 Found 174 hits with Last Name = 'lee' and Initial = 'yk'
Found 174 hits with Last Name = 'lee' and Initial = 'yk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231280
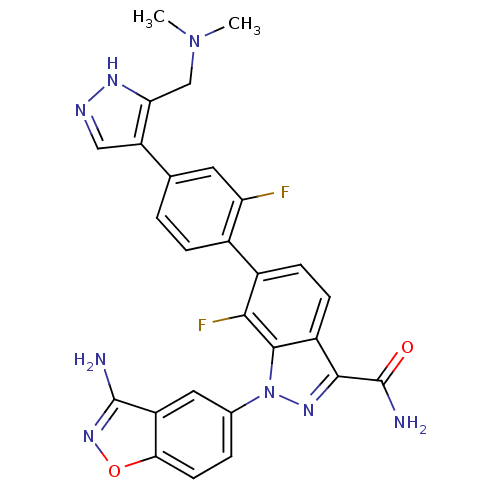
(1-(3-cyano-4-fluorophenyl)-6-[4-(3-dimethylaminome...)Show SMILES CN(C)Cc1[nH]ncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-36(2)12-21-19(11-32-33-21)13-3-5-15(20(28)9-13)16-6-7-17-24(27(31)38)34-37(25(17)23(16)29)14-4-8-22-18(10-14)26(30)35-39-22/h3-11H,12H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231282
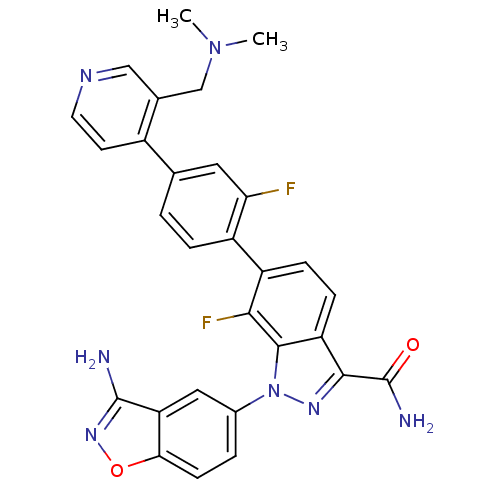
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cnccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-13-34-10-9-18(16)15-3-5-19(23(30)11-15)20-6-7-21-26(29(33)39)35-38(27(21)25(20)31)17-4-8-24-22(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231275
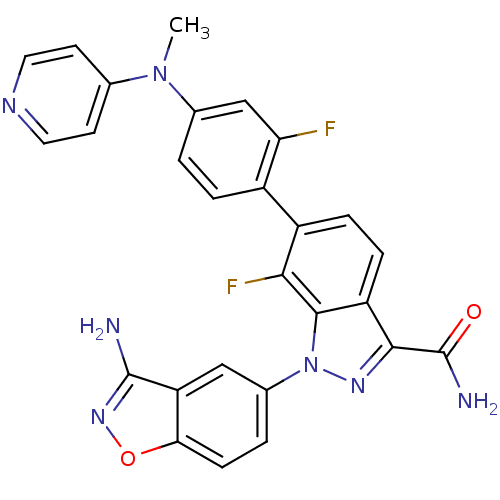
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES CN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H19F2N7O2/c1-35(14-8-10-32-11-9-14)15-2-4-17(21(28)13-15)18-5-6-19-24(27(31)37)33-36(25(19)23(18)29)16-3-7-22-20(12-16)26(30)34-38-22/h2-13H,1H3,(H2,30,34)(H2,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231284
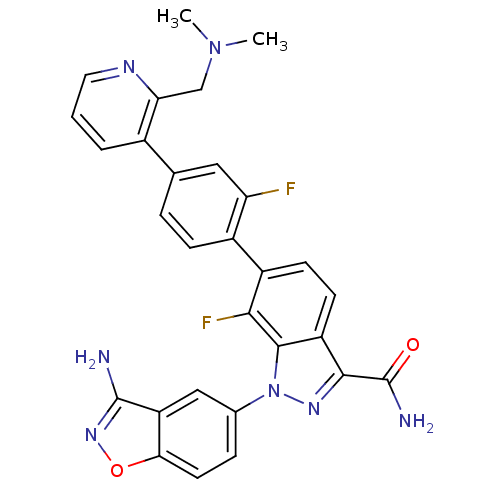
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(2-dimethyla...)Show SMILES CN(C)Cc1ncccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-23-17(4-3-11-34-23)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)25(19)31)16-6-10-24-21(13-16)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231271
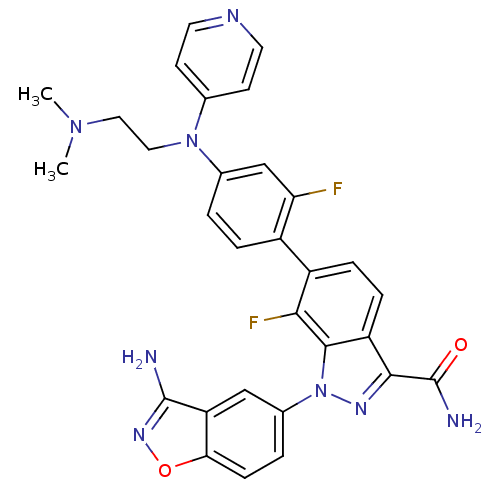
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)13-14-39(17-9-11-35-12-10-17)18-3-5-20(24(31)16-18)21-6-7-22-27(30(34)41)36-40(28(22)26(21)32)19-4-8-25-23(15-19)29(33)37-42-25/h3-12,15-16H,13-14H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231279
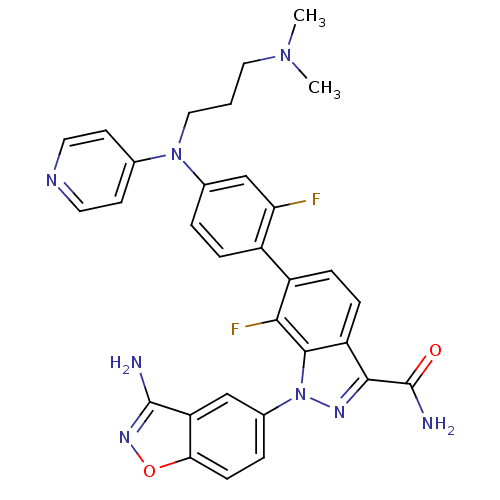
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(3-dimethyl...)Show SMILES CN(C)CCCN(c1ccncc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H28F2N8O2/c1-39(2)14-3-15-40(18-10-12-36-13-11-18)19-4-6-21(25(32)17-19)22-7-8-23-28(31(35)42)37-41(29(23)27(22)33)20-5-9-26-24(16-20)30(34)38-43-26/h4-13,16-17H,3,14-15H2,1-2H3,(H2,34,38)(H2,35,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231283
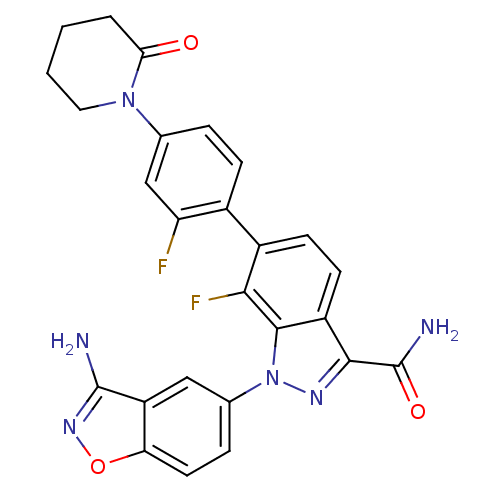
(1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-6-[2-flu...)Show SMILES NC(=O)c1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H20F2N6O3/c27-19-12-13(33-10-2-1-3-21(33)35)4-6-15(19)16-7-8-17-23(26(30)36)31-34(24(17)22(16)28)14-5-9-20-18(11-14)25(29)32-37-20/h4-9,11-12H,1-3,10H2,(H2,29,32)(H2,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086717

(JNJ-39439335 | Mavatrep)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-RTX from human TRPV1 transfected in HEK293 cells after 60 mins by scintillation spectroscopic analysis |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231270

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-(2'-dimethylami...)Show SMILES CN(C)Cc1ccccc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H24F2N6O2/c1-37(2)15-17-5-3-4-6-19(17)16-7-9-20(24(31)13-16)21-10-11-22-27(30(34)39)35-38(28(22)26(21)32)18-8-12-25-23(14-18)29(33)36-40-25/h3-14H,15H2,1-2H3,(H2,33,36)(H2,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231272
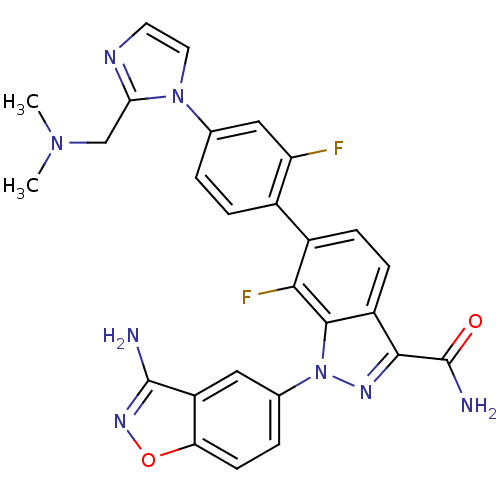
(1-(3-amino-1,2-benzisoxazol-5-yl)-6-(4-{2-[(dimeth...)Show SMILES CN(C)Cc1nccn1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C27H22F2N8O2/c1-35(2)13-22-32-9-10-36(22)14-3-5-16(20(28)12-14)17-6-7-18-24(27(31)38)33-37(25(18)23(17)29)15-4-8-21-19(11-15)26(30)34-39-21/h3-12H,13H2,1-2H3,(H2,30,34)(H2,31,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 15.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231269
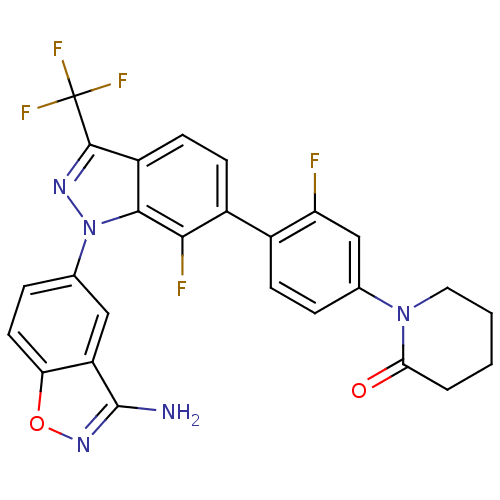
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2ccc(c(F)c12)-c1ccc(cc1F)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C26H18F5N5O2/c27-19-12-13(35-10-2-1-3-21(35)37)4-6-15(19)16-7-8-17-23(22(16)28)36(33-24(17)26(29,30)31)14-5-9-20-18(11-14)25(32)34-38-20/h4-9,11-12H,1-3,10H2,(H2,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231274
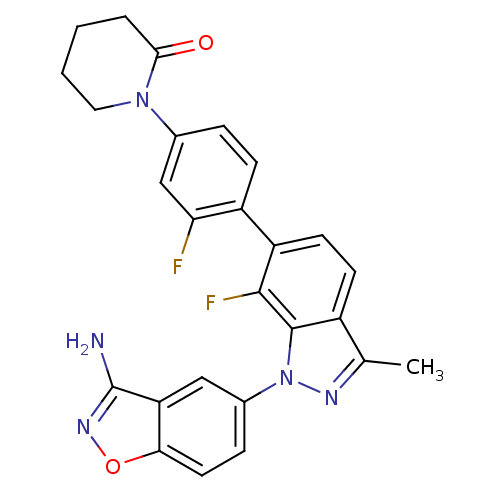
(1-{4-[1-(3-aminobenzo[d]isoxazol-5-yl)-7-fluoro-3-...)Show SMILES Cc1nn(-c2ccc3onc(N)c3c2)c2c(F)c(ccc12)-c1ccc(cc1F)N1CCCCC1=O Show InChI InChI=1S/C26H21F2N5O2/c1-14-17-8-9-19(18-7-5-15(13-21(18)27)32-11-3-2-4-23(32)34)24(28)25(17)33(30-14)16-6-10-22-20(12-16)26(29)31-35-22/h5-10,12-13H,2-4,11H2,1H3,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231286
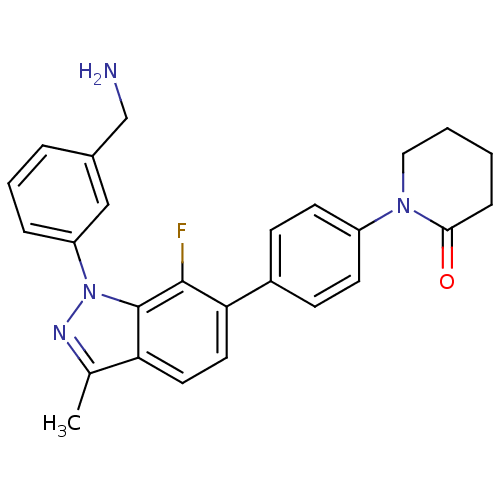
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H25FN4O/c1-17-22-12-13-23(19-8-10-20(11-9-19)30-14-3-2-7-24(30)32)25(27)26(22)31(29-17)21-6-4-5-18(15-21)16-28/h4-6,8-13,15H,2-3,7,14,16,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231268
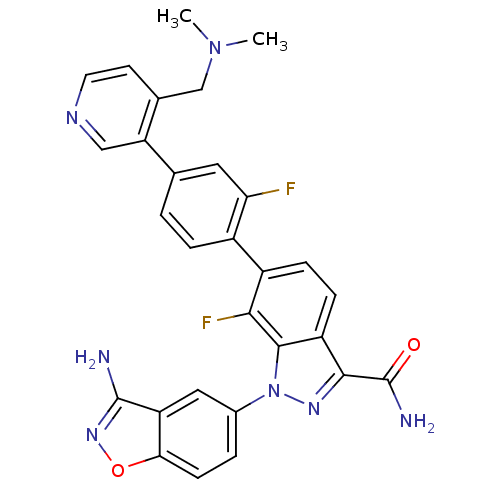
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(4-dimethyla...)Show SMILES CN(C)Cc1ccncc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-9-10-34-13-22(16)15-3-5-18(23(30)11-15)19-6-7-20-26(29(33)39)35-38(27(20)25(19)31)17-4-8-24-21(12-17)28(32)36-40-24/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231278
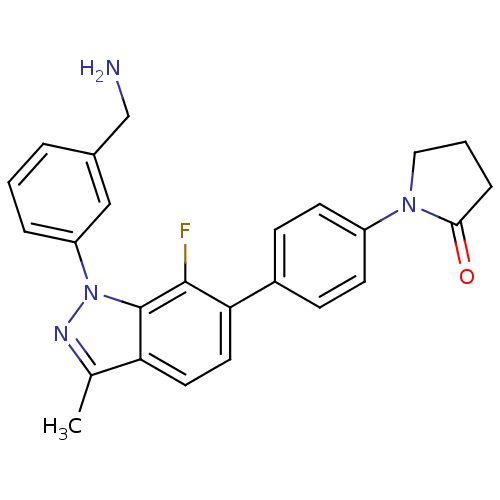
(1-{4-[1-(3-aminomethylphenyl)-7-fluoro-3-methyl-1H...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2c(F)c(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H23FN4O/c1-16-21-11-12-22(18-7-9-19(10-8-18)29-13-3-6-23(29)31)24(26)25(21)30(28-16)20-5-2-4-17(14-20)15-27/h2,4-5,7-12,14H,3,6,13,15,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231281

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1cccnc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C30H26F2N8O2/c1-38(2)12-13-39(19-4-3-11-35-16-19)17-5-7-20(24(31)15-17)21-8-9-22-27(30(34)41)36-40(28(22)26(21)32)18-6-10-25-23(14-18)29(33)37-42-25/h3-11,14-16H,12-13H2,1-2H3,(H2,33,37)(H2,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231267
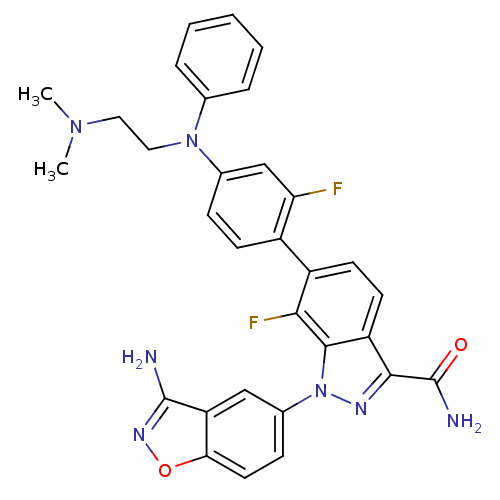
(1-(3-aminobenzo[d]isoxazol-5-yl)-6-{4-[(2-dimethyl...)Show SMILES CN(C)CCN(c1ccccc1)c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C31H27F2N7O2/c1-38(2)14-15-39(18-6-4-3-5-7-18)19-8-10-21(25(32)17-19)22-11-12-23-28(31(35)41)36-40(29(23)27(22)33)20-9-13-26-24(16-20)30(34)37-42-26/h3-13,16-17H,14-15H2,1-2H3,(H2,34,37)(H2,35,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231266

(1-(3-aminobenzo[d]isoxazol-5-yl)-6-[4-(3-dimethyla...)Show SMILES CN(C)Cc1cccnc1-c1ccc(c(F)c1)-c1ccc2c(nn(-c3ccc4onc(N)c4c3)c2c1F)C(N)=O Show InChI InChI=1S/C29H23F2N7O2/c1-37(2)14-16-4-3-11-34-25(16)15-5-7-18(22(30)12-15)19-8-9-20-26(29(33)39)35-38(27(20)24(19)31)17-6-10-23-21(13-17)28(32)36-40-23/h3-13H,14H2,1-2H3,(H2,32,36)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231273
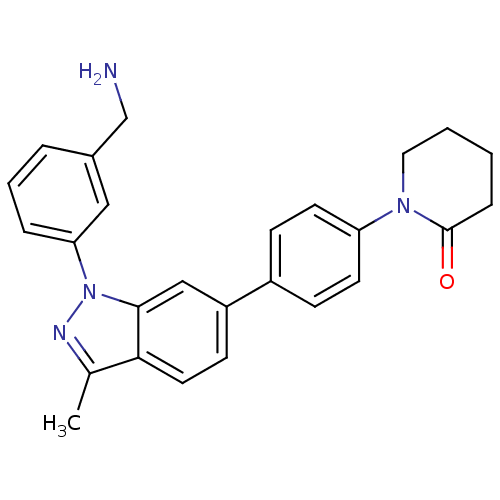
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C26H26N4O/c1-18-24-13-10-21(16-25(24)30(28-18)23-6-4-5-19(15-23)17-27)20-8-11-22(12-9-20)29-14-3-2-7-26(29)31/h4-6,8-13,15-16H,2-3,7,14,17,27H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50231265
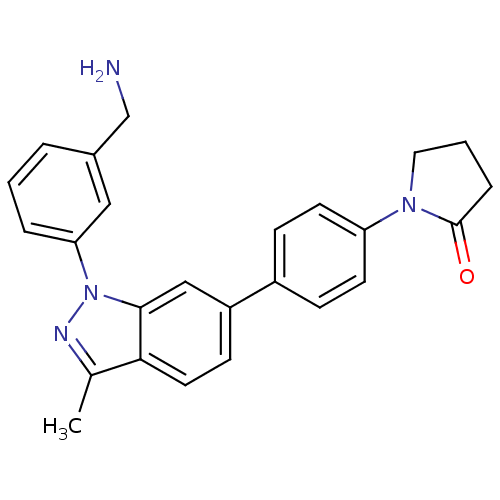
(1-{4-[1-(3-aminomethylphenyl)-3-methyl-1H-indazol-...)Show SMILES Cc1nn(-c2cccc(CN)c2)c2cc(ccc12)-c1ccc(cc1)N1CCCC1=O Show InChI InChI=1S/C25H24N4O/c1-17-23-12-9-20(19-7-10-21(11-8-19)28-13-3-6-25(28)30)15-24(23)29(27-17)22-5-2-4-18(14-22)16-26/h2,4-5,7-12,14-15H,3,6,13,16,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
J Med Chem 51: 282-97 (2008)
Article DOI: 10.1021/jm701217r
BindingDB Entry DOI: 10.7270/Q2K64HTF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193240
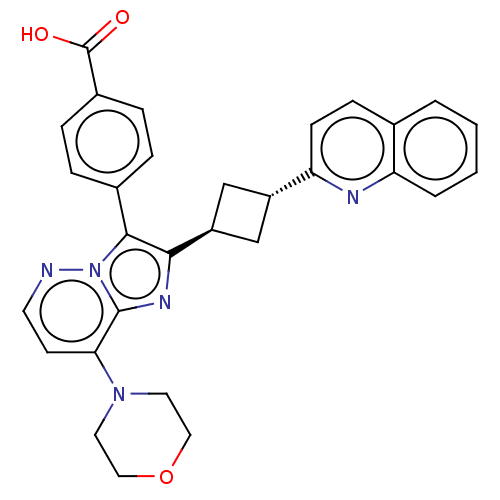
(US9193736, 164)Show SMILES OC(=O)c1ccc(cc1)-c1c(nc2c(ccnn12)N1CCOCC1)[C@H]1C[C@@H](C1)c1ccc2ccccc2n1 |wU:24.27,wD:26.32,(4.46,-12.1,;4.14,-10.59,;2.67,-10.12,;5.28,-9.56,;4.96,-8.06,;6.11,-7.03,;7.57,-7.5,;7.89,-9.01,;6.75,-10.04,;8.72,-6.47,;8.55,-4.94,;9.96,-4.31,;10.99,-5.46,;12.53,-5.46,;13.3,-6.79,;12.53,-8.12,;10.99,-8.12,;10.22,-6.79,;13.3,-4.12,;12.53,-2.79,;13.3,-1.46,;14.84,-1.46,;15.61,-2.79,;14.84,-4.12,;7.22,-4.17,;5.73,-4.57,;5.33,-3.08,;6.82,-2.68,;4,-2.31,;4,-.77,;2.67,,;1.33,-.77,;;-1.33,-.77,;-1.33,-2.31,;,-3.08,;1.33,-2.31,;2.67,-3.08,)| Show InChI InChI=1S/C30H27N5O3/c36-30(37)21-7-5-20(6-8-21)28-27(33-29-26(11-12-31-35(28)29)34-13-15-38-16-14-34)23-17-22(18-23)25-10-9-19-3-1-2-4-24(19)32-25/h1-12,22-23H,13-18H2,(H,36,37)/t22-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193125
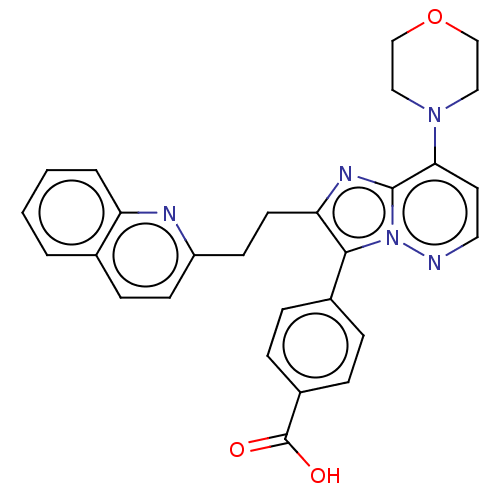
(US9193736, 41)Show SMILES OC(=O)c1ccc(cc1)-c1c(CCc2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C28H25N5O3/c34-28(35)21-7-5-20(6-8-21)26-24(12-11-22-10-9-19-3-1-2-4-23(19)30-22)31-27-25(13-14-29-33(26)27)32-15-17-36-18-16-32/h1-10,13-14H,11-12,15-18H2,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193124

(US9193736, 40)Show SMILES OC(=O)c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C28H23N5O3/c34-28(35)21-7-5-20(6-8-21)26-24(12-11-22-10-9-19-3-1-2-4-23(19)30-22)31-27-25(13-14-29-33(26)27)32-15-17-36-18-16-32/h1-14H,15-18H2,(H,34,35)/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086728
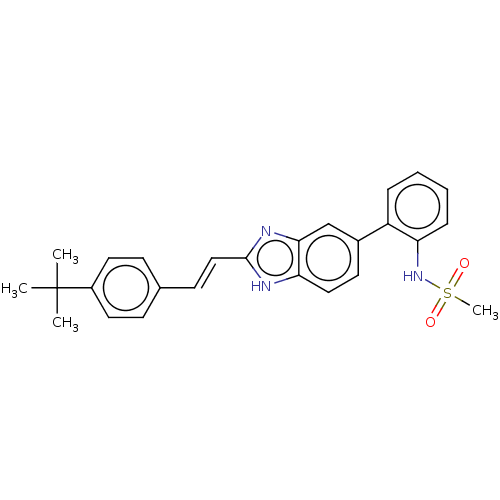
(CHEMBL3426347)Show SMILES CC(C)(C)c1ccc(\C=C\c2nc3cc(ccc3[nH]2)-c2ccccc2NS(C)(=O)=O)cc1 Show InChI InChI=1S/C26H27N3O2S/c1-26(2,3)20-13-9-18(10-14-20)11-16-25-27-23-15-12-19(17-24(23)28-25)21-7-5-6-8-22(21)29-32(4,30)31/h5-17,29H,1-4H3,(H,27,28)/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Rattus norvegicus (rat)) | BDBM193124

(US9193736, 40)Show SMILES OC(=O)c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C28H23N5O3/c34-28(35)21-7-5-20(6-8-21)26-24(12-11-22-10-9-19-3-1-2-4-23(19)30-22)31-27-25(13-14-29-33(26)27)32-15-17-36-18-16-32/h1-14H,15-18H2,(H,34,35)/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of rat PDE10A by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193112
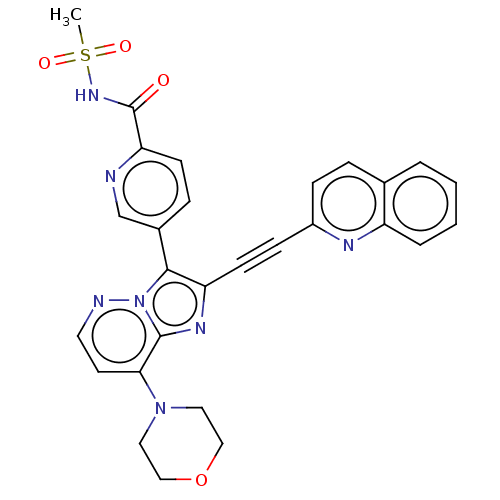
(US9193736, 28)Show SMILES CS(=O)(=O)NC(=O)c1ccc(cn1)-c1c(nc2c(ccnn12)N1CCOCC1)C#Cc1ccc2ccccc2n1 Show InChI InChI=1S/C28H23N7O4S/c1-40(37,38)33-28(36)24-10-7-20(18-29-24)26-23(11-9-21-8-6-19-4-2-3-5-22(19)31-21)32-27-25(12-13-30-35(26)27)34-14-16-39-17-15-34/h2-8,10,12-13,18H,14-17H2,1H3,(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193120
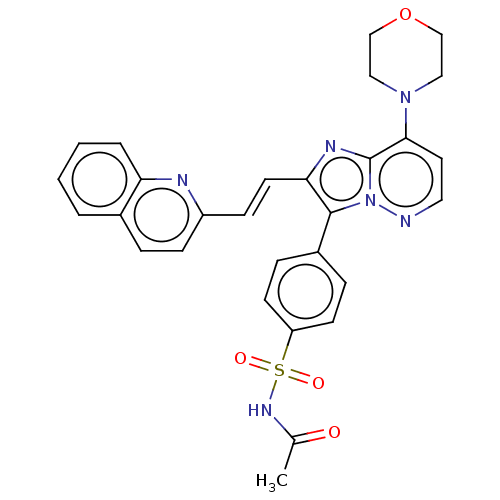
(US9193736, 36)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C29H26N6O4S/c1-20(36)33-40(37,38)24-11-7-22(8-12-24)28-26(13-10-23-9-6-21-4-2-3-5-25(21)31-23)32-29-27(14-15-30-35(28)29)34-16-18-39-19-17-34/h2-15H,16-19H2,1H3,(H,33,36)/b13-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193245
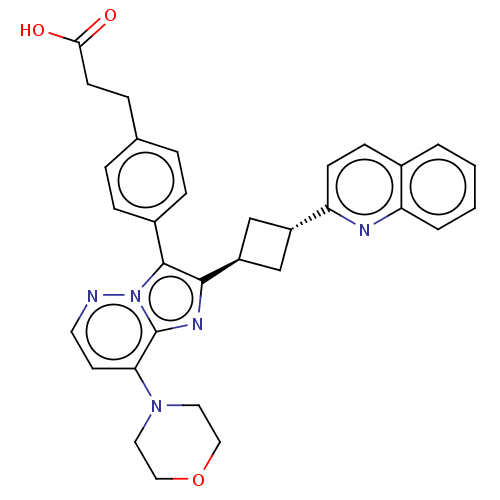
(US9193736, 169)Show SMILES OC(=O)CCc1ccc(cc1)-c1c(nc2c(ccnn12)N1CCOCC1)[C@H]1C[C@@H](C1)c1ccc2ccccc2n1 |wU:26.29,wD:28.34,(-.69,-8.73,;-1.01,-7.22,;-2.48,-6.75,;.13,-6.19,;-.19,-4.69,;.96,-3.66,;.64,-2.15,;1.78,-1.12,;3.24,-1.6,;3.56,-3.1,;2.42,-4.13,;4.39,-.56,;4.23,.97,;5.63,1.59,;6.67,.45,;8.21,.45,;8.98,-.88,;8.21,-2.22,;6.67,-2.22,;5.9,-.88,;8.98,1.78,;8.21,3.12,;8.98,4.45,;10.52,4.45,;11.29,3.12,;10.52,1.78,;2.89,1.74,;1.41,1.34,;1.01,2.83,;2.5,3.22,;-.33,3.6,;-.33,5.14,;-1.66,5.91,;-2.99,5.14,;-4.33,5.91,;-5.66,5.14,;-5.66,3.6,;-4.33,2.83,;-2.99,3.6,;-1.66,2.83,)| Show InChI InChI=1S/C32H31N5O3/c38-29(39)12-7-21-5-8-23(9-6-21)31-30(35-32-28(13-14-33-37(31)32)36-15-17-40-18-16-36)25-19-24(20-25)27-11-10-22-3-1-2-4-26(22)34-27/h1-6,8-11,13-14,24-25H,7,12,15-20H2,(H,38,39)/t24-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086744
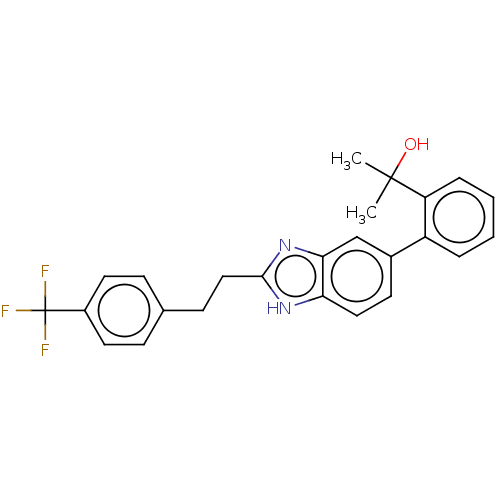
(CHEMBL3426376)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(CCc3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H23F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-8,10-13,15,31H,9,14H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
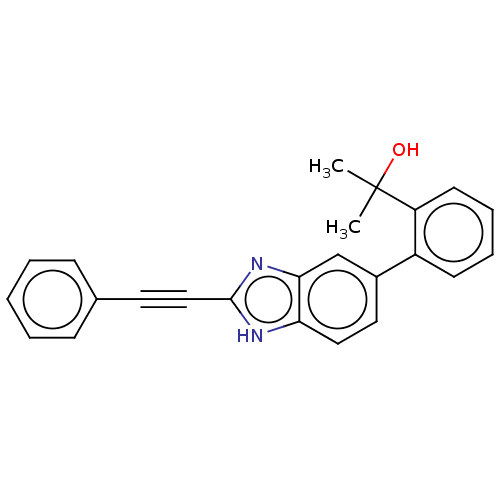
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086740
(CHEMBL3426378)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(nc2c1)C#Cc1ccccc1 Show InChI InChI=1S/C24H20N2O/c1-24(2,27)20-11-7-6-10-19(20)18-13-14-21-22(16-18)26-23(25-21)15-12-17-8-4-3-5-9-17/h3-11,13-14,16,27H,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193096
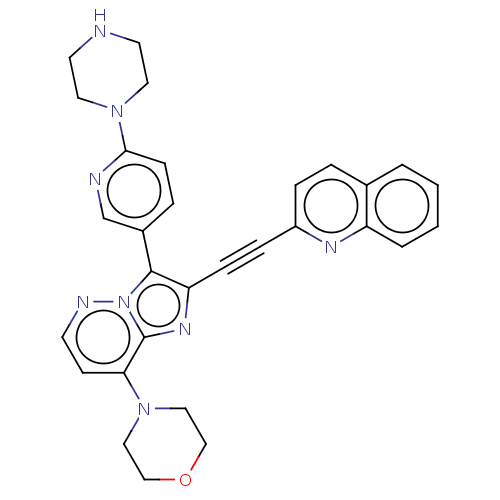
(US9193736, 12)Show SMILES C1CN(CCN1)c1ccc(cn1)-c1c(nc2c(ccnn12)N1CCOCC1)C#Cc1ccc2ccccc2n1 Show InChI InChI=1S/C30H28N8O/c1-2-4-25-22(3-1)5-7-24(34-25)8-9-26-29(23-6-10-28(32-21-23)37-15-13-31-14-16-37)38-30(35-26)27(11-12-33-38)36-17-19-39-20-18-36/h1-7,10-12,21,31H,13-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193244
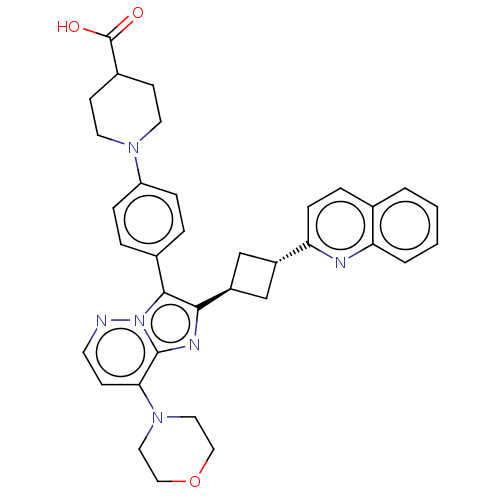
(US9193736, 168)Show SMILES OC(=O)C1CCN(CC1)c1ccc(cc1)-c1c(nc2c(ccnn12)N1CCOCC1)[C@H]1C[C@@H](C1)c1ccc2ccccc2n1 |wU:30.34,wD:32.39,(1.02,-15.19,;.7,-13.68,;-.76,-13.21,;1.85,-12.65,;1.53,-11.15,;2.67,-10.12,;4.14,-10.59,;4.46,-12.1,;3.31,-13.13,;5.28,-9.56,;4.96,-8.06,;6.11,-7.03,;7.57,-7.5,;7.89,-9.01,;6.75,-10.04,;8.72,-6.47,;8.55,-4.94,;9.96,-4.31,;10.99,-5.46,;12.53,-5.46,;13.3,-6.79,;12.53,-8.12,;10.99,-8.12,;10.22,-6.79,;13.3,-4.12,;12.53,-2.79,;13.3,-1.46,;14.84,-1.46,;15.61,-2.79,;14.84,-4.12,;7.22,-4.17,;5.73,-4.57,;5.33,-3.08,;6.82,-2.68,;4,-2.31,;4,-.77,;2.67,,;1.33,-.77,;;-1.33,-.77,;-1.33,-2.31,;,-3.08,;1.33,-2.31,;2.67,-3.08,)| Show InChI InChI=1S/C35H36N6O3/c42-35(43)25-12-15-39(16-13-25)28-8-5-24(6-9-28)33-32(38-34-31(11-14-36-41(33)34)40-17-19-44-20-18-40)27-21-26(22-27)30-10-7-23-3-1-2-4-29(23)37-30/h1-11,14,25-27H,12-13,15-22H2,(H,42,43)/t26-,27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193186
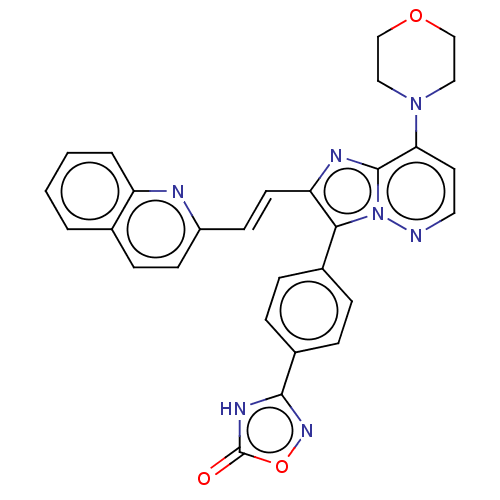
(US9193736, 102)Show SMILES O=c1[nH]c(no1)-c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C29H23N7O3/c37-29-33-27(34-39-29)21-7-5-20(6-8-21)26-24(12-11-22-10-9-19-3-1-2-4-23(19)31-22)32-28-25(13-14-30-36(26)28)35-15-17-38-18-16-35/h1-14H,15-18H2,(H,33,34,37)/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193184
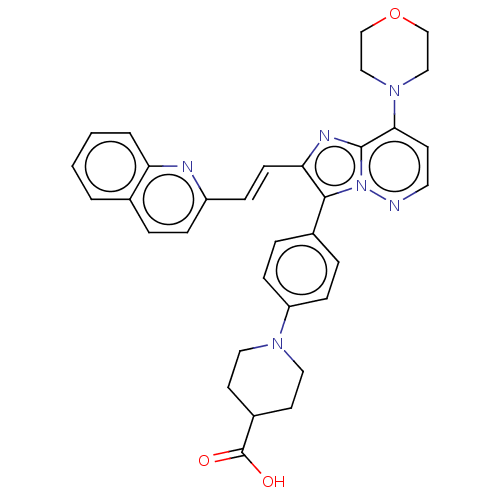
(US9193736, 100)Show SMILES OC(=O)C1CCN(CC1)c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C33H32N6O3/c40-33(41)25-14-17-37(18-15-25)27-10-6-24(7-11-27)31-29(12-9-26-8-5-23-3-1-2-4-28(23)35-26)36-32-30(13-16-34-39(31)32)38-19-21-42-22-20-38/h1-13,16,25H,14-15,17-22H2,(H,40,41)/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086739

(CHEMBL3426379)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(nc2c1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C25H19F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-8,10-13,15,31H,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086717

(JNJ-39439335 | Mavatrep)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086716
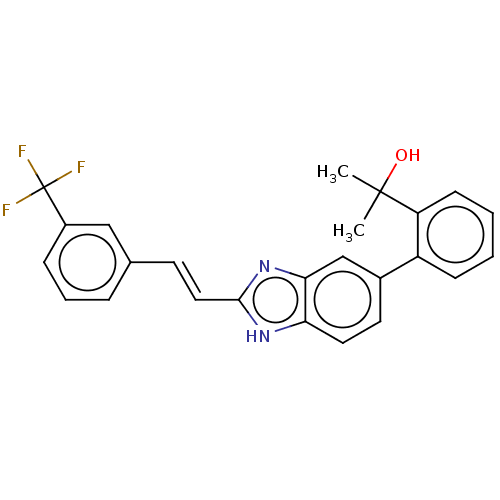
(CHEMBL3426361)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3cccc(c3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-9-4-3-8-19(20)17-11-12-21-22(15-17)30-23(29-21)13-10-16-6-5-7-18(14-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b13-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27135

(2-[(2R)-2-methylpyrrolidin-2-yl]-1H-1,3-benzodiazo...)Show InChI InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Malaya
Curated by ChEMBL
| Assay Description
Inhibition of PARP-1 (unknown origin) assessed as incorporation of biotinylated poly (ADP-ribose) onto histone protein after 60 mins by TACS-Sapphire... |
Bioorg Med Chem 23: 4669-80 (2015)
Article DOI: 10.1016/j.bmc.2015.05.051
BindingDB Entry DOI: 10.7270/Q2S75J2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086713

(CHEMBL3426364)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(Cl)cc3)nc2c1 Show InChI InChI=1S/C24H21ClN2O/c1-24(2,28)20-6-4-3-5-19(20)17-10-13-21-22(15-17)27-23(26-21)14-9-16-7-11-18(25)12-8-16/h3-15,28H,1-2H3,(H,26,27)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086714
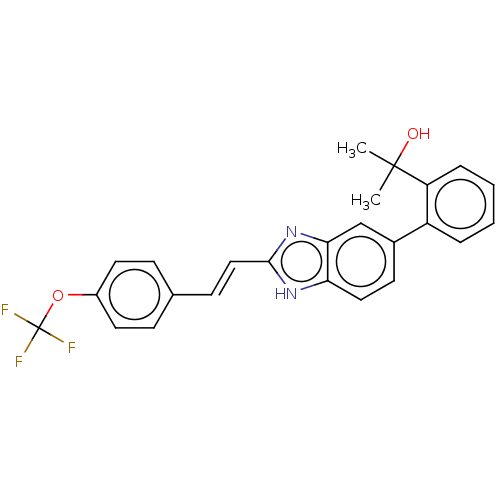
(CHEMBL3426363)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(OC(F)(F)F)cc3)nc2c1 Show InChI InChI=1S/C25H21F3N2O2/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)32-25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086742
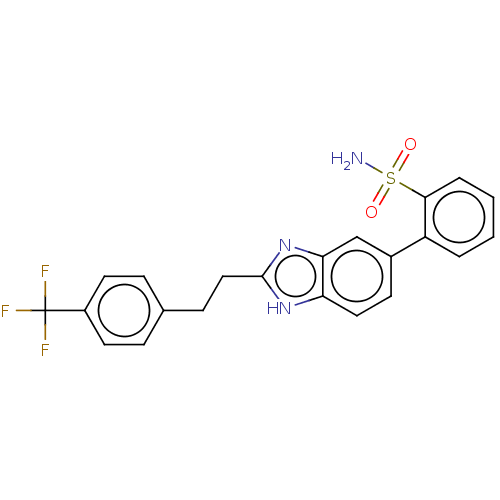
(CHEMBL3426377)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc2[nH]c(CCc3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C22H18F3N3O2S/c23-22(24,25)16-9-5-14(6-10-16)7-12-21-27-18-11-8-15(13-19(18)28-21)17-3-1-2-4-20(17)31(26,29)30/h1-6,8-11,13H,7,12H2,(H,27,28)(H2,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086749
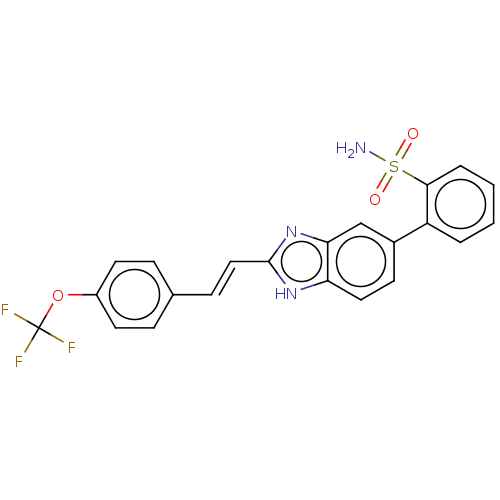
(CHEMBL3426369)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(OC(F)(F)F)cc3)nc2c1 Show InChI InChI=1S/C22H16F3N3O3S/c23-22(24,25)31-16-9-5-14(6-10-16)7-12-21-27-18-11-8-15(13-19(18)28-21)17-3-1-2-4-20(17)32(26,29)30/h1-13H,(H,27,28)(H2,26,29,30)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086717

(JNJ-39439335 | Mavatrep)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of pH 5-induced current measured for 150 secs by patc... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086864

(CHEMBL3426367)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(F)cc3)nc2c1 Show InChI InChI=1S/C21H16FN3O2S/c22-16-9-5-14(6-10-16)7-12-21-24-18-11-8-15(13-19(18)25-21)17-3-1-2-4-20(17)28(23,26)27/h1-13H,(H,24,25)(H2,23,26,27)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086848

(CHEMBL3426368)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc2[nH]c(\C=C\c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C22H16F3N3O2S/c23-22(24,25)16-9-5-14(6-10-16)7-12-21-27-18-11-8-15(13-19(18)28-21)17-3-1-2-4-20(17)31(26,29)30/h1-13H,(H,27,28)(H2,26,29,30)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086745

(CHEMBL3426375)Show SMILES CC(C)(O)c1ccccc1-c1ccc2[nH]c(\C=C/c3ccc(cc3)C(F)(F)F)nc2c1 Show InChI InChI=1S/C25H21F3N2O/c1-24(2,31)20-6-4-3-5-19(20)17-10-13-21-22(15-17)30-23(29-21)14-9-16-7-11-18(12-8-16)25(26,27)28/h3-15,31H,1-2H3,(H,29,30)/b14-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM193138
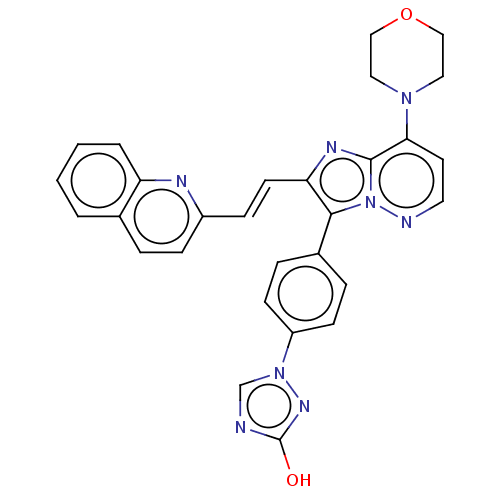
(US9193736, 54)Show SMILES Oc1ncn(n1)-c1ccc(cc1)-c1c(\C=C\c2ccc3ccccc3n2)nc2c(ccnn12)N1CCOCC1 Show InChI InChI=1S/C29H24N8O2/c38-29-30-19-36(34-29)23-10-6-21(7-11-23)27-25(12-9-22-8-5-20-3-1-2-4-24(20)32-22)33-28-26(13-14-31-37(27)28)35-15-17-39-18-16-35/h1-14,19H,15-18H2,(H,34,38)/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length PDE10A expressed in sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 26: 4216-22 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.054
BindingDB Entry DOI: 10.7270/Q2X63RGK |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50086748

(CHEMBL3426370)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc2[nH]c(\C=C\c3cccc(OC(F)(F)F)c3)nc2c1 Show InChI InChI=1S/C22H16F3N3O3S/c23-22(24,25)31-16-5-3-4-14(12-16)8-11-21-27-18-10-9-15(13-19(18)28-21)17-6-1-2-7-20(17)32(26,29)30/h1-13H,(H,27,28)(H2,26,29,30)/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx preincubated for 5 m... |
J Med Chem 58: 3859-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00132
BindingDB Entry DOI: 10.7270/Q2154JS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data