 Found 2846 hits with Last Name = 'lim' and Initial = 'k'
Found 2846 hits with Last Name = 'lim' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394718
(CHEMBL2165801)Show SMILES Nc1nc2-c3cc(Cc4cnccc4Cl)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-8-9-26-12-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333930
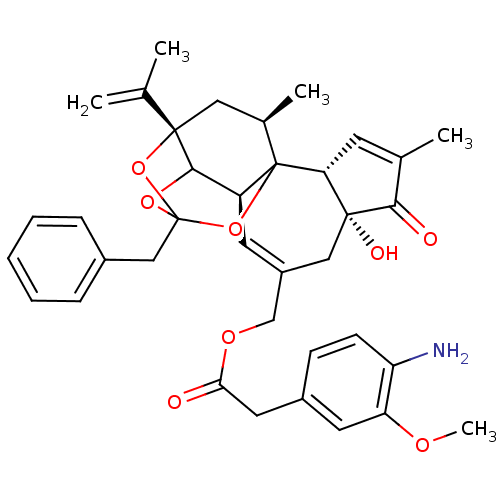
(CHEMBL1644419 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1N |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H41NO8/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,41H,1,16-20,38H2,2-5H3/t23-,27+,30-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333927
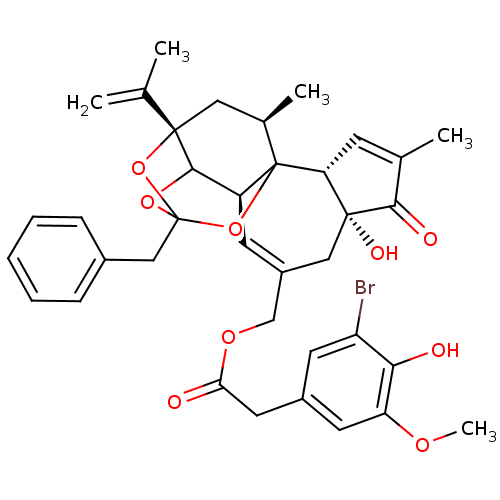
(CHEMBL1644416 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1O |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H39BrO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333925
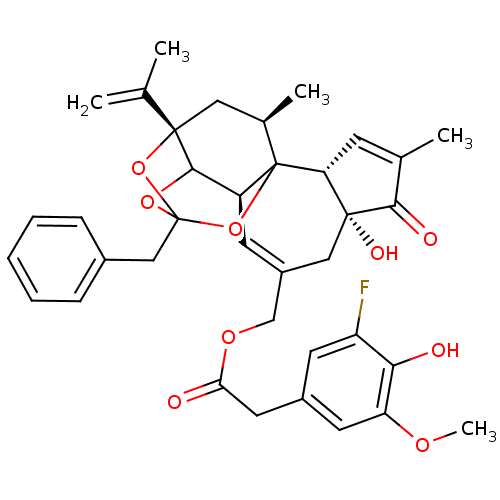
(CHEMBL1644414 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(F)c1O |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H39FO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521937

(CHEMBL4564687)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(CCF)C3)C2)ncc1F |r| Show InChI InChI=1S/C25H30F2N8/c1-17-4-2-3-8-35-23(17)19(11-30-35)22-20(27)12-29-24(32-22)31-21-6-5-18(10-28-21)34-15-25(16-34)13-33(14-25)9-7-26/h5-6,10-12,17H,2-4,7-9,13-16H2,1H3,(H,28,29,31,32)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521925

(CHEMBL4529532)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(CCCF)C3)C2)ncc1F |r| Show InChI InChI=1S/C26H32F2N8/c1-18-5-2-3-10-36-24(18)20(12-31-36)23-21(28)13-30-25(33-23)32-22-7-6-19(11-29-22)35-16-26(17-35)14-34(15-26)9-4-8-27/h6-7,11-13,18H,2-5,8-10,14-17H2,1H3,(H,29,30,32,33)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521933

(CHEMBL4440980)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCN(C)CC2)ncc1F |r| Show InChI InChI=1S/C23H29FN8/c1-16-5-3-4-8-32-22(16)18(14-27-32)21-19(24)15-26-23(29-21)28-20-7-6-17(13-25-20)31-11-9-30(2)10-12-31/h6-7,13-16H,3-5,8-12H2,1-2H3,(H,25,26,28,29)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333928
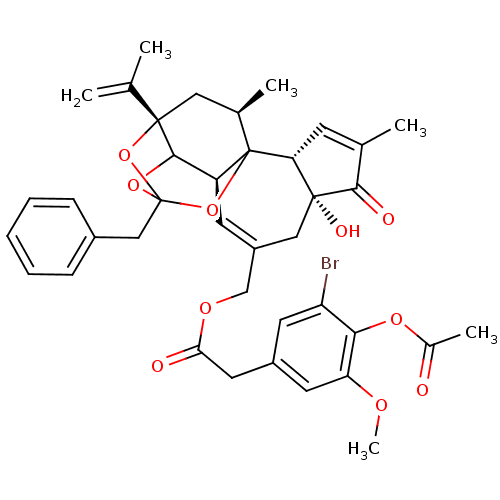
(CHEMBL1644417 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1OC(C)=O |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C39H41BrO10/c1-21(2)37-17-23(4)39-28(35(37)48-38(49-37,50-39)19-25-10-8-7-9-11-25)13-27(18-36(44)31(39)12-22(3)34(36)43)20-46-32(42)16-26-14-29(40)33(47-24(5)41)30(15-26)45-6/h7-15,23,28,31,35,44H,1,16-20H2,2-6H3/t23-,28+,31-,35?,36-,37+,38?,39?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521938
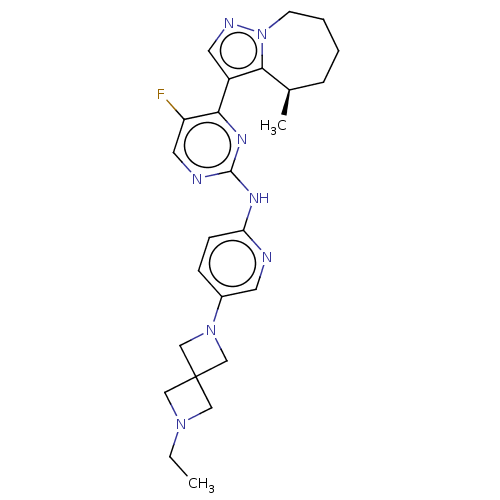
(CHEMBL4545456)Show SMILES CCN1CC2(C1)CN(C2)c1ccc(Nc2ncc(F)c(n2)-c2cnn3CCCC[C@@H](C)c23)nc1 |r| Show InChI InChI=1S/C25H31FN8/c1-3-32-13-25(14-32)15-33(16-25)18-7-8-21(27-10-18)30-24-28-12-20(26)22(31-24)19-11-29-34-9-5-4-6-17(2)23(19)34/h7-8,10-12,17H,3-6,9,13-16H2,1-2H3,(H,27,28,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521935

(CHEMBL4589135)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(C)C3)C2)ncc1F |r| Show InChI InChI=1S/C24H29FN8/c1-16-5-3-4-8-33-22(16)18(10-28-33)21-19(25)11-27-23(30-21)29-20-7-6-17(9-26-20)32-14-24(15-32)12-31(2)13-24/h6-7,9-11,16H,3-5,8,12-15H2,1-2H3,(H,26,27,29,30)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394722
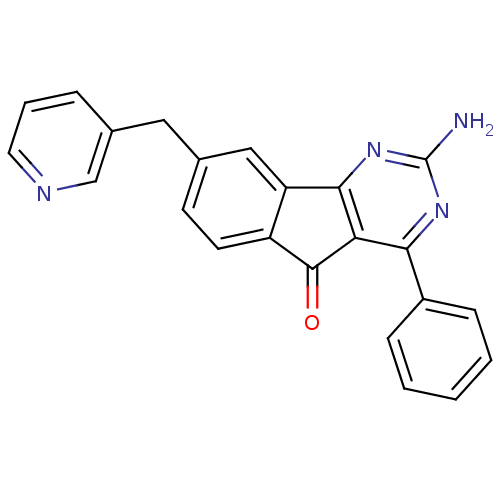
(CHEMBL2165807)Show SMILES Nc1nc2-c3cc(Cc4cccnc4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H16N4O/c24-23-26-20(16-6-2-1-3-7-16)19-21(27-23)18-12-14(8-9-17(18)22(19)28)11-15-5-4-10-25-13-15/h1-10,12-13H,11H2,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521936

(CHEMBL4560219)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(C3)C3COC3)C2)ncc1F |r| Show InChI InChI=1S/C26H31FN8O/c1-17-4-2-3-7-35-24(17)20(9-30-35)23-21(27)10-29-25(32-23)31-22-6-5-18(8-28-22)33-13-26(14-33)15-34(16-26)19-11-36-12-19/h5-6,8-10,17,19H,2-4,7,11-16H2,1H3,(H,28,29,31,32)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521934
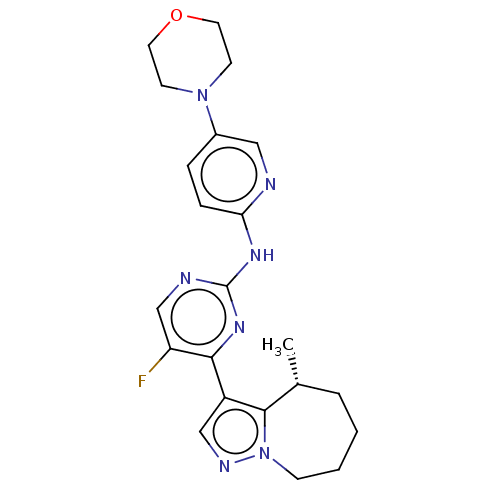
(CHEMBL4546255)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCOCC2)ncc1F |r| Show InChI InChI=1S/C22H26FN7O/c1-15-4-2-3-7-30-21(15)17(13-26-30)20-18(23)14-25-22(28-20)27-19-6-5-16(12-24-19)29-8-10-31-11-9-29/h5-6,12-15H,2-4,7-11H2,1H3,(H,24,25,27,28)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394717
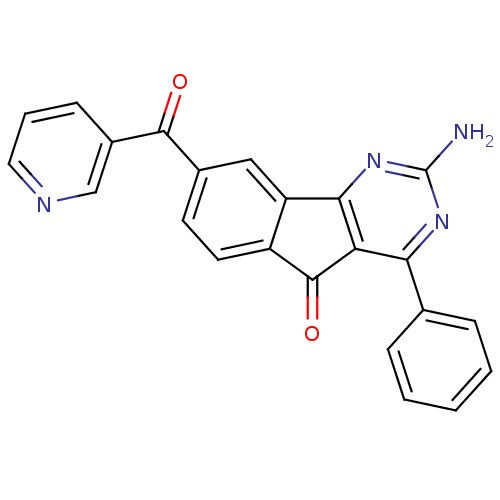
(CHEMBL2165802)Show SMILES Nc1nc2-c3cc(ccc3C(=O)c2c(n1)-c1ccccc1)C(=O)c1cccnc1 Show InChI InChI=1S/C23H14N4O2/c24-23-26-19(13-5-2-1-3-6-13)18-20(27-23)17-11-14(8-9-16(17)22(18)29)21(28)15-7-4-10-25-12-15/h1-12H,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50394718

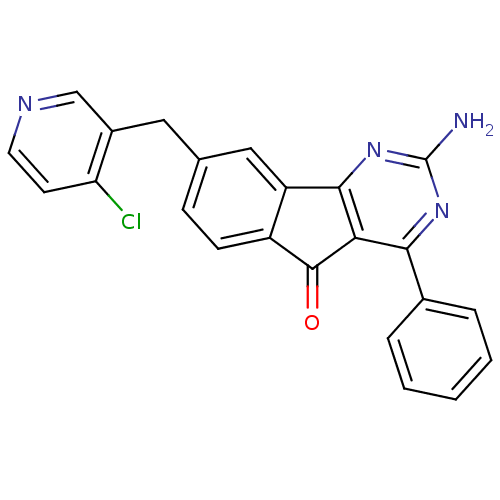
(CHEMBL2165801)Show SMILES Nc1nc2-c3cc(Cc4cnccc4Cl)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15ClN4O/c24-18-8-9-26-12-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440401
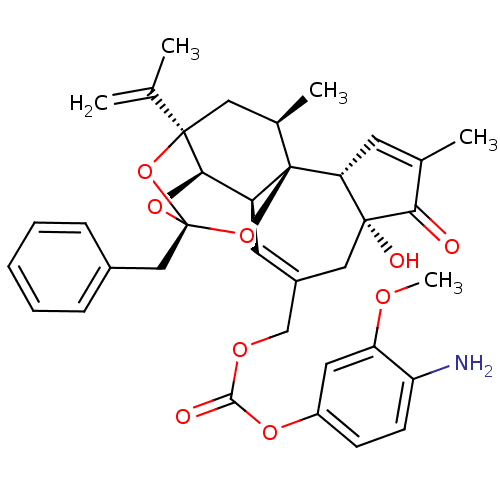
(CHEMBL2425550)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1N |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H39NO9/c1-20(2)34-16-22(4)36-26(31(34)44-35(45-34,46-36)18-23-9-7-6-8-10-23)14-24(17-33(40)29(36)13-21(3)30(33)38)19-42-32(39)43-25-11-12-27(37)28(15-25)41-5/h6-15,22,26,29,31,40H,1,16-19,37H2,2-5H3/t22-,26+,29-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333924
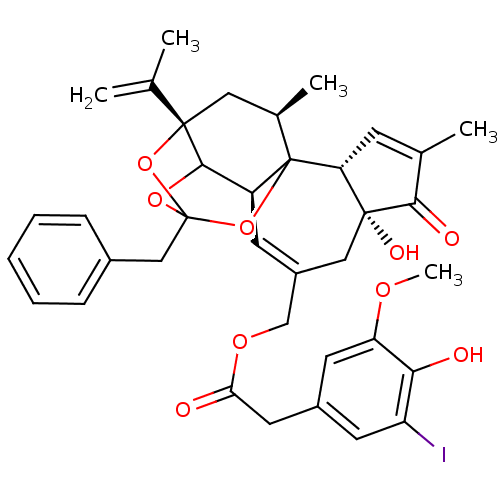
(2-iodo Resiniferatoxin | CHEMBL1644413)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(I)c1O |r,t:10,35,TLB:39:24:12:29.14.15,THB:27:26:12:29.14.15,23:24:12:29.14.15,23:15:12:24.25.26| Show InChI InChI=1S/C37H39IO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333931
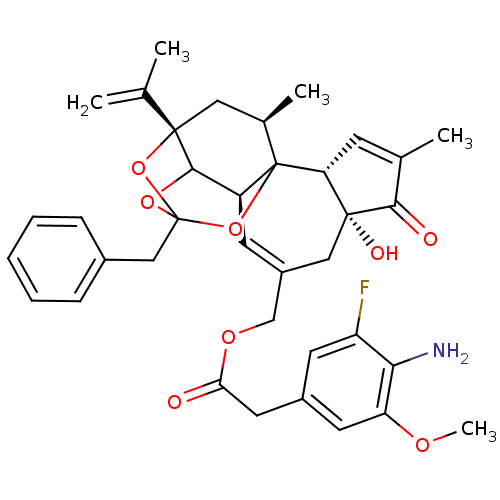
(CHEMBL1644420 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(F)c1N |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H40FNO8/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(40)15-24-13-27(38)31(39)28(14-24)43-5/h6-14,22,26,29,33,42H,1,15-19,39H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394719
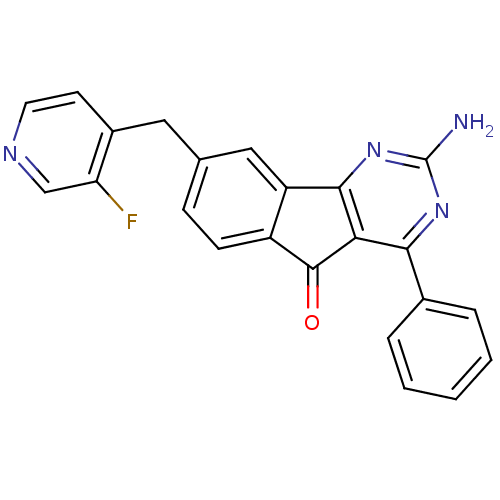
(CHEMBL2165800)Show SMILES Nc1nc2-c3cc(Cc4ccncc4F)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-18-12-26-9-8-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521927

(CHEMBL4466797)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)C2CCN(C)CC2)ncc1F |r| Show InChI InChI=1S/C24H30FN7/c1-16-5-3-4-10-32-23(16)19(14-28-32)22-20(25)15-27-24(30-22)29-21-7-6-18(13-26-21)17-8-11-31(2)12-9-17/h6-7,13-17H,3-5,8-12H2,1-2H3,(H,26,27,29,30)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333932
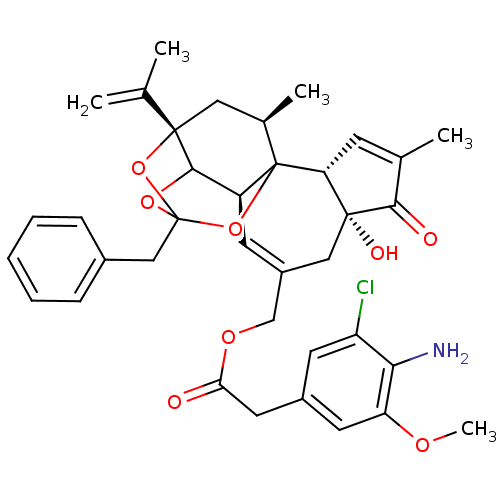
(CHEMBL1644421 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Cl)c1N |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H40ClNO8/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(40)15-24-13-27(38)31(39)28(14-24)43-5/h6-14,22,26,29,33,42H,1,15-19,39H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521940

(CHEMBL4539133)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCC(CC2)N(C)C)ncc1F |r| Show InChI InChI=1S/C25H33FN8/c1-17-6-4-5-11-34-24(17)20(15-29-34)23-21(26)16-28-25(31-23)30-22-8-7-19(14-27-22)33-12-9-18(10-13-33)32(2)3/h7-8,14-18H,4-6,9-13H2,1-3H3,(H,27,28,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521924

(CHEMBL4578267)Show SMILES C[C@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCN(C)CC2)ncc1F |r| Show InChI InChI=1S/C23H29FN8/c1-16-5-3-4-8-32-22(16)18(14-27-32)21-19(24)15-26-23(29-21)28-20-7-6-17(13-25-20)31-11-9-30(2)10-12-31/h6-7,13-16H,3-5,8-12H2,1-2H3,(H,25,26,28,29)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333926
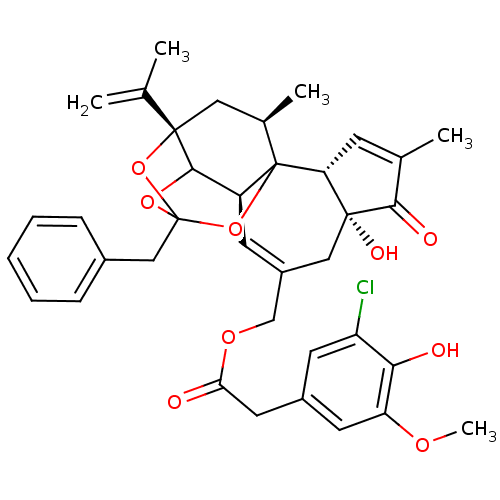
(CHEMBL1644415 | [(2R,6R,10S,15S,17R)-13-benzyl-6-h...)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Cl)c1O |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H39ClO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440402
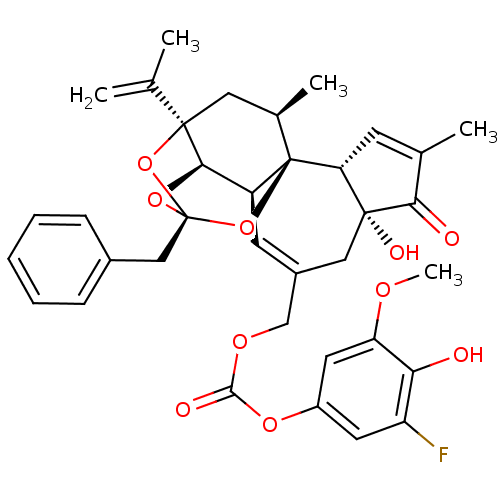
(CHEMBL2425547)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(F)c1O |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H37FO10/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,38,41H,1,15-18H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50366620

(RESINIFERATOXIN)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3[C@H]4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1O |r,t:10,35,TLB:23:15:12:24.25.26,THB:16:15:12:24.25.26| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35+,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from human TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521939
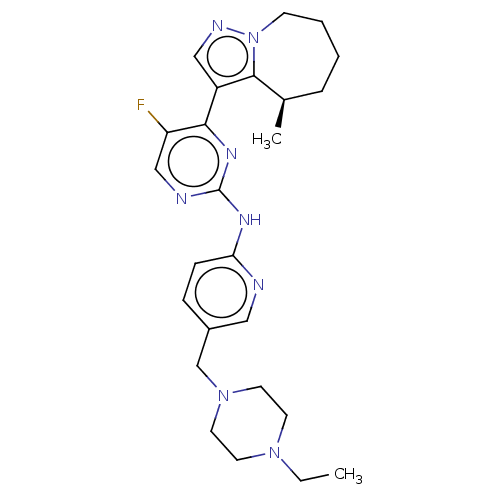
(CHEMBL4520067)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cnn4CCCC[C@@H](C)c34)nc2)CC1 |r| Show InChI InChI=1S/C25H33FN8/c1-3-32-10-12-33(13-11-32)17-19-7-8-22(27-14-19)30-25-28-16-21(26)23(31-25)20-15-29-34-9-5-4-6-18(2)24(20)34/h7-8,14-16,18H,3-6,9-13,17H2,1-2H3,(H,27,28,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394720
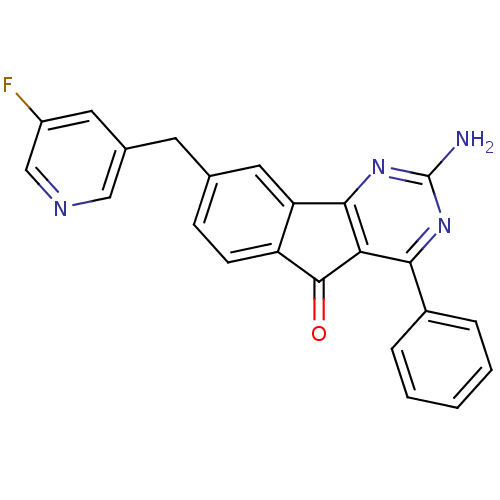
(CHEMBL2165799)Show SMILES Nc1nc2-c3cc(Cc4cncc(F)c4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-16-9-14(11-26-12-16)8-13-6-7-17-18(10-13)21-19(22(17)29)20(27-23(25)28-21)15-4-2-1-3-5-15/h1-7,9-12H,8H2,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440400
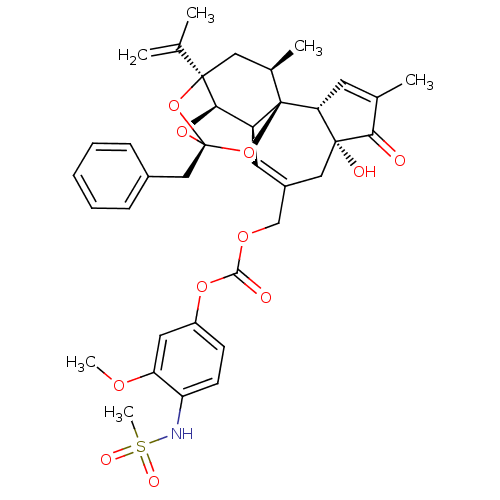
(CHEMBL2425551)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)ccc1NS(C)(=O)=O |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C37H41NO11S/c1-21(2)35-17-23(4)37-27(32(35)47-36(48-35,49-37)19-24-10-8-7-9-11-24)15-25(18-34(41)30(37)14-22(3)31(34)39)20-45-33(40)46-26-12-13-28(29(16-26)44-5)38-50(6,42)43/h7-16,23,27,30,32,38,41H,1,17-20H2,2-6H3/t23-,27+,30-,32-,34-,35-,36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521926
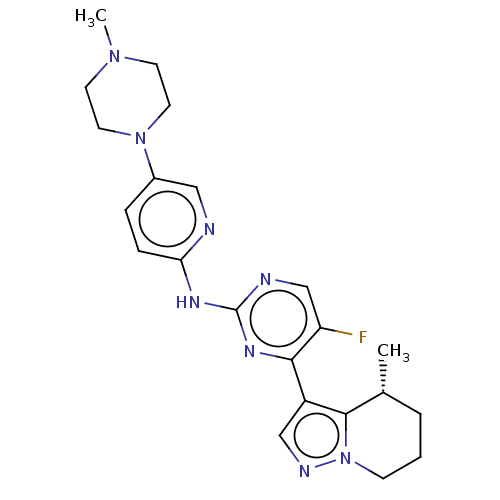
(CHEMBL4561999)Show SMILES C[C@@H]1CCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CCN(C)CC2)ncc1F |r| Show InChI InChI=1S/C22H27FN8/c1-15-4-3-7-31-21(15)17(13-26-31)20-18(23)14-25-22(28-20)27-19-6-5-16(12-24-19)30-10-8-29(2)9-11-30/h5-6,12-15H,3-4,7-11H2,1-2H3,(H,24,25,27,28)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394721
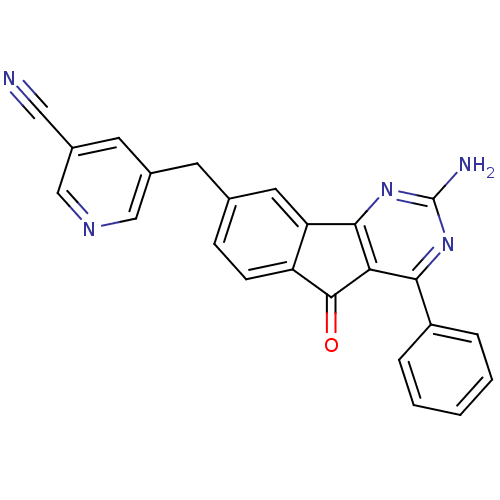
(CHEMBL2165808)Show SMILES Nc1nc2-c3cc(Cc4cncc(c4)C#N)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C24H15N5O/c25-11-16-9-15(12-27-13-16)8-14-6-7-18-19(10-14)22-20(23(18)30)21(28-24(26)29-22)17-4-2-1-3-5-17/h1-7,9-10,12-13H,8H2,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394716
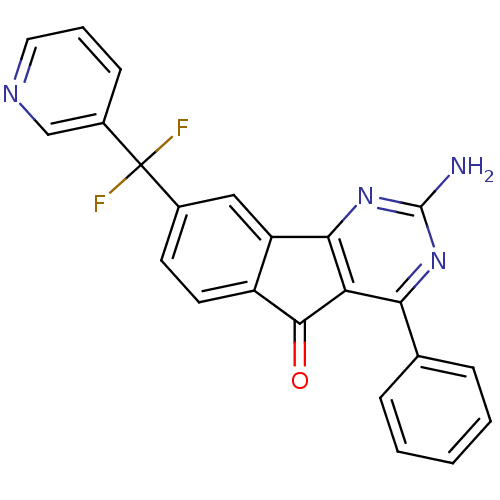
(CHEMBL2165803)Show SMILES Nc1nc2-c3cc(ccc3C(=O)c2c(n1)-c1ccccc1)C(F)(F)c1cccnc1 Show InChI InChI=1S/C23H14F2N4O/c24-23(25,15-7-4-10-27-12-15)14-8-9-16-17(11-14)20-18(21(16)30)19(28-22(26)29-20)13-5-2-1-3-6-13/h1-12H,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521937

(CHEMBL4564687)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(CCF)C3)C2)ncc1F |r| Show InChI InChI=1S/C25H30F2N8/c1-17-4-2-3-8-35-23(17)19(11-30-35)22-20(27)12-29-24(32-22)31-21-6-5-18(10-28-21)34-15-25(16-34)13-33(14-25)9-7-26/h5-6,10-12,17H,2-4,7-9,13-16H2,1H3,(H,28,29,31,32)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK6 (1 to 326(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440397
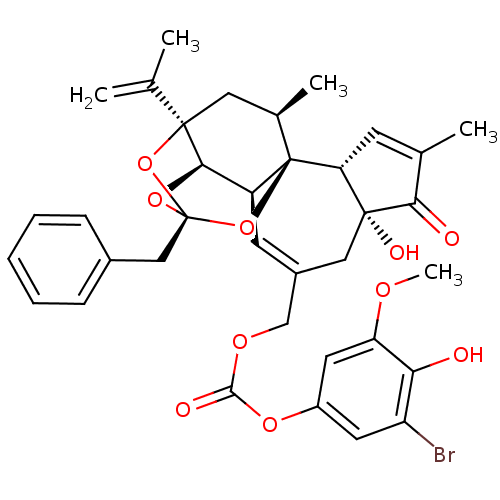
(CHEMBL2425549)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1O |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H37BrO10/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,38,41H,1,15-18H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440398
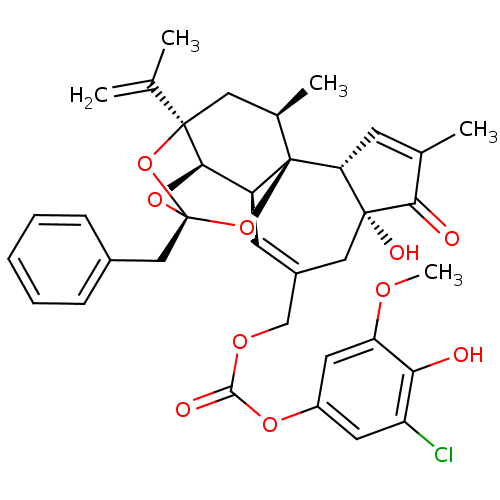
(CHEMBL2425548)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Cl)c1O |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H37ClO10/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,38,41H,1,15-18H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50305285
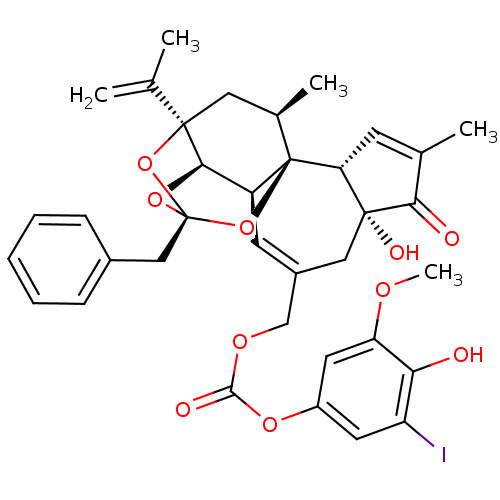
(CHEMBL595262 | [(1R,2R,6R,10S,11R,13S,15R,17R)-13-...)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(I)c1O |r,t:10,35,TLB:16:15:12:25.26.24,THB:23:15:12:25.26.24| Show InChI InChI=1S/C36H37IO10/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,38,41H,1,15-18H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521935

(CHEMBL4589135)Show SMILES C[C@@H]1CCCCn2ncc(c12)-c1nc(Nc2ccc(cn2)N2CC3(CN(C)C3)C2)ncc1F |r| Show InChI InChI=1S/C24H29FN8/c1-16-5-3-4-8-33-22(16)18(10-28-33)21-19(25)11-27-23(30-21)29-20-7-6-17(9-26-20)32-14-24(15-32)12-31(2)13-24/h6-7,9-11,16H,3-5,8,12-15H2,1-2H3,(H,26,27,29,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK6 (1 to 326(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50394717

(CHEMBL2165802)Show SMILES Nc1nc2-c3cc(ccc3C(=O)c2c(n1)-c1ccccc1)C(=O)c1cccnc1 Show InChI InChI=1S/C23H14N4O2/c24-23-26-19(13-5-2-1-3-6-13)18-20(27-23)17-11-14(8-9-16(17)22(18)29)21(28)15-7-4-10-25-12-15/h1-12H,(H2,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50394715
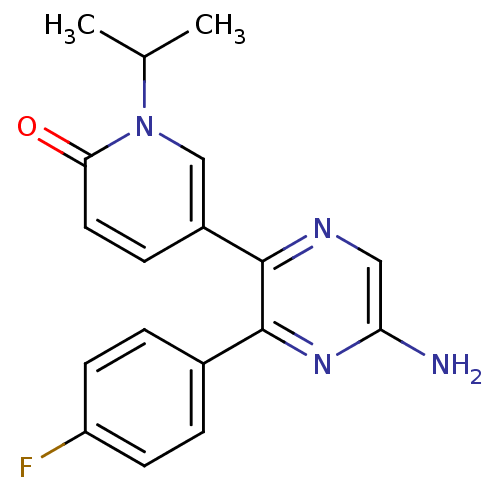
(CHEMBL2165804)Show InChI InChI=1S/C18H17FN4O/c1-11(2)23-10-13(5-8-16(23)24)17-18(22-15(20)9-21-17)12-3-6-14(19)7-4-12/h3-11H,1-2H3,(H2,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521932
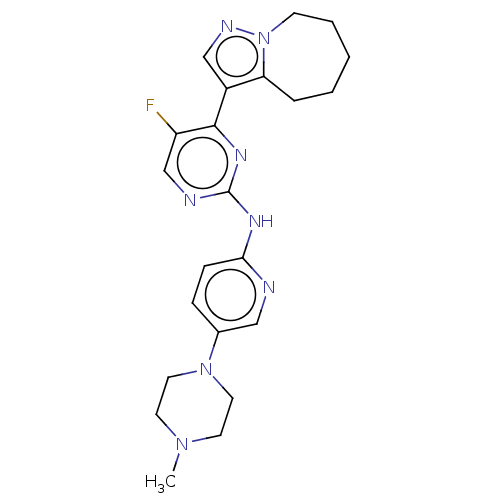
(CHEMBL4588989)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(F)c(n2)-c2cnn3CCCCCc23)nc1 Show InChI InChI=1S/C22H27FN8/c1-29-9-11-30(12-10-29)16-6-7-20(24-13-16)27-22-25-15-18(23)21(28-22)17-14-26-31-8-4-2-3-5-19(17)31/h6-7,13-15H,2-5,8-12H2,1H3,(H,24,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK4 (1 to 303(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440395
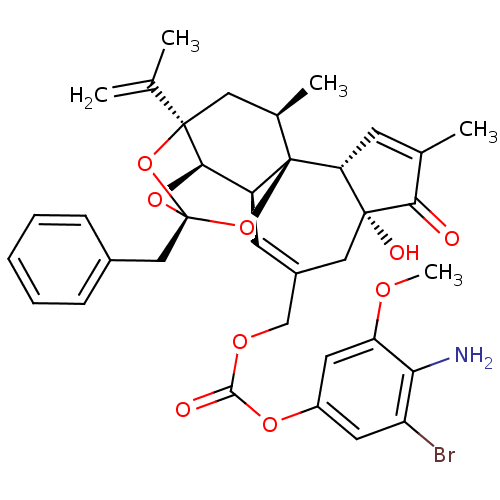
(CHEMBL2425546)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1N |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H38BrNO9/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,41H,1,15-18,38H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50333933
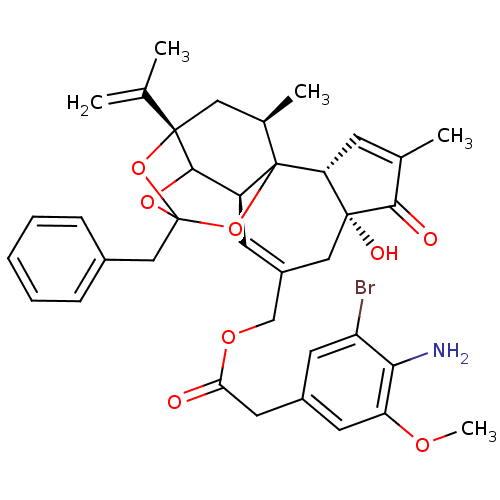
(5-bromo-4-amino Resiniferatoxin | CHEMBL1644422)Show SMILES COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1N |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15| Show InChI InChI=1S/C37H40BrNO8/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(40)15-24-13-27(38)31(39)28(14-24)43-5/h6-14,22,26,29,33,42H,1,15-19,39H2,2-5H3/t22-,26+,29-,33?,34-,35+,36?,37?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRV1 expressed in CHO cells by competitive binding assay |
Bioorg Med Chem Lett 21: 299-302 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.012
BindingDB Entry DOI: 10.7270/Q2J967BB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50305285

(CHEMBL595262 | [(1R,2R,6R,10S,11R,13S,15R,17R)-13-...)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(I)c1O |r,t:10,35,TLB:16:15:12:25.26.24,THB:23:15:12:25.26.24| Show InChI InChI=1S/C36H37IO10/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,38,41H,1,15-18H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-stimulated Ca2+ uptake |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50521938

(CHEMBL4545456)Show SMILES CCN1CC2(C1)CN(C2)c1ccc(Nc2ncc(F)c(n2)-c2cnn3CCCC[C@@H](C)c23)nc1 |r| Show InChI InChI=1S/C25H31FN8/c1-3-32-13-25(14-32)15-33(16-25)18-7-8-21(27-10-18)30-24-28-12-20(26)22(31-24)19-11-29-34-9-5-4-6-17(2)23(19)34/h7-8,10-12,17H,3-6,9,13-16H2,1-2H3,(H,27,28,30,31)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human full length GST-tagged CDK6 (1 to 326(end) amino acids)/Cyclin D3 (1 to 292(end) amino acids) expressed in baculovirus expression... |
Bioorg Med Chem Lett 29: 2294-2301 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.021
BindingDB Entry DOI: 10.7270/Q2C250VT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440399
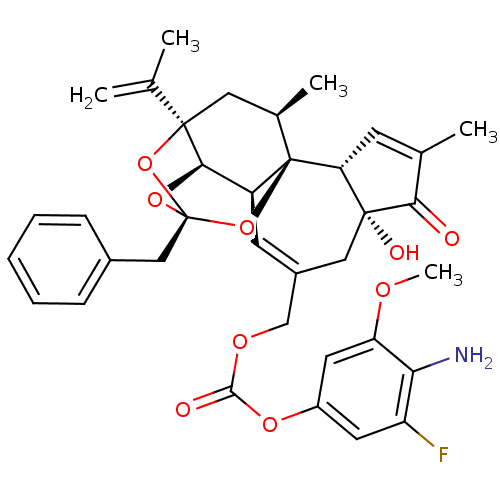
(CHEMBL2425544)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(F)c1N |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H38FNO9/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,41H,1,15-18,38H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50394722

(CHEMBL2165807)Show SMILES Nc1nc2-c3cc(Cc4cccnc4)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H16N4O/c24-23-26-20(16-6-2-1-3-7-16)19-21(27-23)18-12-14(8-9-17(18)22(19)28)11-15-5-4-10-25-13-15/h1-10,12-13H,11H2,(H2,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440396
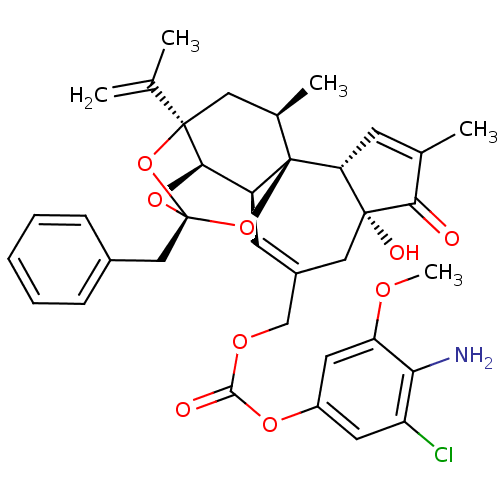
(CHEMBL2425545)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Cl)c1N |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H38ClNO9/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,41H,1,15-18,38H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from rat TRPV1 expressed in CHO cells |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM50440395

(CHEMBL2425546)Show SMILES COc1cc(OC(=O)OCC2=C[C@H]3[C@H]4O[C@]5(Cc6ccccc6)O[C@]4(C[C@@H](C)[C@]3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1N |r,t:10,35,TLB:16:15:12:24.25.26,THB:23:15:12:24.25.26| Show InChI InChI=1S/C36H38BrNO9/c1-19(2)34-15-21(4)36-25(31(34)45-35(46-34,47-36)17-22-9-7-6-8-10-22)12-23(16-33(41)28(36)11-20(3)30(33)39)18-43-32(40)44-24-13-26(37)29(38)27(14-24)42-5/h6-14,21,25,28,31,41H,1,15-18,38H2,2-5H3/t21-,25+,28-,31-,33-,34-,35-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-stimulated Ca2+ uptake |
Eur J Med Chem 68: 233-43 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.042
BindingDB Entry DOI: 10.7270/Q2RB761G |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50394719

(CHEMBL2165800)Show SMILES Nc1nc2-c3cc(Cc4ccncc4F)ccc3C(=O)c2c(n1)-c1ccccc1 Show InChI InChI=1S/C23H15FN4O/c24-18-12-26-9-8-15(18)10-13-6-7-16-17(11-13)21-19(22(16)29)20(27-23(25)28-21)14-4-2-1-3-5-14/h1-9,11-12H,10H2,(H2,25,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production |
J Med Chem 55: 1402-17 (2012)
Article DOI: 10.1021/jm201640m
BindingDB Entry DOI: 10.7270/Q2CZ388Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data