 Found 132 hits with Last Name = 'lieberman' and Initial = 'rl'
Found 132 hits with Last Name = 'lieberman' and Initial = 'rl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50117215
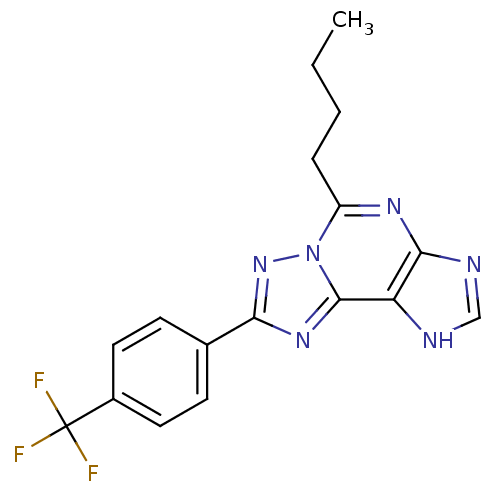
(5-Butyl-8-(4-trifluoromethyl-phenyl)-3H-[1,2,4]tri...)Show SMILES CCCCc1nc2nc[nH]c2c2nc(nn12)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H15F3N6/c1-2-3-4-12-23-15-13(21-9-22-15)16-24-14(25-26(12)16)10-5-7-11(8-6-10)17(18,19)20/h5-9H,2-4H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM108255
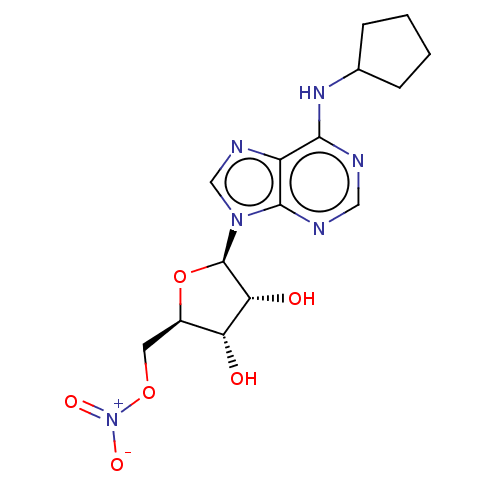
(US8609833, 86)Show SMILES O[C@@H]1[C@@H](CO[N+]([O-])=O)O[C@H]([C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C16H23N5O3/c1-2-10-12(22)13(23)16(24-10)21-8-19-11-14(17-7-18-15(11)21)20-9-5-3-4-6-9/h7-10,12-13,16,22-23H,2-6H2,1H3,(H,17,18,20)/t10-,12-,13-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A1 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ZM241385 from human adenosine A2A receptor by scintillation spectroscopy |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A3 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50118810
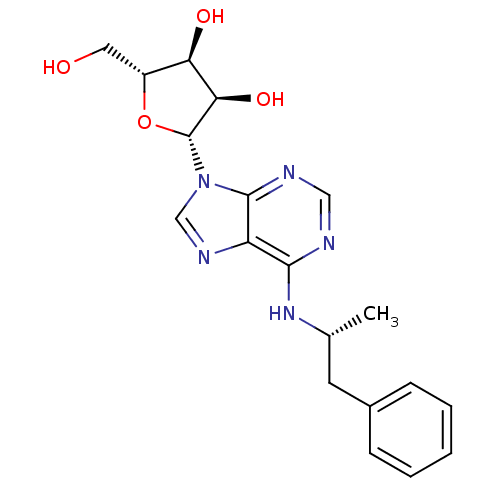
((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...)Show SMILES C[C@H](Cc1ccccc1)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23)/t11-,13-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A1 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A1 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50148580
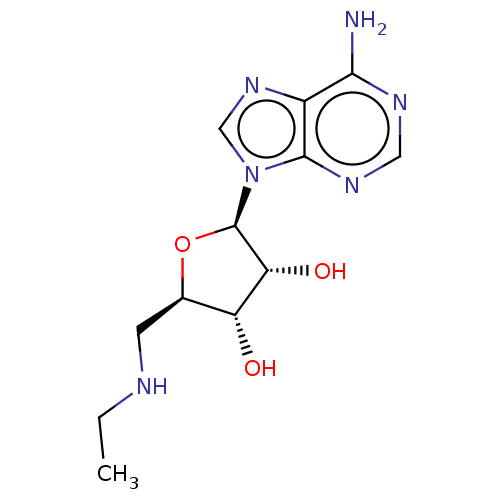
(CHEMBL3770601)Show SMILES CCNC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-2-14-3-6-8(19)9(20)12(21-6)18-5-17-7-10(13)15-4-16-11(7)18/h4-6,8-9,12,14,19-20H,2-3H2,1H3,(H2,13,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PIA from human recombinant adenosine A1 receptor after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50118810

((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-1-phenyl...)Show SMILES C[C@H](Cc1ccccc1)Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23)/t11-,13-,15-,16-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A3 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50074225

(6-Ethyl-5-ethylsulfanylcarbonyl-2-phenyl-4-propyl-...)Show SMILES CCCOC(=O)c1c(CCC)c(C(=O)SCC)c(CC)nc1-c1ccccc1 Show InChI InChI=1S/C23H29NO3S/c1-5-12-17-19(23(26)28-8-4)18(7-3)24-21(16-13-10-9-11-14-16)20(17)22(25)27-15-6-2/h9-11,13-14H,5-8,12,15H2,1-4H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A3 receptor in human NPE cells assessed as inhibition of adenosine-triggered cell shrinkage |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50148580

(CHEMBL3770601)Show SMILES CCNC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-2-14-3-6-8(19)9(20)12(21-6)18-5-17-7-10(13)15-4-16-11(7)18/h4-6,8-9,12,14,19-20H,2-3H2,1H3,(H2,13,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine 2A receptor after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50148580

(CHEMBL3770601)Show SMILES CCNC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-2-14-3-6-8(19)9(20)12(21-6)18-5-17-7-10(13)15-4-16-11(7)18/h4-6,8-9,12,14,19-20H,2-3H2,1H3,(H2,13,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from human recombinant adenosine 2A receptor after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50148601

(CHEMBL3771367)Show SMILES CCOC(=O)C1=C(C)NC(=C(C1C#Cc1ccccc1)C(=O)Oc1ccccc1)c1ccccc1 |c:5,9| Show InChI InChI=1S/C30H25NO4/c1-3-34-29(32)26-21(2)31-28(23-15-9-5-10-16-23)27(30(33)35-24-17-11-6-12-18-24)25(26)20-19-22-13-7-4-8-14-22/h4-18,25,31H,3H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human brain adenosine A3 receptor expressed in HEK293 cells |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50453216
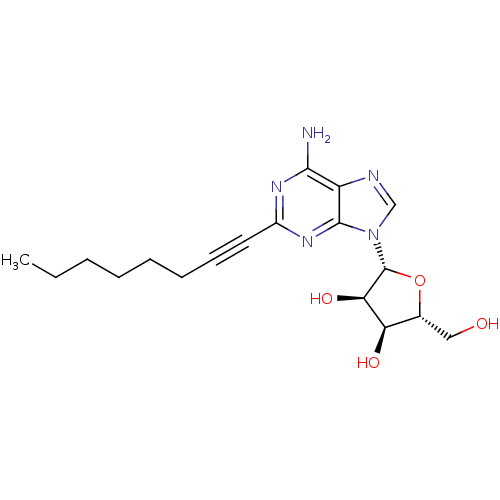
(2-(l-hexyn-l-yl)adenosine (8, YT-146) | CHEMBL2113...)Show SMILES CCCCCCC#Cc1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 |r| Show InChI InChI=1S/C18H25N5O4/c1-2-3-4-5-6-7-8-12-21-16(19)13-17(22-12)23(10-20-13)18-15(26)14(25)11(9-24)27-18/h10-11,14-15,18,24-26H,2-6,9H2,1H3,(H2,19,21,22)/t11-,14-,15-,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGS2168 from human recombinant adenosine A2A receptor |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50148599

(CHEMBL3770782)Show SMILES Nc1nc(nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C#CCCCCC#N |r| Show InChI InChI=1S/C17H20N6O4/c18-7-5-3-1-2-4-6-11-21-15(19)12-16(22-11)23(9-20-12)17-14(26)13(25)10(8-24)27-17/h9-10,13-14,17,24-26H,1-3,5,8H2,(H2,19,21,22)/t10-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGS2168 from human recombinant adenosine A2A receptor |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50118812

((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O4/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Binding affinity to adenosine A1 receptor (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM35804

((CGS21680) 3-(4-{2-[6-Amino-9-(5-ethylcarbamoylmet...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(NCCc3ccc(CCC(O)=O)cc3)nc12 Show InChI InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50148600

(CHEMBL3770003)Show SMILES CCOC(=O)C1=C(C)NC(=C(C1\C=C\c1ccccc1)C(=O)OCC)c1ccccc1 |c:5,9| Show InChI InChI=1S/C26H27NO4/c1-4-30-25(28)22-18(3)27-24(20-14-10-7-11-15-20)23(26(29)31-5-2)21(22)17-16-19-12-8-6-9-13-19/h6-17,21,27H,4-5H2,1-3H3/b17-16+ | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human brain adenosine A3 receptor expressed in HEK293 cells |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50148580

(CHEMBL3770601)Show SMILES CCNC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-2-14-3-6-8(19)9(20)12(21-6)18-5-17-7-10(13)15-4-16-11(7)18/h4-6,8-9,12,14,19-20H,2-3H2,1H3,(H2,13,15,16)/t6-,8-,9-,12-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant human adenosine A2B receptor assessed as cAMP accumulation preincubated for 45 mins followed by forskolin addition me... |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50148602
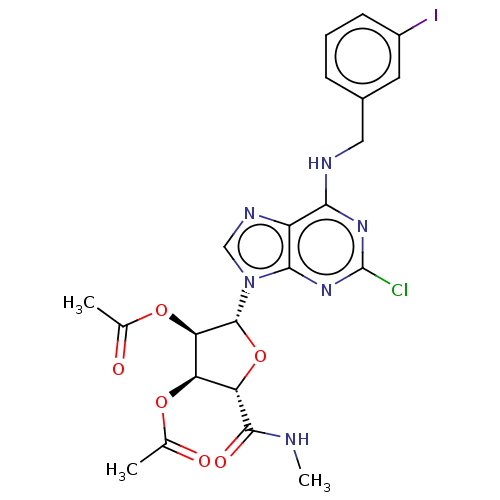
(CHEMBL3770194)Show SMILES CNC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H]1OC(C)=O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C22H22ClIN6O6/c1-10(31)34-15-16(20(33)25-3)36-21(17(15)35-11(2)32)30-9-27-14-18(28-22(23)29-19(14)30)26-8-12-5-4-6-13(24)7-12/h4-7,9,15-17,21H,8H2,1-3H3,(H,25,33)(H,26,28,29)/t15-,16+,17-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113762

(1-Deoxygalactonojirimycin (DGJ) | 2-(hydroxymethyl...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50148579
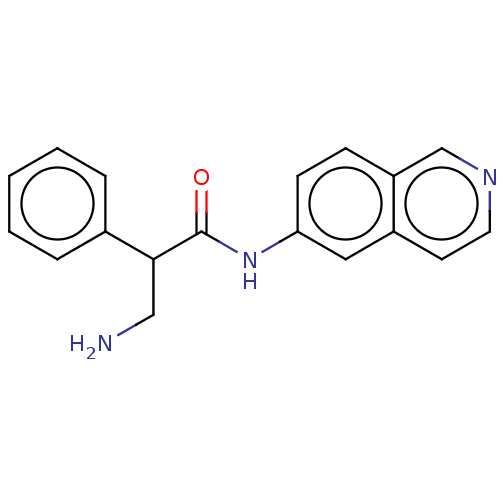
(CHEMBL3770836)Show InChI InChI=1S/C18H17N3O/c19-11-17(13-4-2-1-3-5-13)18(22)21-16-7-6-15-12-20-9-8-14(15)10-16/h1-10,12,17H,11,19H2,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50148579

(CHEMBL3770836)Show InChI InChI=1S/C18H17N3O/c19-11-17(13-4-2-1-3-5-13)18(22)21-16-7-6-15-12-20-9-8-14(15)10-16/h1-10,12,17H,11,19H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113758
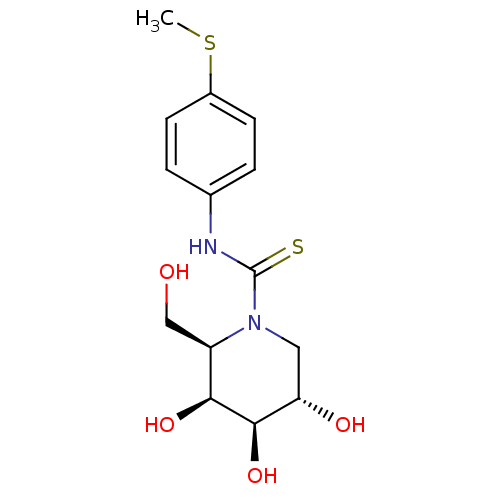
(DGJ-pMe SPhT)Show SMILES CSc1ccc(NC(=S)N2C[C@H](O)[C@@H](O)[C@@H](O)[C@H]2CO)cc1 |r| Show InChI InChI=1S/C14H20N2O4S2/c1-22-9-4-2-8(3-5-9)15-14(21)16-6-11(18)13(20)12(19)10(16)7-17/h2-5,10-13,17-20H,6-7H2,1H3,(H,15,21)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50148578
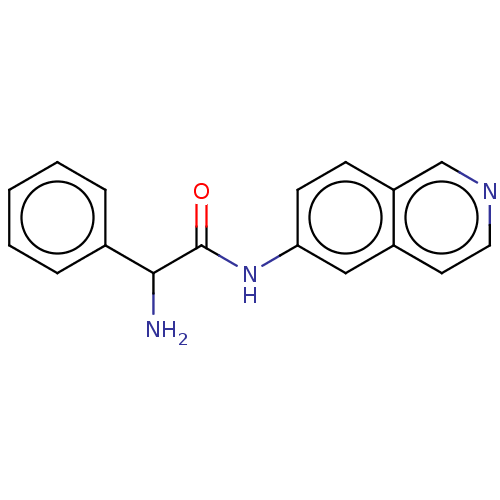
(CHEMBL3770730)Show InChI InChI=1S/C17H15N3O/c18-16(12-4-2-1-3-5-12)17(21)20-15-7-6-14-11-19-9-8-13(14)10-15/h1-11,16H,18H2,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM25474

(JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...)Show SMILES CN(C)CCOc1cc(ccc1NC(=O)C1COc2ccccc2O1)-c1cn[nH]c1 Show InChI InChI=1S/C22H24N4O4/c1-26(2)9-10-28-20-11-15(16-12-23-24-13-16)7-8-17(20)25-22(27)21-14-29-18-5-3-4-6-19(18)30-21/h3-8,11-13,21H,9-10,14H2,1-2H3,(H,23,24)(H,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) using STK2 as substrate after 1 hr |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113762

(1-Deoxygalactonojirimycin (DGJ) | 2-(hydroxymethyl...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50148578

(CHEMBL3770730)Show InChI InChI=1S/C17H15N3O/c18-16(12-4-2-1-3-5-12)17(21)20-15-7-6-14-11-19-9-8-13(14)10-15/h1-11,16H,18H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113758

(DGJ-pMe SPhT)Show SMILES CSc1ccc(NC(=S)N2C[C@H](O)[C@@H](O)[C@@H](O)[C@H]2CO)cc1 |r| Show InChI InChI=1S/C14H20N2O4S2/c1-22-9-4-2-8(3-5-9)15-14(21)16-6-11(18)13(20)12(19)10(16)7-17/h2-5,10-13,17-20H,6-7H2,1H3,(H,15,21)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113757
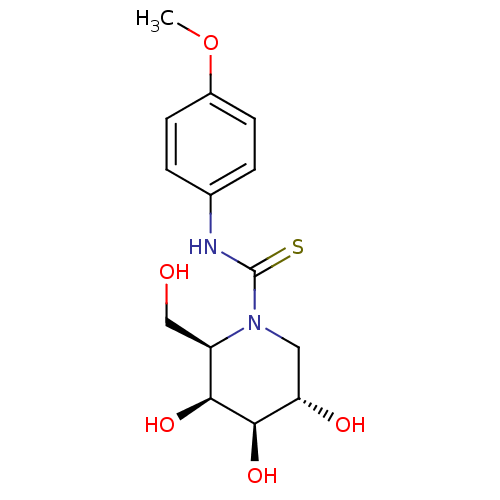
(DGJ-pMe OPhT)Show SMILES COc1ccc(NC(=S)N2C[C@H](O)[C@@H](O)[C@@H](O)[C@H]2CO)cc1 |r| Show InChI InChI=1S/C14H20N2O5S/c1-21-9-4-2-8(3-5-9)15-14(22)16-6-11(18)13(20)12(19)10(16)7-17/h2-5,10-13,17-20H,6-7H2,1H3,(H,15,22)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50087135

(CHEMBL3426621)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(F)c12 |r| Show InChI InChI=1S/C15H18FN3O2S/c1-11-8-17-6-3-7-19(11)22(20,21)14-5-2-4-12-9-18-10-13(16)15(12)14/h2,4-5,9-11,17H,3,6-8H2,1H3/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Transmembrane domain-containing protein TMIGD3
(Homo sapiens (Human)) | BDBM50118803

((2R,3R,4S,5S)-3,4-dihydroxy-2-[6-(3-iodo-benzylami...)Show SMILES O[C@@H]1[C@H](O)[C@]2(CCNC2=O)O[C@H]1n1cnc2c(NCc3cccc(I)c3)ncnc12 Show InChI InChI=1S/C19H19IN6O4/c20-11-3-1-2-10(6-11)7-22-15-12-16(24-8-23-15)26(9-25-12)17-13(27)14(28)19(30-17)4-5-21-18(19)29/h1-3,6,8-9,13-14,17,27-28H,4-5,7H2,(H,21,29)(H,22,23,24)/t13-,14+,17-,19+/m1/s1 | UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at adenosine A3 receptor in human NPE cells assessed as inhibition of adenosine-triggered cell shrinkage |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113759

(DGJ-pF PhT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H17FN2O4S/c14-7-1-3-8(4-2-7)15-13(21)16-5-10(18)12(20)11(19)9(16)6-17/h1-4,9-12,17-20H,5-6H2,(H,15,21)/t9-,10+,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50087135

(CHEMBL3426621)Show SMILES C[C@H]1CNCCCN1S(=O)(=O)c1cccc2cncc(F)c12 |r| Show InChI InChI=1S/C15H18FN3O2S/c1-11-8-17-6-3-7-19(11)22(20,21)14-5-2-4-12-9-18-10-13(16)15(12)14/h2,4-5,9-11,17H,3,6-8H2,1H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM25474

(JMC516642 Compound 5 | N-{2-[2-(dimethylamino)etho...)Show SMILES CN(C)CCOc1cc(ccc1NC(=O)C1COc2ccccc2O1)-c1cn[nH]c1 Show InChI InChI=1S/C22H24N4O4/c1-26(2)9-10-28-20-11-15(16-12-23-24-13-16)7-8-17(20)25-22(27)21-14-29-18-5-3-4-6-19(18)30-21/h3-8,11-13,21H,9-10,14H2,1-2H3,(H,23,24)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50182801
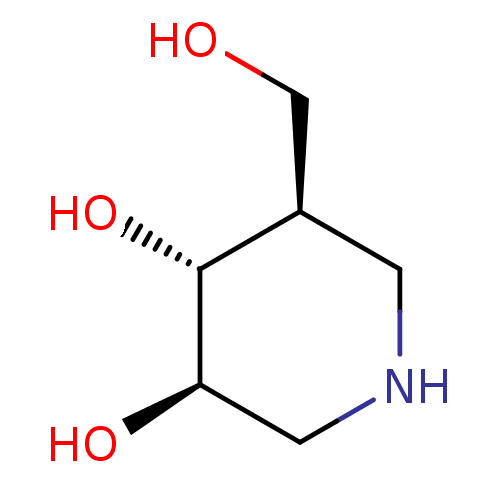
((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of GCase assessed as 4-methylumbelliferone release assay after 30 mins by fluorimetry |
Nat Chem Biol 3: 101-7 (2007)
Article DOI: 10.1038/nchembio850
BindingDB Entry DOI: 10.7270/Q2639PX9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113757

(DGJ-pMe OPhT)Show SMILES COc1ccc(NC(=S)N2C[C@H](O)[C@@H](O)[C@@H](O)[C@H]2CO)cc1 |r| Show InChI InChI=1S/C14H20N2O5S/c1-21-9-4-2-8(3-5-9)15-14(22)16-6-11(18)13(20)12(19)10(16)7-17/h2-5,10-13,17-20H,6-7H2,1H3,(H,15,22)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50148577

(CHEMBL1994526)Show SMILES CC(N)C1CCC(CC1)C(=O)Nc1ccncc1 |(2.67,4.62,;1.33,3.85,;,4.62,;1.33,2.31,;,1.54,;;1.33,-.77,;2.67,,;2.67,1.54,;1.33,-2.31,;,-3.08,;2.67,-3.08,;2.67,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;4,-5.39,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50148577

(CHEMBL1994526)Show SMILES CC(N)C1CCC(CC1)C(=O)Nc1ccncc1 |(2.67,4.62,;1.33,3.85,;,4.62,;1.33,2.31,;,1.54,;;1.33,-.77,;2.67,,;2.67,1.54,;1.33,-2.31,;,-3.08,;2.67,-3.08,;2.67,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;4,-5.39,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14027
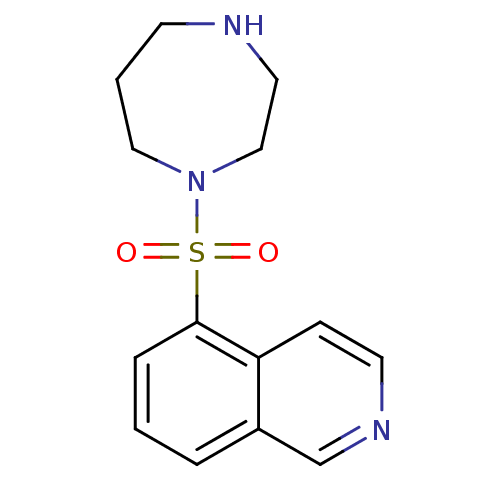
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14027

(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) |
J Med Chem 59: 788-809 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00828
BindingDB Entry DOI: 10.7270/Q25M67MM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113759

(DGJ-pF PhT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)Nc1ccc(F)cc1 |r| Show InChI InChI=1S/C13H17FN2O4S/c14-7-1-3-8(4-2-7)15-13(21)16-5-10(18)12(20)11(19)9(16)6-17/h1-4,9-12,17-20H,5-6H2,(H,15,21)/t9-,10+,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113756

(DGJ-NphT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C17H20N2O4S/c20-9-13-15(22)16(23)14(21)8-19(13)17(24)18-12-7-3-5-10-4-1-2-6-11(10)12/h1-7,13-16,20-23H,8-9H2,(H,18,24)/t13-,14+,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113756

(DGJ-NphT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)Nc1cccc2ccccc12 |r| Show InChI InChI=1S/C17H20N2O4S/c20-9-13-15(22)16(23)14(21)8-19(13)17(24)18-12-7-3-5-10-4-1-2-6-11(10)12/h1-7,13-16,20-23H,8-9H2,(H,18,24)/t13-,14+,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113760

(pF PhIM-DGJ)Show SMILES O[C@H]1CN2C(CS\C2=N\c2ccc(F)cc2)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H15FN2O3S/c14-7-1-3-8(4-2-7)15-13-16-5-10(17)12(19)11(18)9(16)6-20-13/h1-4,9-12,17-19H,5-6H2/b15-13+/t9?,10-,11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113761
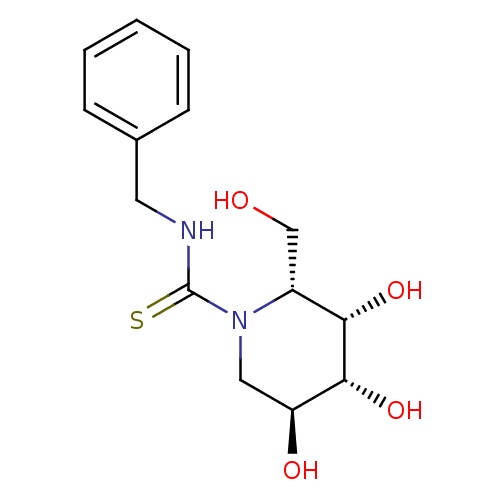
(DGJ-BnT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)NCc1ccccc1 |r| Show InChI InChI=1S/C14H20N2O4S/c17-8-10-12(19)13(20)11(18)7-16(10)14(21)15-6-9-4-2-1-3-5-9/h1-5,10-13,17-20H,6-8H2,(H,15,21)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.41E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113760

(pF PhIM-DGJ)Show SMILES O[C@H]1CN2C(CS\C2=N\c2ccc(F)cc2)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H15FN2O3S/c14-7-1-3-8(4-2-7)15-13-16-5-10(17)12(19)11(18)9(16)6-20-13/h1-4,9-12,17-19H,5-6H2/b15-13+/t9?,10-,11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.67E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM113761

(DGJ-BnT)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1C(=S)NCc1ccccc1 |r| Show InChI InChI=1S/C14H20N2O4S/c17-8-10-12(19)13(20)11(18)7-16(10)14(21)15-6-9-4-2-1-3-5-9/h1-5,10-13,17-20H,6-8H2,(H,15,21)/t10-,11+,12+,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Tottori University , Yonago 683-8503, Japan
| Assay Description
For inhibition assay, 0.1% Triton X-100 extracts were mixed with 4-MU substrates ( 5 mM 4-MU α-ᴅ-galactopyranoside and 0.1 M N-acetyl-J... |
ACS Chem Biol 9: 1460-9 (2014)
Article DOI: 10.1021/cb500143h
BindingDB Entry DOI: 10.7270/Q2XS5T16 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50087812
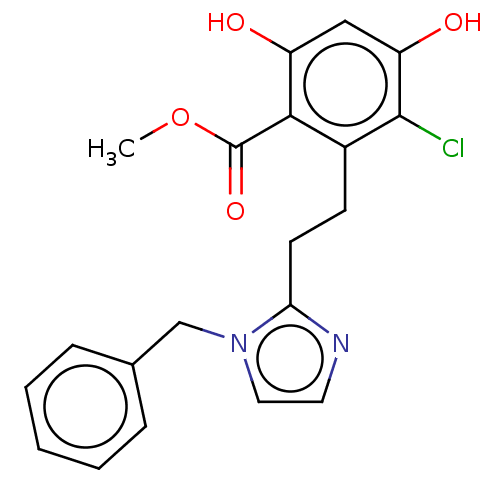
(CHEMBL3426787)Show SMILES COC(=O)c1c(O)cc(O)c(Cl)c1CCc1nccn1Cc1ccccc1 Show InChI InChI=1S/C20H19ClN2O4/c1-27-20(26)18-14(19(21)16(25)11-15(18)24)7-8-17-22-9-10-23(17)12-13-5-3-2-4-6-13/h2-6,9-11,24-25H,7-8,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Apparent binding affinity to Hsp90alpha (unknown origin) after 24 hrs by fluorescence polarization assay using FITC-GDA as tracer |
J Med Chem 59: 3471-88 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00085
BindingDB Entry DOI: 10.7270/Q2H133X3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50169783

(CHEMBL3805570)Show SMILES COC(=O)c1c(O)cc(O)c(Cl)c1CCc1nccn1Cc1ccc(F)cc1 Show InChI InChI=1S/C20H18ClFN2O4/c1-28-20(27)18-14(19(21)16(26)10-15(18)25)6-7-17-23-8-9-24(17)11-12-2-4-13(22)5-3-12/h2-5,8-10,25-26H,6-7,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a |
The University of Kansas
Curated by ChEMBL
| Assay Description
Apparent binding affinity to Hsp90alpha (unknown origin) after 24 hrs by fluorescence polarization assay using FITC-GDA as tracer |
J Med Chem 59: 3471-88 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00085
BindingDB Entry DOI: 10.7270/Q2H133X3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data