

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878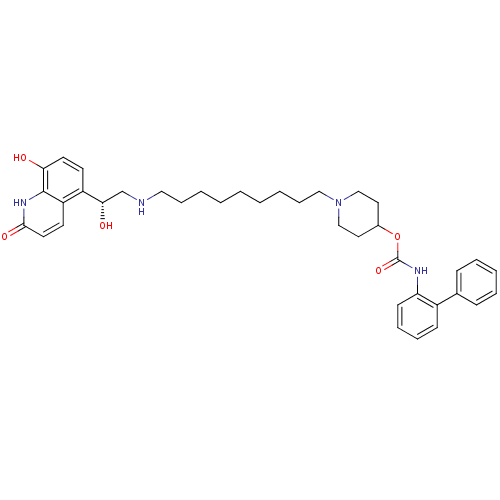 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084436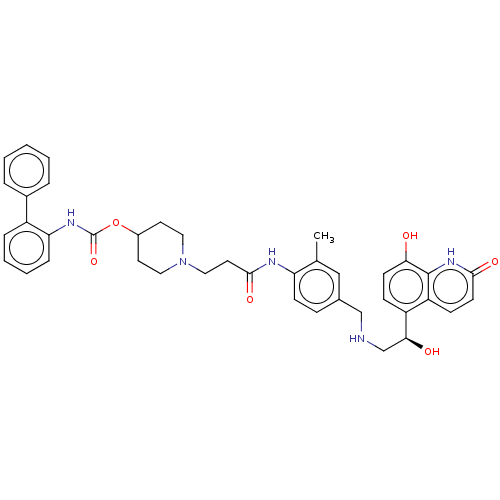 (CHEMBL3426693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084443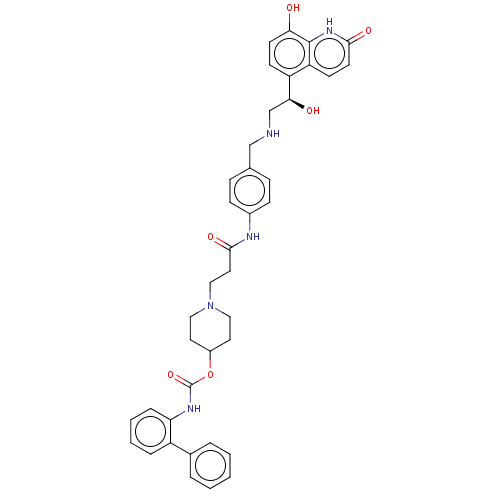 (CHEMBL3426687) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084432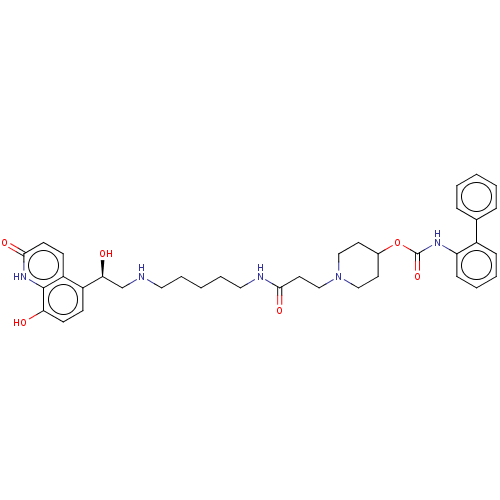 (CHEMBL3426697) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337881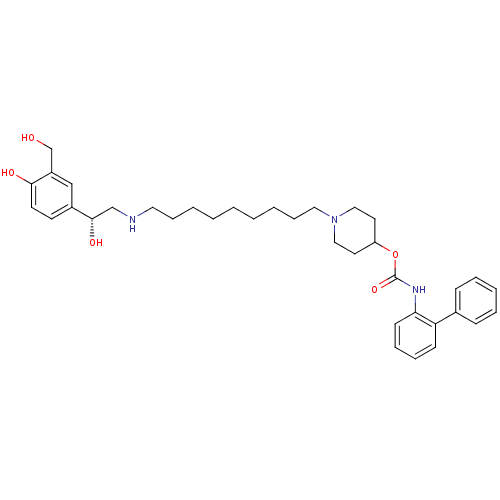 ((R)-1-(9-(2-hydroxy-2-(4-hydroxy-3-(hydroxymethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337880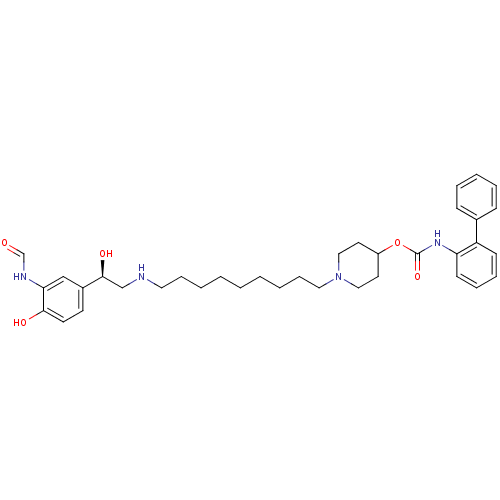 ((R)-1-(9-(2-(3-formamido-4-hydroxyphenyl)-2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084439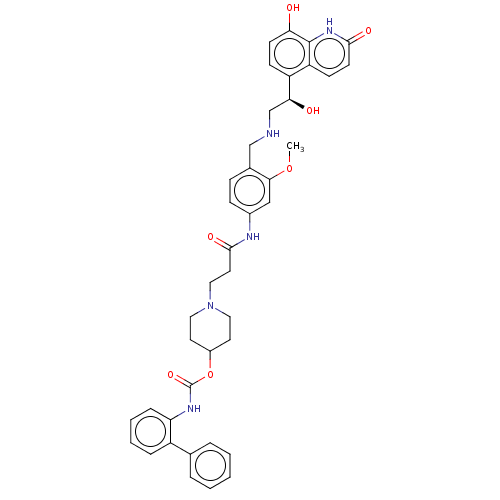 (CHEMBL3426691) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084440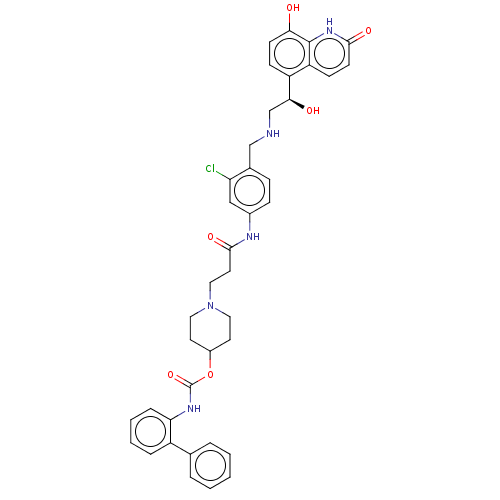 (CHEMBL3426690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084442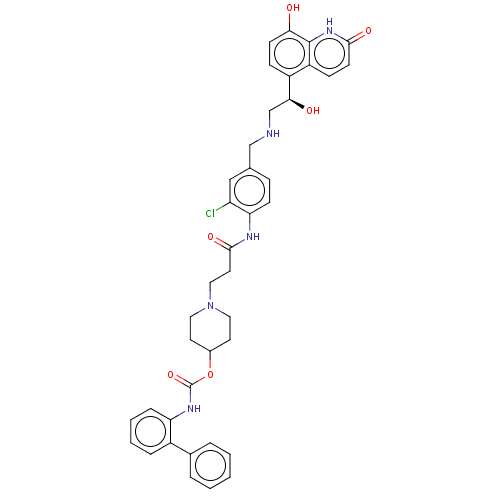 (CHEMBL3426688) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084424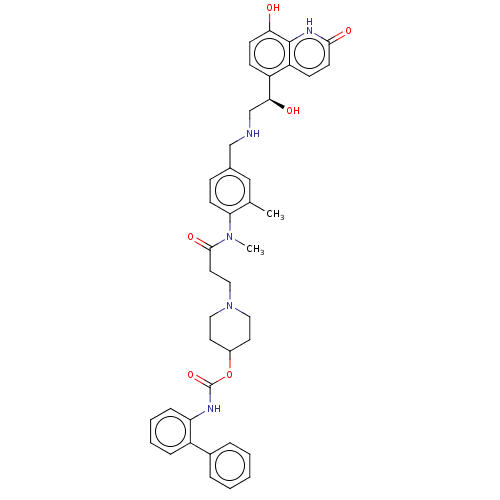 (CHEMBL3426705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084426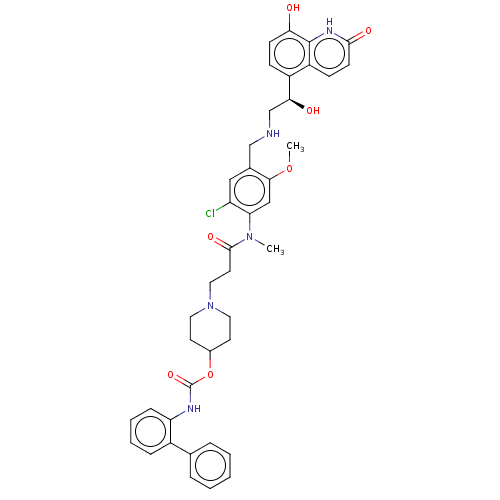 (CHEMBL3426703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084433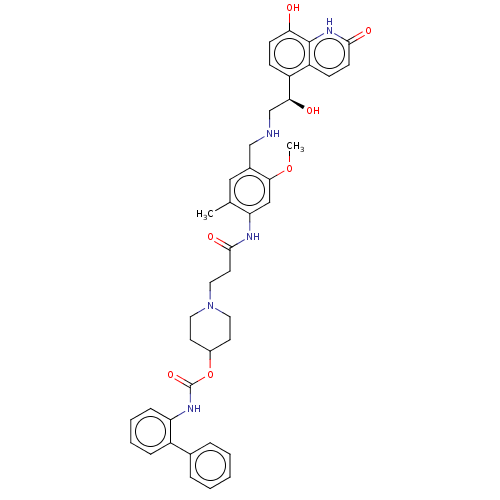 (CHEMBL3426696) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084431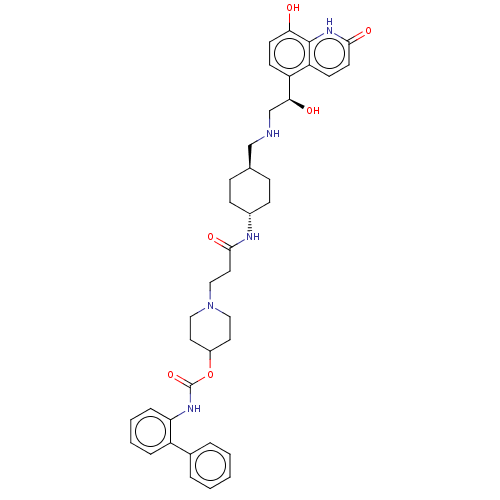 (CHEMBL3426698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337877 ((R)-1-(8-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084434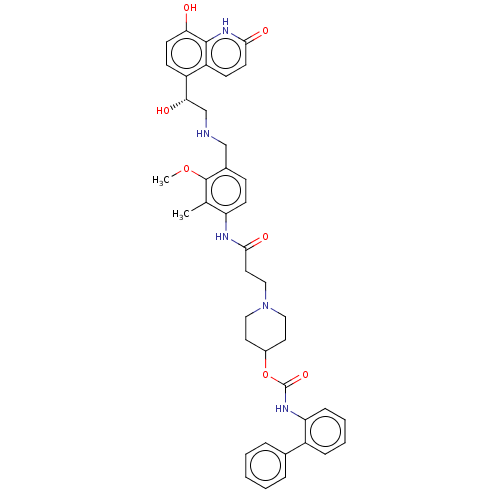 (CHEMBL3426695) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337870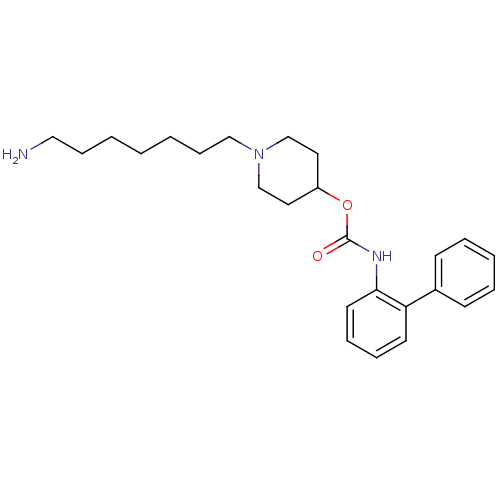 (1-(7-aminoheptyl)piperidin-4-yl biphenyl-2-ylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084438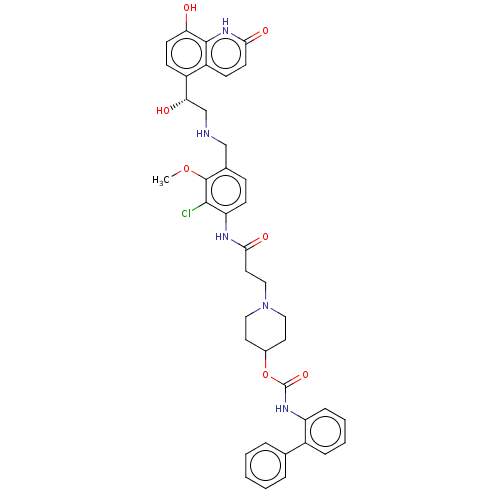 (CHEMBL3426692) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084425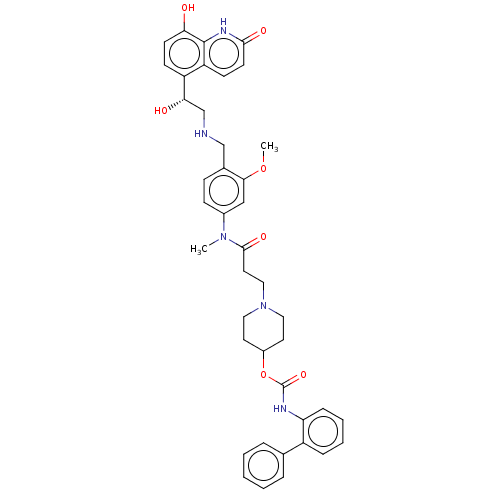 (CHEMBL3426704 | US9394275, I-25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084430 (CHEMBL3426699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337886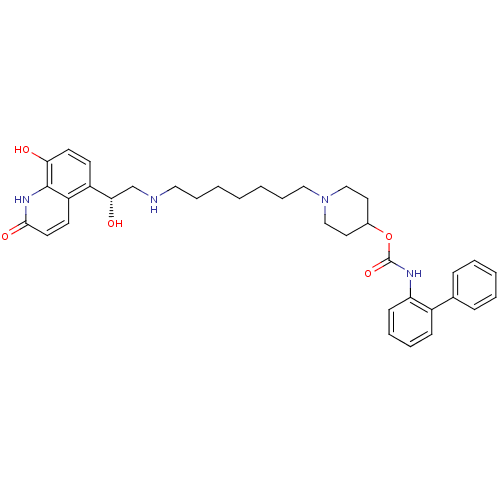 ((R)-1-(7-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337872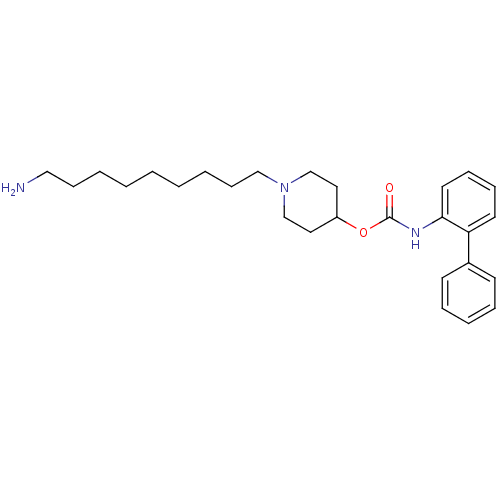 (1-(9-aminononyl)piperidin-4-yl biphenyl-2-ylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084427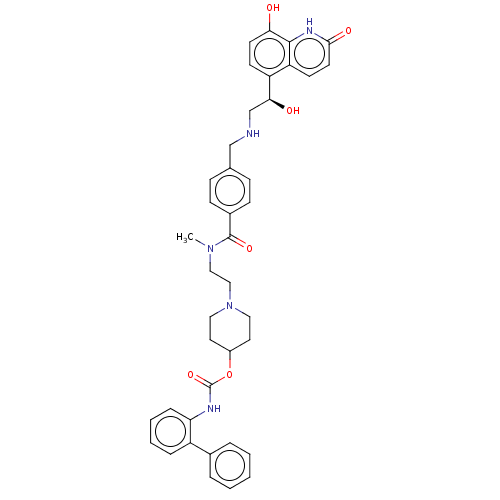 (CHEMBL3426702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084423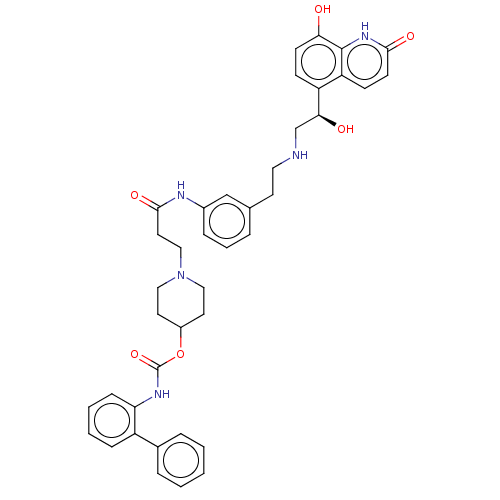 (CHEMBL3426706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084435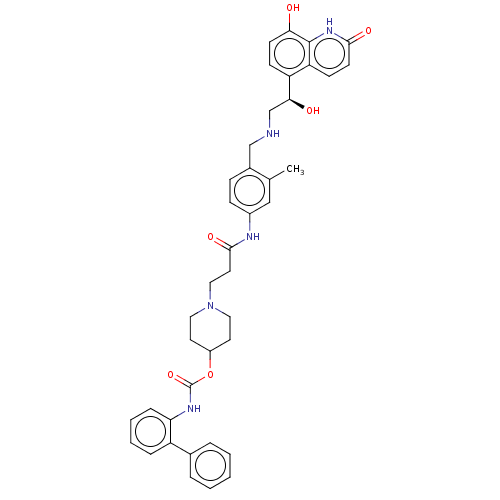 (CHEMBL3426694) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337873 (1-(10-aminodecyl)piperidin-4-yl biphenyl-2-ylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084441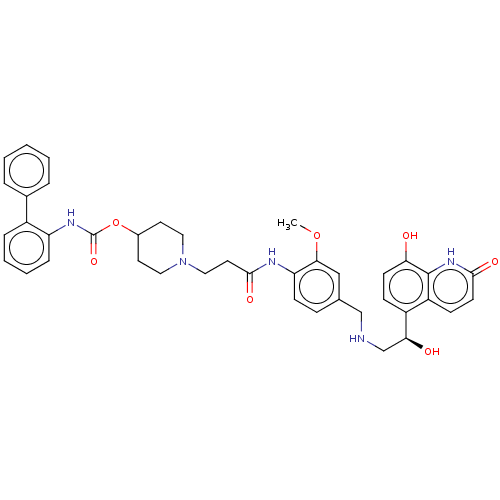 (CHEMBL3426689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337871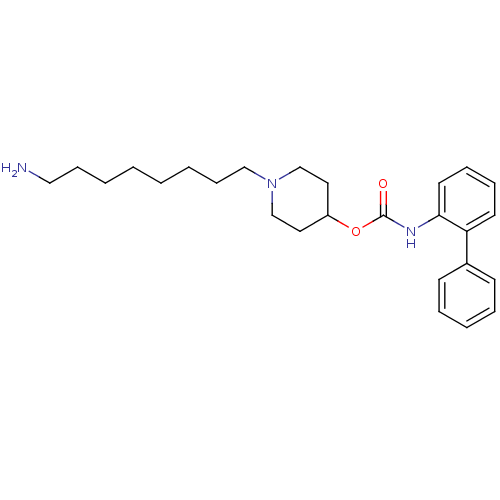 (1-(8-aminooctyl)piperidin-4-yl biphenyl-2-ylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084437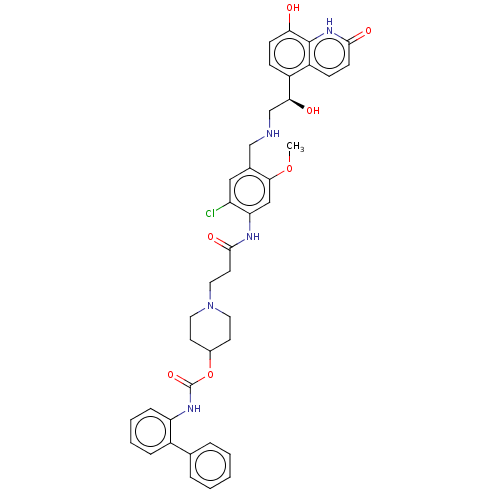 (Batefenterol | GSK961081 | GSK961081A | TD-5959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084421 (CHEMBL3426708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25771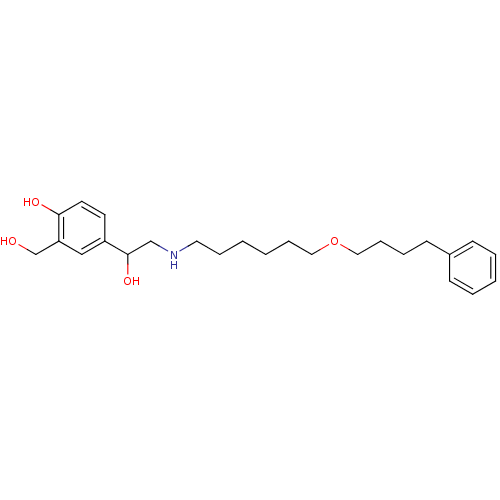 (1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337874 (1-(11-aminoundecyl)piperidin-4-yl biphenyl-2-ylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337875 (1-(12-aminododecyl)piperidin-4-yl biphenyl-2-ylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084428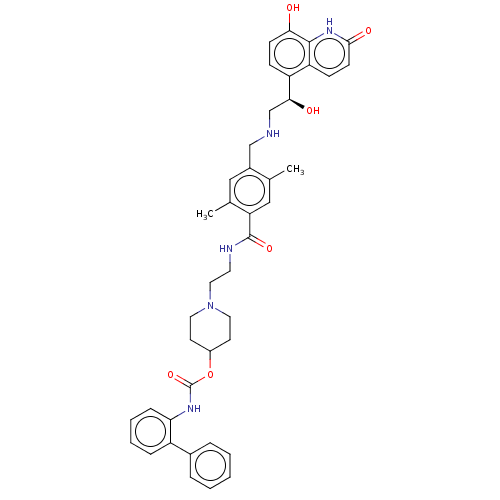 (CHEMBL3426701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337877 ((R)-1-(8-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337878 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337885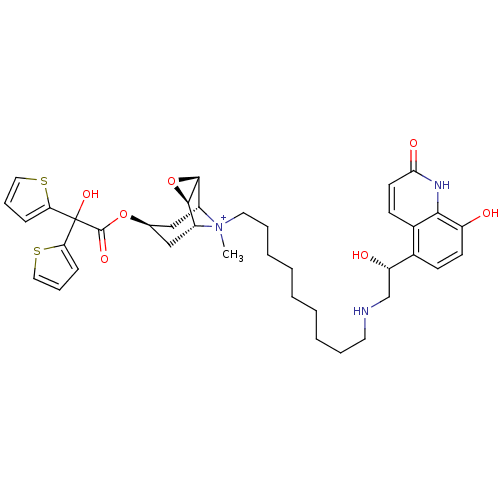 ((1R,2R,4S,5R,7S)-7-(2-Hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337859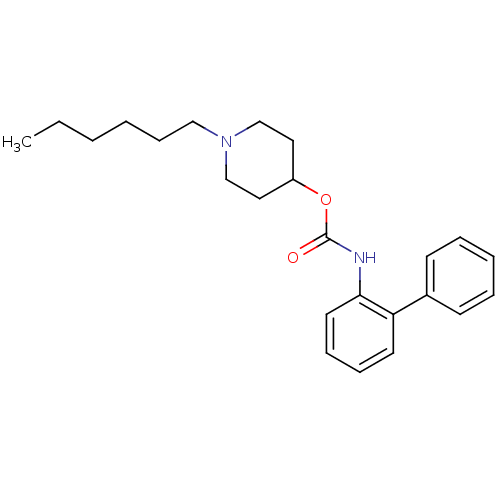 (1-hexylpiperidin-4-yl biphenyl-2-ylcarbamate | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337860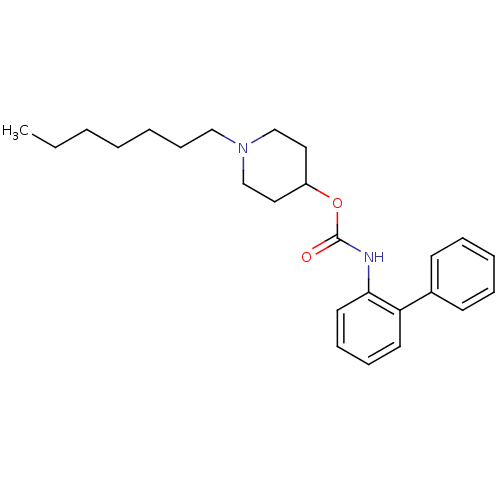 (1-heptylpiperidin-4-yl biphenyl-2-ylcarbamate | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084429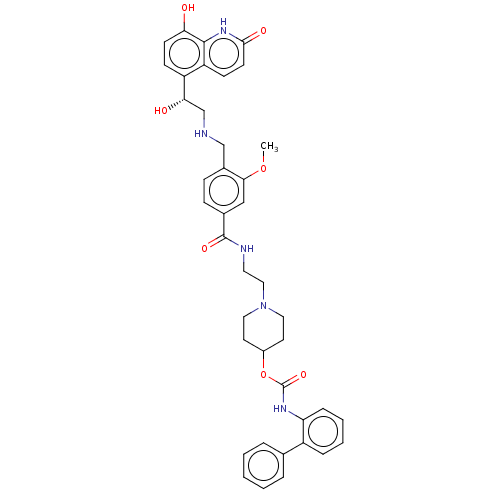 (CHEMBL3426700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337861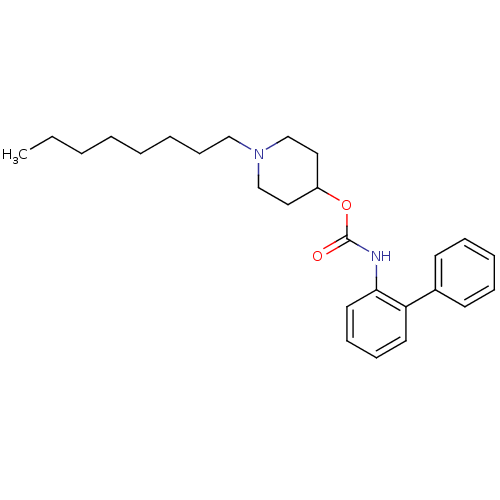 (1-octylpiperidin-4-yl biphenyl-2-ylcarbamate | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337883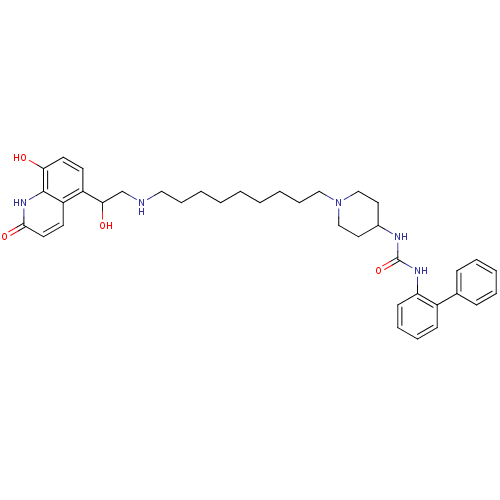 (1-(biphenyl-2-yl)-3-(1-(9-(2-hydroxy-2-(8-hydroxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084422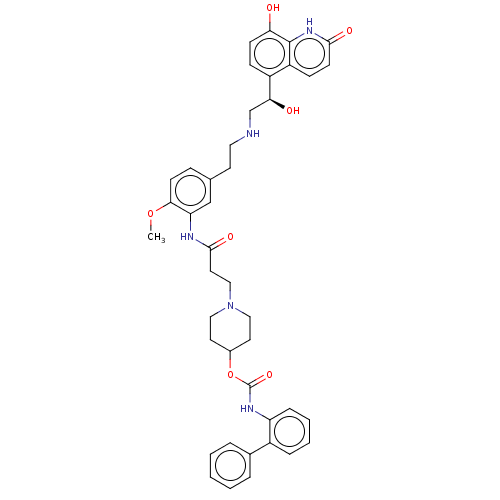 (CHEMBL3426707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337862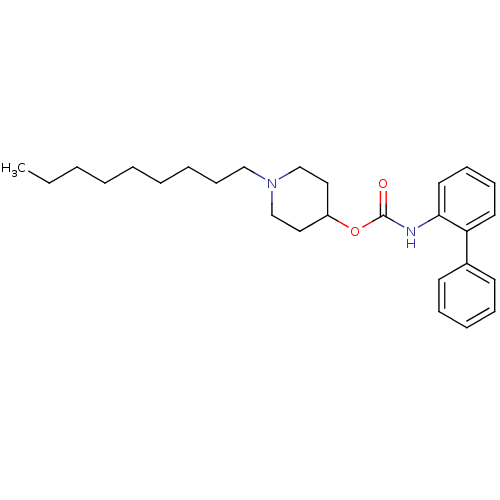 (1-nonylpiperidin-4-yl biphenyl-2-ylcarbamate | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337886 ((R)-1-(7-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337878 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-dihydroalprenolol from human beta2-adrenoceptor expressed in HEK cells after 4 hrs | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337858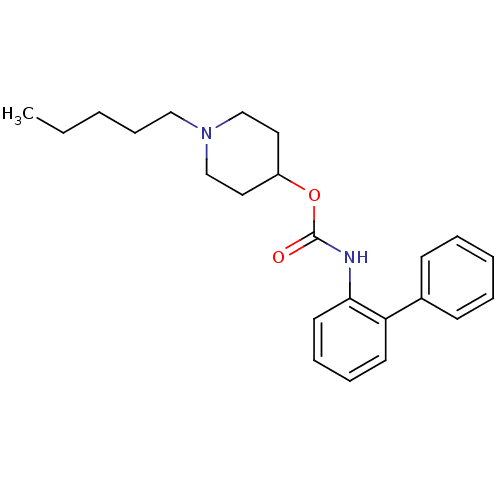 (1-pentylpiperidin-4-yl biphenyl-2-ylcarbamate | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl-scopolamine from human muscarinic M3 receptor after 6 hrs by cell based assay | Bioorg Med Chem Lett 21: 1354-8 (2011) Checked by Author Article DOI: 10.1016/j.bmcl.2011.01.043 BindingDB Entry DOI: 10.7270/Q2NC61HQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 804 total ) | Next | Last >> |