)(H2,49,50,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck SH2-domain in ELISA |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50373221
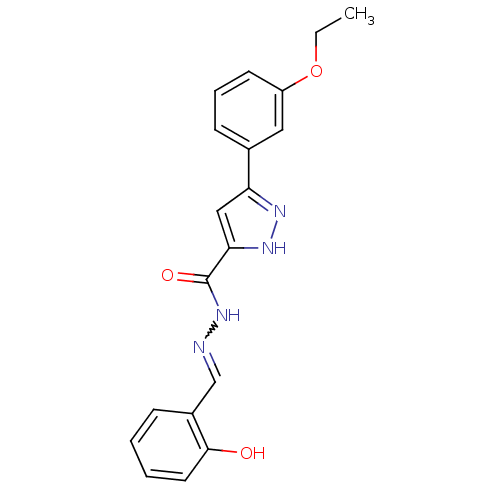
(CHEMBL408233)Show SMILES CCOc1cccc(c1)-c1cc([nH]n1)C(=O)NN=Cc1ccccc1O |w:17.18| Show InChI InChI=1S/C19H18N4O3/c1-2-26-15-8-5-7-13(10-15)16-11-17(22-21-16)19(25)23-20-12-14-6-3-4-9-18(14)24/h3-12,24H,2H2,1H3,(H,21,22)(H,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50086195
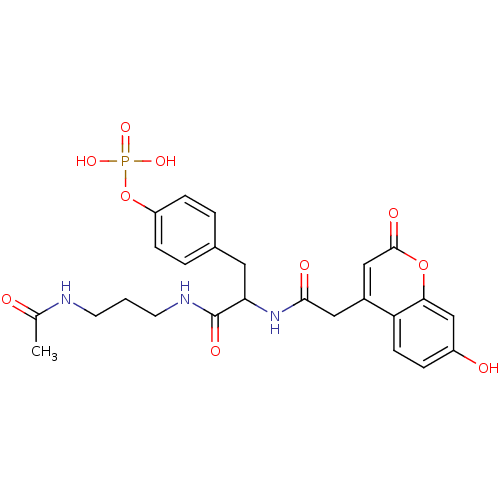
(CHEMBL17578 | Phosphoric acid mono-(4-{2-(3-acetyl...)Show SMILES CC(=O)NCCCNC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)Cc1cc(=O)oc2cc(O)ccc12 Show InChI InChI=1S/C25H28N3O10P/c1-15(29)26-9-2-10-27-25(33)21(11-16-3-6-19(7-4-16)38-39(34,35)36)28-23(31)12-17-13-24(32)37-22-14-18(30)5-8-20(17)22/h3-8,13-14,21,30H,2,9-12H2,1H3,(H,26,29)(H,27,33)(H,28,31)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck SH2-domain in ELISA |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50086193
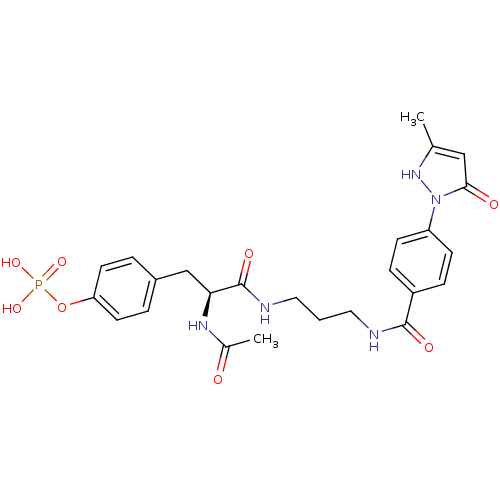
(CHEMBL17637 | Phosphoric acid mono-[4-(2-acetylami...)Show SMILES CC(=O)N[C@@H](Cc1ccc(OP(O)(O)=O)cc1)C(=O)NCCCNC(=O)c1ccc(cc1)-n1[nH]c(C)cc1=O Show InChI InChI=1S/C25H30N5O8P/c1-16-14-23(32)30(29-16)20-8-6-19(7-9-20)24(33)26-12-3-13-27-25(34)22(28-17(2)31)15-18-4-10-21(11-5-18)38-39(35,36)37/h4-11,14,22,29H,3,12-13,15H2,1-2H3,(H,26,33)(H,27,34)(H,28,31)(H2,35,36,37)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck SH2-domain in ELISA |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50373222

(CHEMBL408232)Show SMILES Oc1cccc(C=NNC(=S)NN=Cc2cccc(O)c2O)c1O |w:12.11,6.5| Show InChI InChI=1S/C15H14N4O4S/c20-11-5-1-3-9(13(11)22)7-16-18-15(24)19-17-8-10-4-2-6-12(21)14(10)23/h1-8,20-23H,(H2,18,19,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50236527
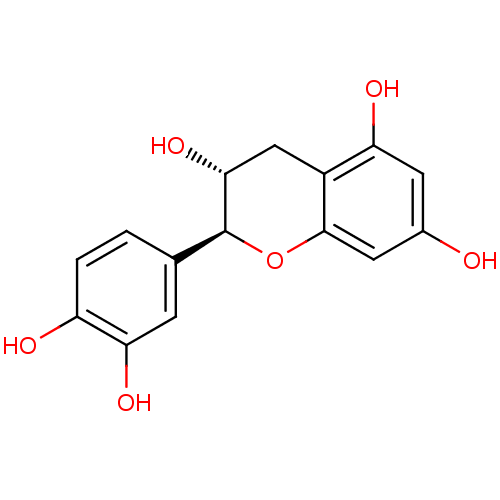
((-)-catechin | (2S,3R)-2-(3,4-dihydroxyphenyl)-3,4...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM23417
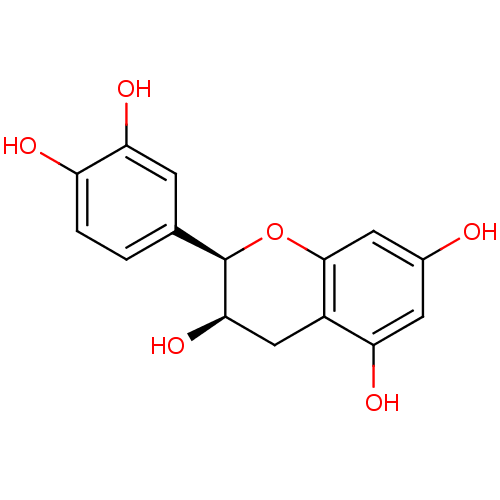
(α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50187665
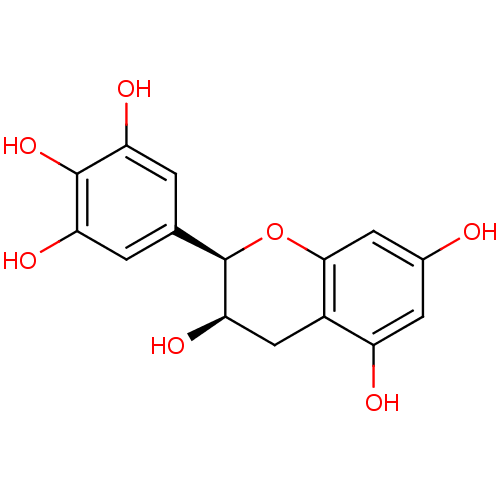
((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM23417

(α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50236527

((-)-catechin | (2S,3R)-2-(3,4-dihydroxyphenyl)-3,4...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1ccc(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O6/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7/h1-5,13,15-20H,6H2/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50373220
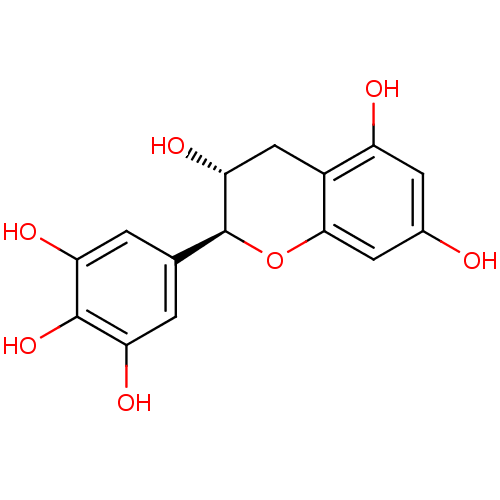
((-)-GALLOCATECHIN)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
6-phosphogluconate dehydrogenase, decarboxylating
(Homo sapiens (Human)) | BDBM50187665

((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 6PGD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Glucose-6-phosphate 1-dehydrogenase
(Saccharomyces cerevisiae S288c) | BDBM50373220

((-)-GALLOCATECHIN)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific Corporation
Curated by ChEMBL
| Assay Description
Inhibition of yeast G6PD |
Bioorg Med Chem 16: 3580-6 (2008)
Article DOI: 10.1016/j.bmc.2008.02.030
BindingDB Entry DOI: 10.7270/Q2F190K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50074329

(CH3-COOH | CHEMBL539 | Essigsaeure | Ethylic acid ...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50074329

(CH3-COOH | CHEMBL539 | Essigsaeure | Ethylic acid ...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50022182

((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid | 7-...)Show InChI InChI=1S/C11H8O5/c12-7-1-2-8-6(3-10(13)14)4-11(15)16-9(8)5-7/h1-2,4-5,12H,3H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Growth factor receptor-bound protein 2
(Homo sapiens (Human)) | BDBM50022182

((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid | 7-...)Show InChI InChI=1S/C11H8O5/c12-7-1-2-8-6(3-10(13)14)4-11(15)16-9(8)5-7/h1-2,4-5,12H,3H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Growth factor receptor bound protein 2, Grb2 |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50472532
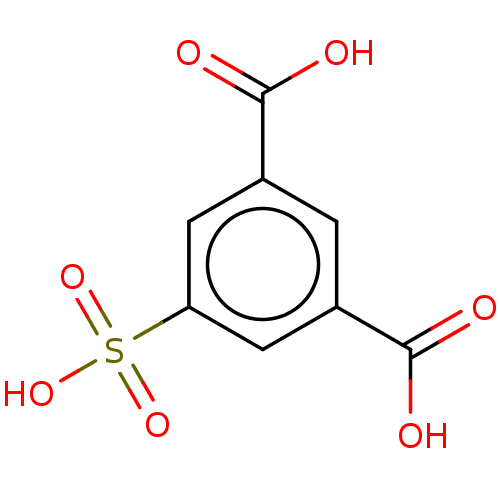
(5-Sulfo-Isophthalic Acid | CHEMBL165399)Show InChI InChI=1S/C8H6O7S/c9-7(10)4-1-5(8(11)12)3-6(2-4)16(13,14)15/h1-3H,(H,9,10)(H,11,12)(H,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50472533

(CHEMBL349371)Show InChI InChI=1S/C9H9BrO4/c1-13-7-3-5(9(11)12)6(10)4-8(7)14-2/h3-4H,1-2H3,(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50022182

((7-Hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid | 7-...)Show InChI InChI=1S/C11H8O5/c12-7-1-2-8-6(3-10(13)14)4-11(15)16-9(8)5-7/h1-2,4-5,12H,3H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50016603

((4-Nitro-benzoylamino)-acetic acid | 4-Nitrohippur...)Show InChI InChI=1S/C9H8N2O5/c12-8(13)5-10-9(14)6-1-3-7(4-2-6)11(15)16/h1-4H,5H2,(H,10,14)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50022173
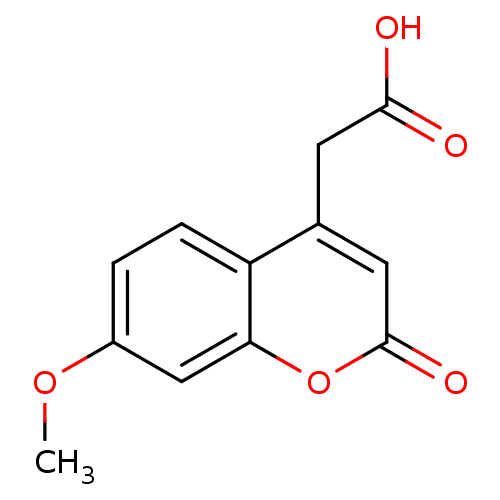
((7-methoxy-2-oxo-2H-chromen-4-yl)acetic acid | 7-M...)Show InChI InChI=1S/C12H10O5/c1-16-8-2-3-9-7(4-11(13)14)5-12(15)17-10(9)6-8/h2-3,5-6H,4H2,1H3,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50074329

(CH3-COOH | CHEMBL539 | Essigsaeure | Ethylic acid ...) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50074328

(CHEMBL164585 | Thiophene-3-carboxylic acid)Show InChI InChI=1S/C5H4O2S/c6-5(7)4-1-2-8-3-4/h1-3H,(H,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50472533

(CHEMBL349371)Show InChI InChI=1S/C9H9BrO4/c1-13-7-3-5(9(11)12)6(10)4-8(7)14-2/h3-4H,1-2H3,(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50472532

(5-Sulfo-Isophthalic Acid | CHEMBL165399)Show InChI InChI=1S/C8H6O7S/c9-7(10)4-1-5(8(11)12)3-6(2-4)16(13,14)15/h1-3H,(H,9,10)(H,11,12)(H,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50016603

((4-Nitro-benzoylamino)-acetic acid | 4-Nitrohippur...)Show InChI InChI=1S/C9H8N2O5/c12-8(13)5-10-9(14)6-1-3-7(4-2-6)11(15)16/h1-4H,5H2,(H,10,14)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of Fyn protein kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50086192

(CHEMBL277350 | Phosphoric acid mono-[4-(2-[2-(7-hy...)Show SMILES Cc1cc(=O)n([nH]1)-c1ccc(cc1)C(=O)NCCCNC(=O)C(Cc1ccc(OP(O)(O)=O)cc1)NC(=O)Cc1cc(=O)oc2cc(O)ccc12 Show InChI InChI=1S/C34H34N5O11P/c1-20-15-31(42)39(38-20)24-7-5-22(6-8-24)33(44)35-13-2-14-36-34(45)28(16-21-3-10-26(11-4-21)50-51(46,47)48)37-30(41)17-23-18-32(43)49-29-19-25(40)9-12-27(23)29/h3-12,15,18-19,28,38,40H,2,13-14,16-17H2,1H3,(H,35,44)(H,36,45)(H,37,41)(H2,46,47,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity for p56 Lck tyrosine kinase SH2 domain and Ac-pTyr-Glu-Glu-Ile-amide. |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50074328

(CHEMBL164585 | Thiophene-3-carboxylic acid)Show InChI InChI=1S/C5H4O2S/c6-5(7)4-1-2-8-3-4/h1-3H,(H,6,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine of Yeshiva University
Curated by ChEMBL
| Assay Description
Dissociation constant for binding to SH2 domain of p56 lck tyrosine kinase |
J Med Chem 42: 784-7 (1999)
Article DOI: 10.1021/jm980663f
BindingDB Entry DOI: 10.7270/Q2D79F4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50369796

(CHEMBL1793996)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(N)=O Show InChI InChI=1S/C32H46N6O11/c1-4-17(2)27(28(33)45)37-30(47)22(12-14-26(43)44)34-29(46)21(11-13-25(41)42)35-31(48)23(16-19-7-9-20(40)10-8-19)36-32(49)24-6-5-15-38(24)18(3)39/h7-10,17,21-24,27,40H,4-6,11-16H2,1-3H3,(H2,33,45)(H,34,46)(H,35,48)(H,36,49)(H,37,47)(H,41,42)(H,43,44)/t17-,21-,22-,23-,24-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
The Albert Einstein College of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity for p56 Lck tyrosine kinase SH2 domain and Ac-pTyr-Glu-Glu-Ile-amide. |
J Med Chem 43: 1173-9 (2000)
BindingDB Entry DOI: 10.7270/Q2GF0V6G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data