

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096606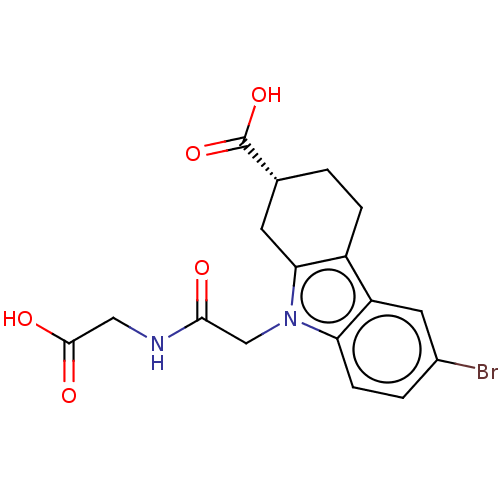 (CHEMBL3577279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096604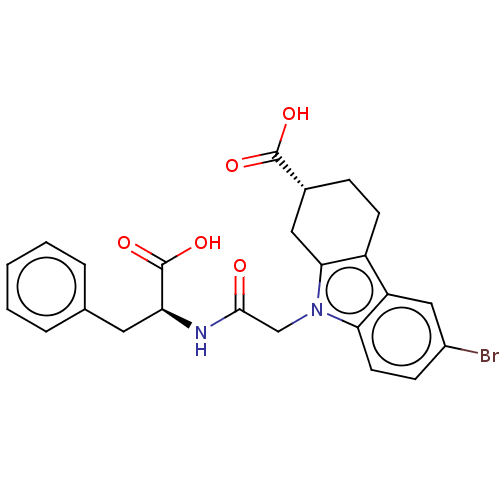 (CHEMBL3577290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096605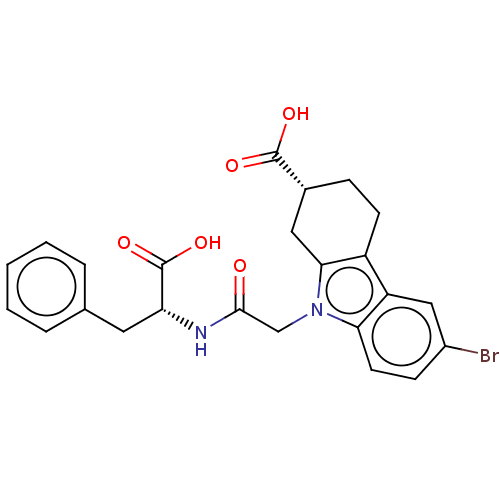 (CHEMBL3577289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096608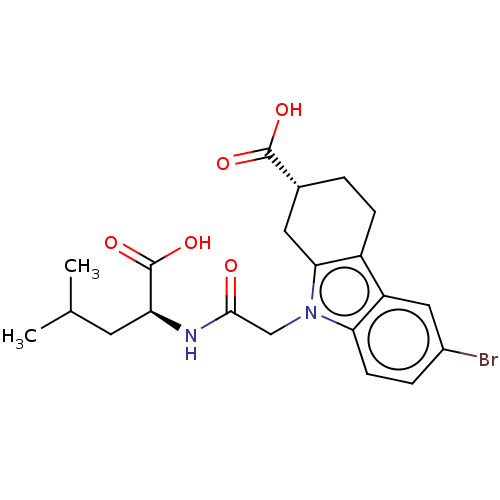 (CHEMBL3577288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096611 (CHEMBL3577285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096607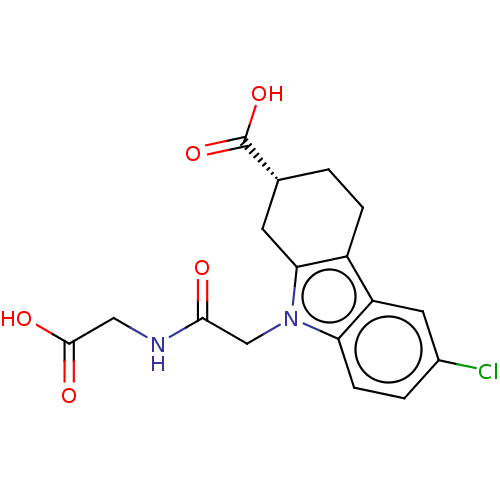 (CHEMBL3577278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096609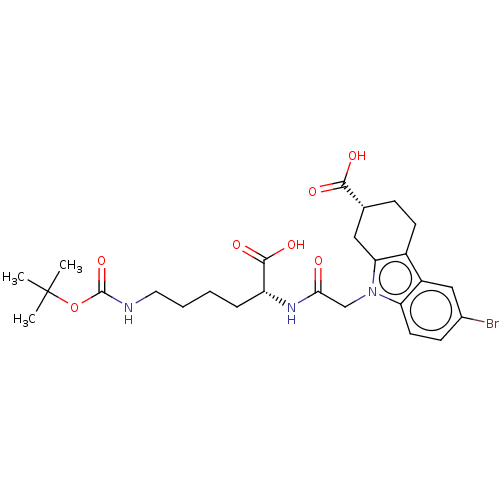 (CHEMBL3577287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096638 (CHEMBL3577268) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096614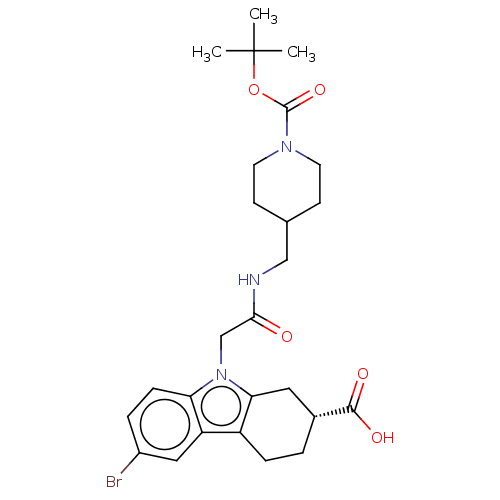 (CHEMBL3577282) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096634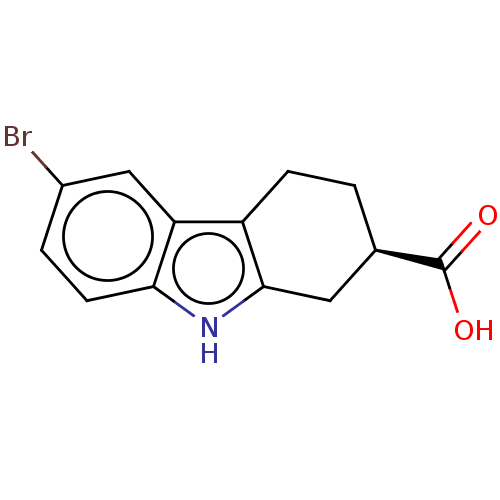 (CHEMBL3577272) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096612 (CHEMBL3577284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096635 (CHEMBL3577271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096613 (CHEMBL3577283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096618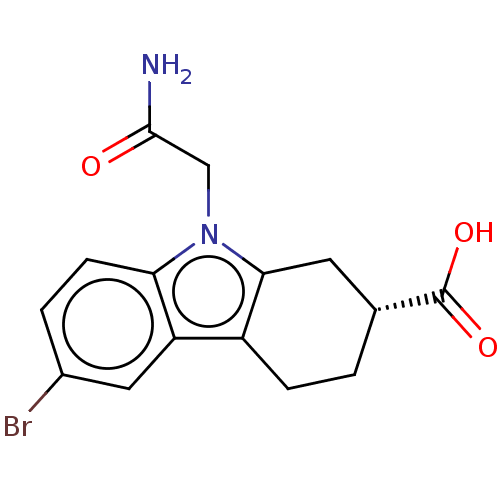 (CHEMBL3577276) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096617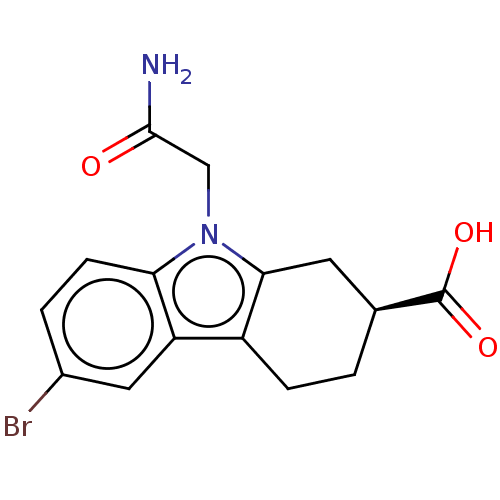 (CHEMBL3577277) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096641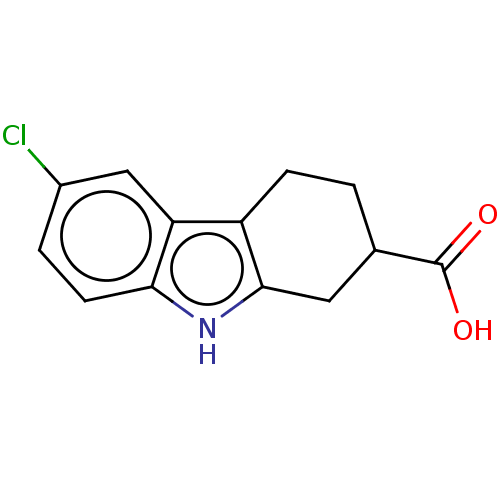 (CHEMBL3277053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096610 (CHEMBL3577286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096633 (CHEMBL3577273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096616 (CHEMBL3577280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096615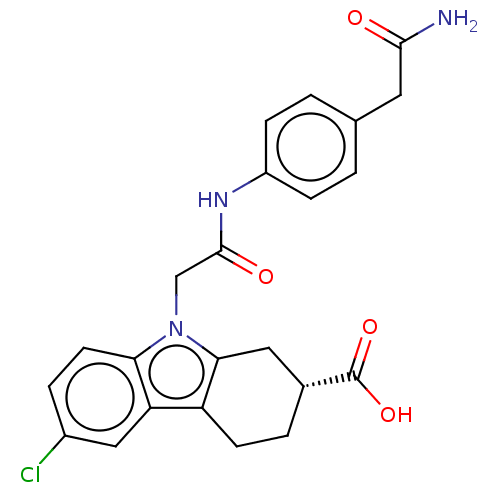 (CHEMBL3577281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096632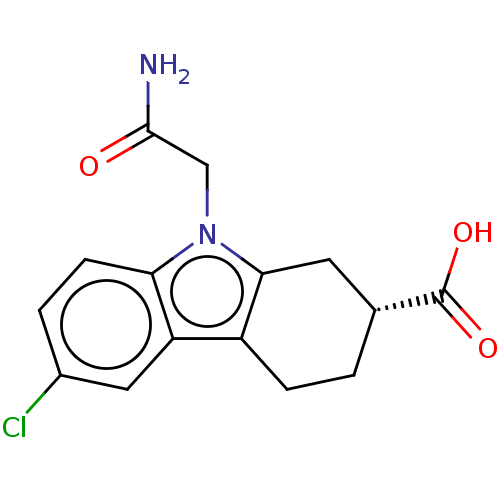 (CHEMBL3577274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096637 (CHEMBL3577269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096636 (CHEMBL3577270) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta sliding clamp (Escherichia coli (strain K12)) | BDBM50096631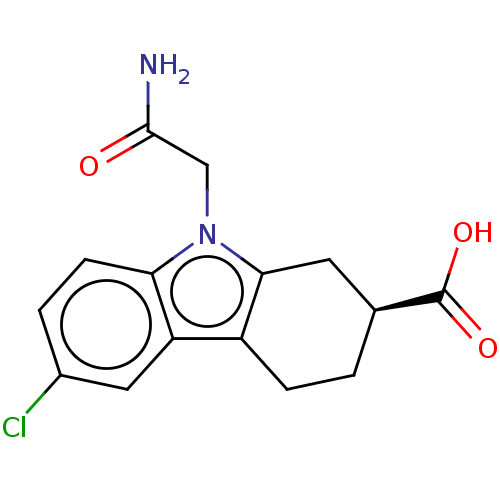 (CHEMBL3577275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong and The Illawarra Health and Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA sliding clamp protein using N-fluorescein (FAM)-QLDLF-OH tracer by fluorescence polarization assay | J Med Chem 58: 4693-702 (2015) Article DOI: 10.1021/acs.jmedchem.5b00232 BindingDB Entry DOI: 10.7270/Q2KK9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit [3H]haloperidol binding to dopamine receptor in rat striatal homogenate | J Med Chem 28: 1811-7 (1985) BindingDB Entry DOI: 10.7270/Q2KW5J7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50334150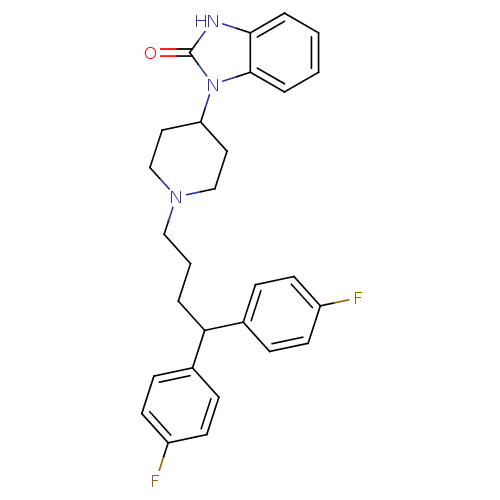 (1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. | J Med Chem 28: 606-12 (1985) BindingDB Entry DOI: 10.7270/Q2TB193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50334150 (1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. | J Med Chem 28: 606-12 (1985) BindingDB Entry DOI: 10.7270/Q2TB193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225369 (CHEMBL295712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. | J Med Chem 28: 606-12 (1985) BindingDB Entry DOI: 10.7270/Q2TB193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353767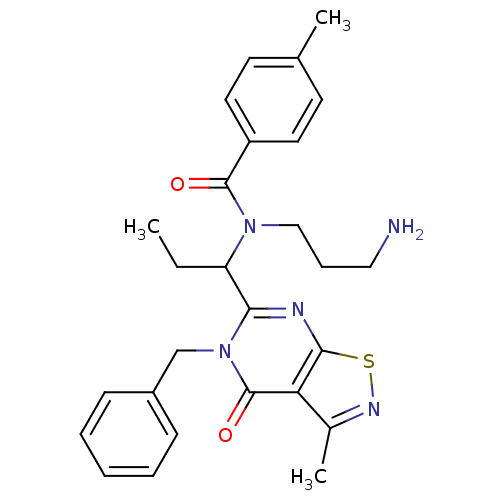 (CHEMBL1829430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353767 (CHEMBL1829430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353788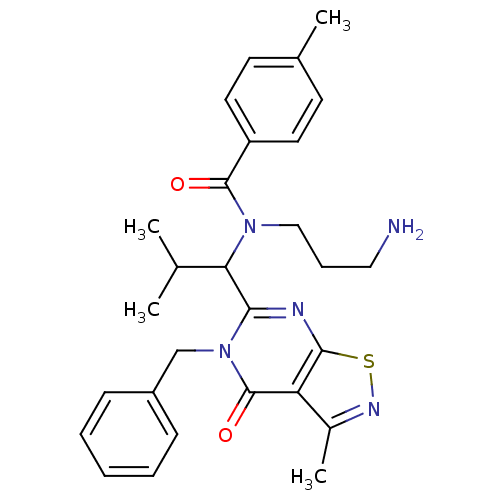 (CHEMBL1829433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353788 (CHEMBL1829433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353767 (CHEMBL1829430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353799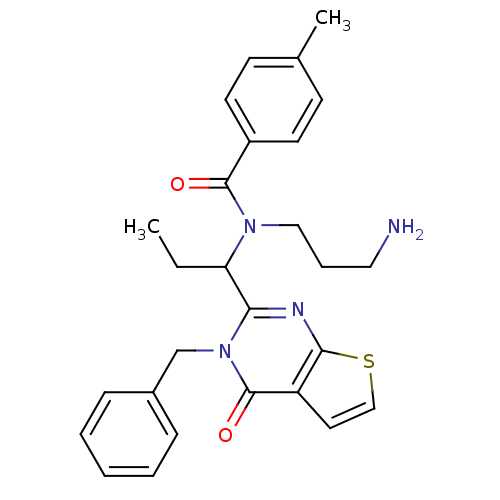 (CHEMBL1829424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353799 (CHEMBL1829424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353820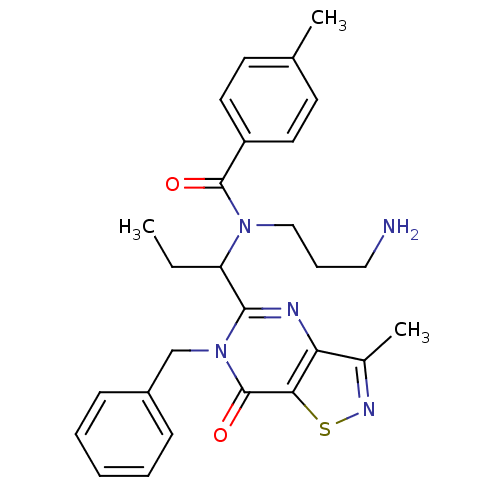 (CHEMBL1829432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353799 (CHEMBL1829424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353821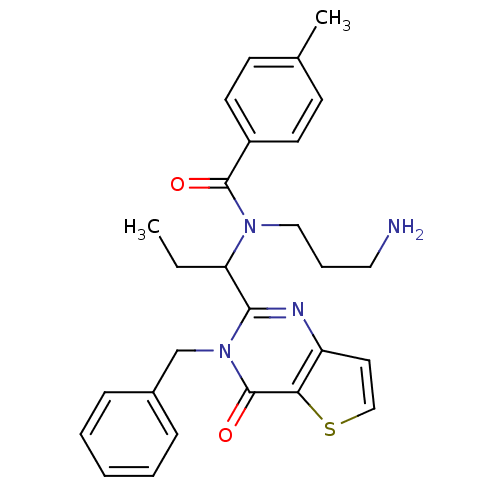 (CHEMBL1829246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353823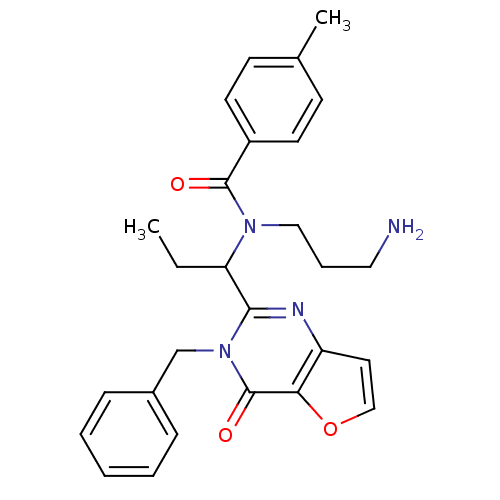 (CHEMBL1829422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225510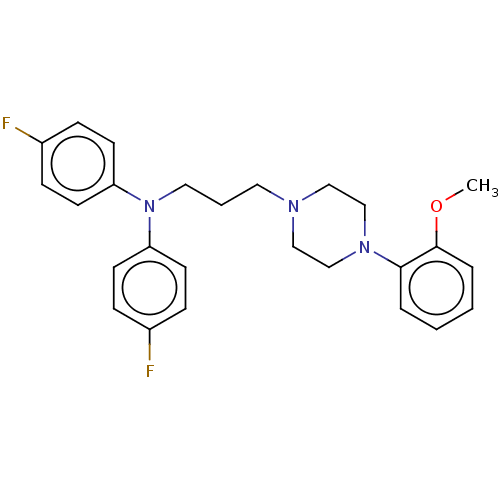 (CHEMBL156395) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. | J Med Chem 28: 606-12 (1985) BindingDB Entry DOI: 10.7270/Q2TB193Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353822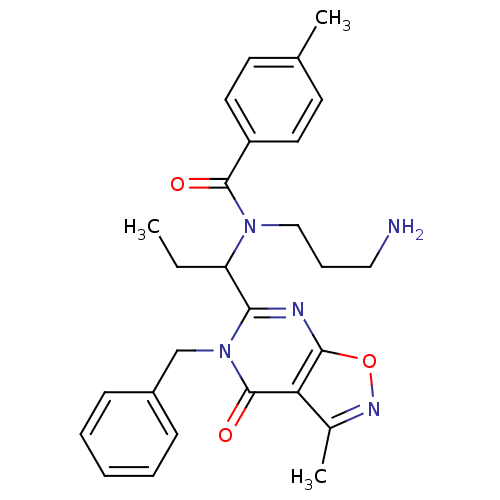 (CHEMBL1829431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353761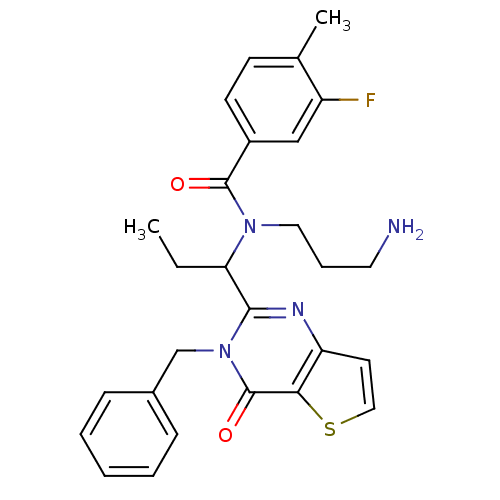 (CHEMBL1829406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of [3H]WB-4101 binding to alpha-1 adrenergic receptor of rat frontal cortex | J Med Chem 28: 1811-7 (1985) BindingDB Entry DOI: 10.7270/Q2KW5J7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353760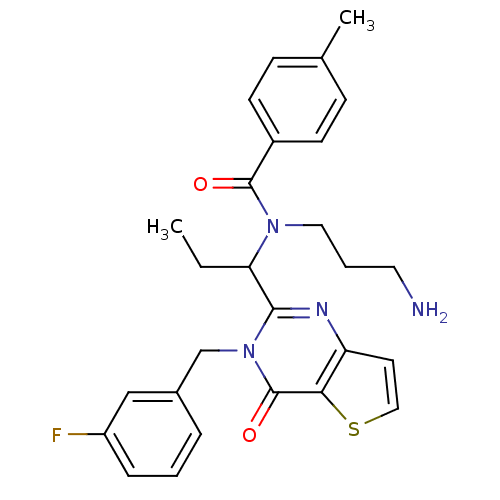 (CHEMBL1829248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353762 (CHEMBL1829423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353763 (CHEMBL1829401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353764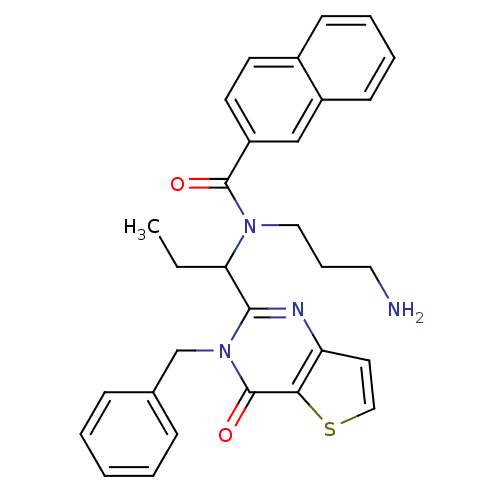 (CHEMBL1829420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein KIF11 (Homo sapiens (Human)) | BDBM50353765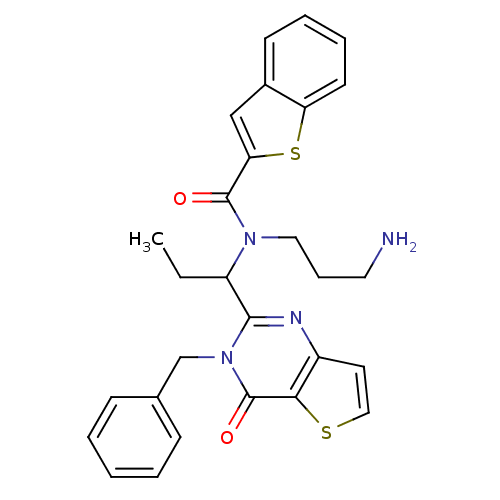 (CHEMBL1829418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His6-tagged KSP ATPase activity after 1 hr by malachite green assay | J Med Chem 54: 6734-50 (2011) Article DOI: 10.1021/jm200629m BindingDB Entry DOI: 10.7270/Q2ZC838Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50113978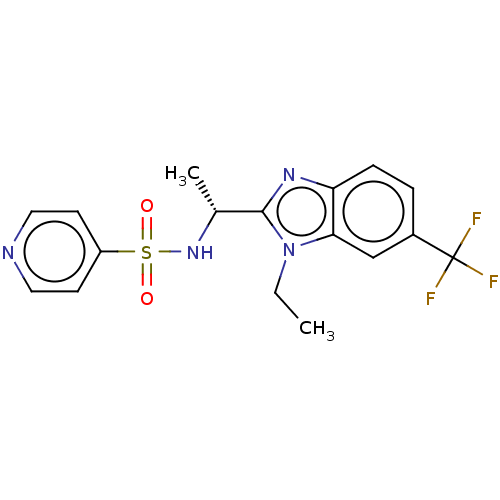 (CHEMBL3605543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for inhibition of [3H]QNB binding to Muscarinic acetylcholine receptor | J Med Chem 28: 1811-7 (1985) BindingDB Entry DOI: 10.7270/Q2KW5J7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 210 total ) | Next | Last >> |