 Found 111 hits with Last Name = 'lombroso' and Initial = 'pj'
Found 111 hits with Last Name = 'lombroso' and Initial = 'pj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103445

(CHEMBL3398243)Show InChI InChI=1S/C7H3F3S5/c8-7(9,10)4-1-2-5-6(3-4)12-14-15-13-11-5/h1-3H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50067512
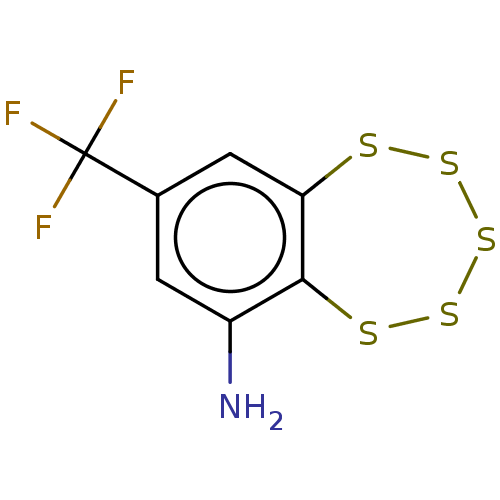
(CHEMBL3401893)Show InChI InChI=1S/C7H4F3NS5/c8-7(9,10)3-1-4(11)6-5(2-3)12-14-16-15-13-6/h1-2H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103444
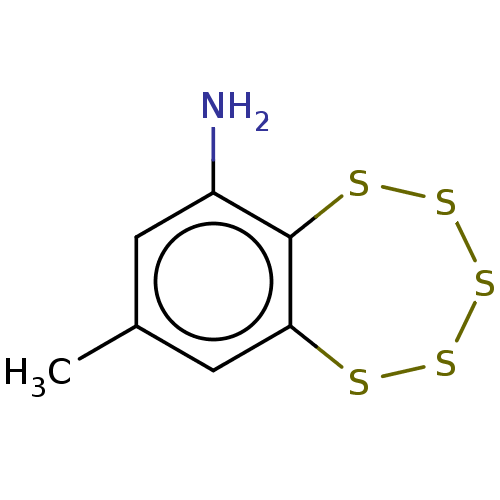
(CHEMBL3398244)Show InChI InChI=1S/C7H7NS5.ClH/c1-4-2-5(8)7-6(3-4)9-11-13-12-10-7;/h2-3H,8H2,1H3;1H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103448
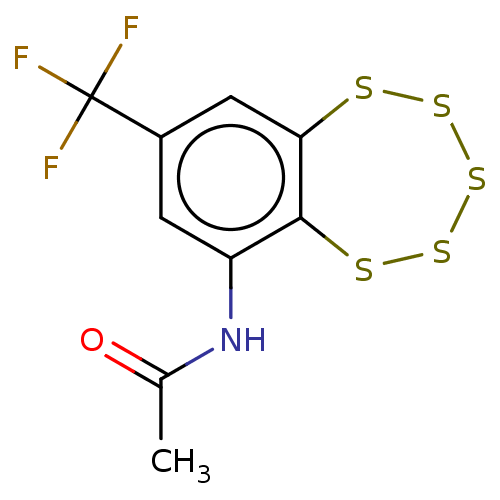
(CHEMBL3398247)Show InChI InChI=1S/C9H6F3NOS5/c1-4(14)13-6-2-5(9(10,11)12)3-7-8(6)16-18-19-17-15-7/h2-3H,1H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103447

(CHEMBL3398250)Show SMILES Cl.Cl.Nc1cc(cc2SSSc3c(N)cc(cc3SSSc12)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C14H8F6N2S6.2ClH/c15-13(16,17)5-1-7(21)11-9(3-5)23-28-26-12-8(22)2-6(14(18,19)20)4-10(12)24-27-25-11;;/h1-4H,21-22H2;2*1H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103450

(CHEMBL3398249)Show InChI InChI=1S/C14H10F3NS5/c15-14(16,17)10-6-11(18-8-9-4-2-1-3-5-9)13-12(7-10)19-21-23-22-20-13/h1-7,18H,8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103443
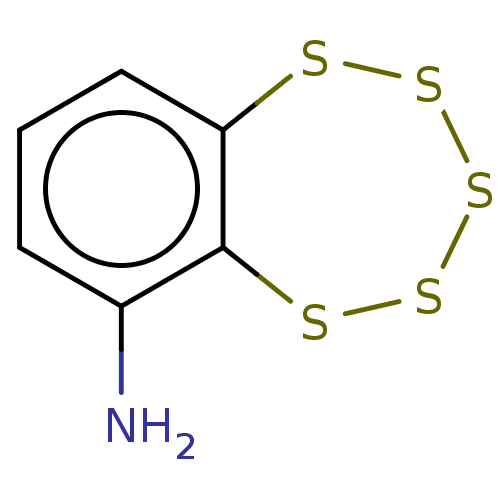
(CHEMBL3398245)Show InChI InChI=1S/C6H5NS5.ClH/c7-4-2-1-3-5-6(4)9-11-12-10-8-5;/h1-3H,7H2;1H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103446
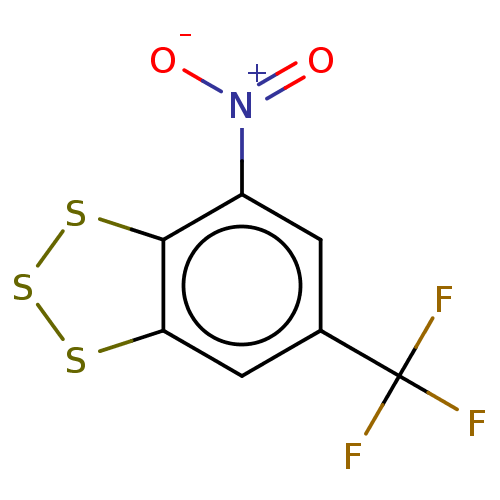
(CHEMBL3398251)Show InChI InChI=1S/C7H2F3NO2S3/c8-7(9,10)3-1-4(11(12)13)6-5(2-3)14-16-15-6/h1-2H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50103449

(CHEMBL3398248)Show InChI InChI=1S/C9H8F3NS5/c1-2-13-6-3-5(9(10,11)12)4-7-8(6)15-17-18-16-14-7/h3-4,13H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50067504

(CHEMBL3398246)Show InChI InChI=1S/C9H3F6NOS5/c10-8(11,12)3-1-4(16-7(17)9(13,14)15)6-5(2-3)18-20-22-21-19-6/h1-2H,(H,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) in absence of GSH by p-nitrophenyl phosphate hydrolysis assay |
Bioorg Med Chem Lett 25: 1044-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.020
BindingDB Entry DOI: 10.7270/Q2QN68JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250610

(CHEMBL4076072)Show SMILES OP(O)(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H22Cl3FNO6PS/c25-20-14-22(27)21(26)13-18(20)12-17-2-1-3-19(23(17)28)15-4-6-16(7-5-15)24(36(30,31)32)37(33,34)29-8-10-35-11-9-29/h1-7,13-14,24H,8-12H2,(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250608
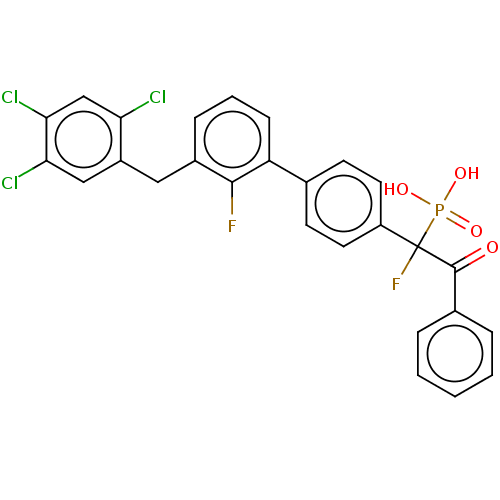
(CHEMBL4092589)Show SMILES OP(O)(=O)C(F)(C(=O)c1ccccc1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C27H18Cl3F2O4P/c28-22-15-24(30)23(29)14-19(22)13-18-7-4-8-21(25(18)31)16-9-11-20(12-10-16)27(32,37(34,35)36)26(33)17-5-2-1-3-6-17/h1-12,14-15H,13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250601
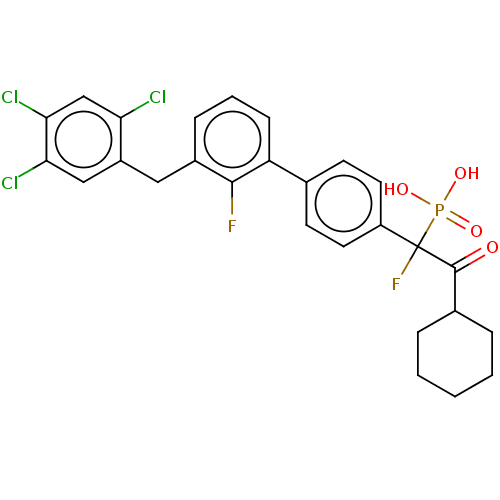
(CHEMBL4070970)Show SMILES OP(O)(=O)C(F)(C(=O)C1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C27H24Cl3F2O4P/c28-22-15-24(30)23(29)14-19(22)13-18-7-4-8-21(25(18)31)16-9-11-20(12-10-16)27(32,37(34,35)36)26(33)17-5-2-1-3-6-17/h4,7-12,14-15,17H,1-3,5-6,13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250600
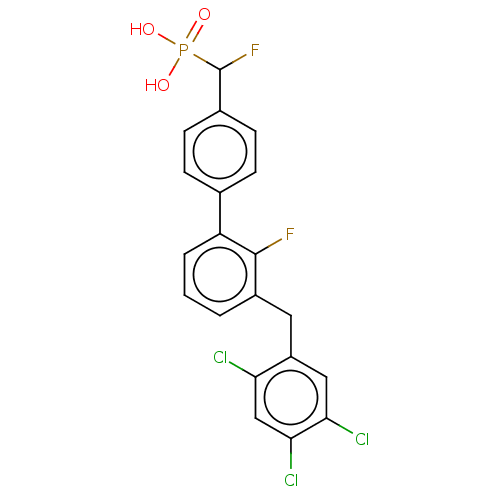
(CHEMBL4103050)Show SMILES OP(O)(=O)C(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C20H14Cl3F2O3P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20H,8H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50250606
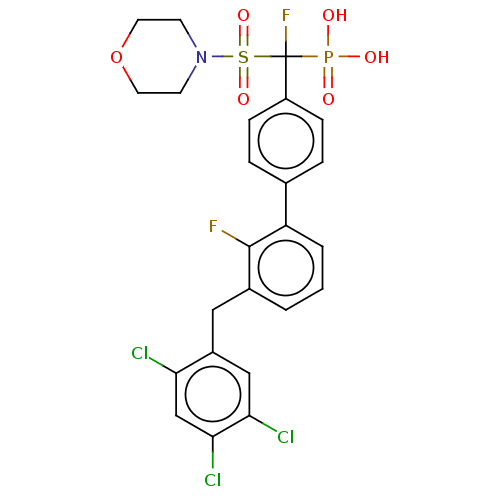
(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of LAR (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrophot... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250603
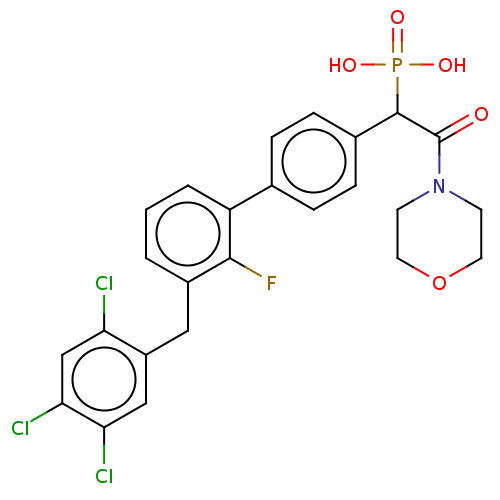
(CHEMBL4102693)Show SMILES OP(O)(=O)C(C(=O)N1CCOCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C25H22Cl3FNO5P/c26-20-14-22(28)21(27)13-18(20)12-17-2-1-3-19(23(17)29)15-4-6-16(7-5-15)24(36(32,33)34)25(31)30-8-10-35-11-9-30/h1-7,13-14,24H,8-12H2,(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250607
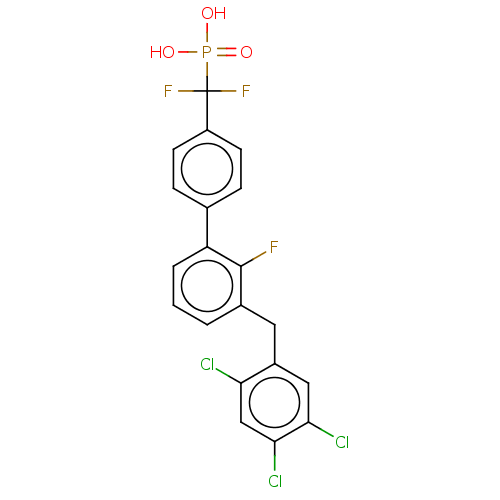
(CHEMBL4069436)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C20H13Cl3F3O3P/c21-16-10-18(23)17(22)9-13(16)8-12-2-1-3-15(19(12)24)11-4-6-14(7-5-11)20(25,26)30(27,28)29/h1-7,9-10H,8H2,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250604
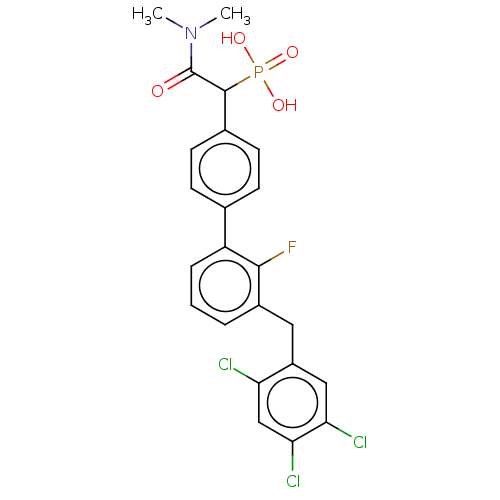
(CHEMBL4084768)Show SMILES CN(C)C(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O Show InChI InChI=1S/C23H20Cl3FNO4P/c1-28(2)23(29)22(33(30,31)32)14-8-6-13(7-9-14)17-5-3-4-15(21(17)27)10-16-11-19(25)20(26)12-18(16)24/h3-9,11-12,22H,10H2,1-2H3,(H2,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250599
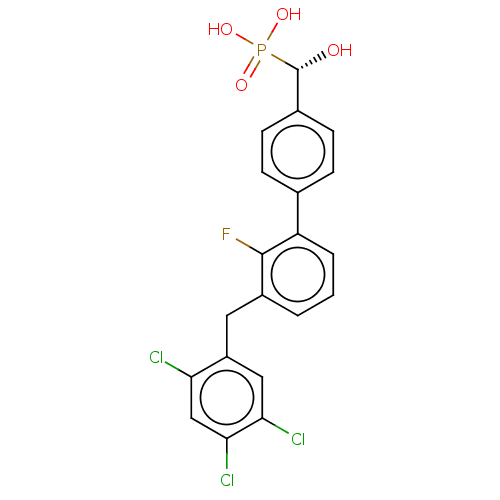
(CHEMBL4088863)Show SMILES O[C@H](c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O |r| Show InChI InChI=1S/C20H15Cl3FO4P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20,25H,8H2,(H2,26,27,28)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250609

(CHEMBL4105477)Show SMILES OP(O)(=O)C(C(=O)N1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C26H24Cl3FNO4P/c27-21-15-23(29)22(28)14-19(21)13-18-5-4-6-20(24(18)30)16-7-9-17(10-8-16)25(36(33,34)35)26(32)31-11-2-1-3-12-31/h4-10,14-15,25H,1-3,11-13H2,(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250602

(CHEMBL4087599)Show SMILES OP(O)(=O)C(F)(C(=O)N1CCCCC1)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F Show InChI InChI=1S/C26H23Cl3F2NO4P/c27-21-15-23(29)22(28)14-18(21)13-17-5-4-6-20(24(17)30)16-7-9-19(10-8-16)26(31,37(34,35)36)25(33)32-11-2-1-3-12-32/h4-10,14-15H,1-3,11-13H2,(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250597
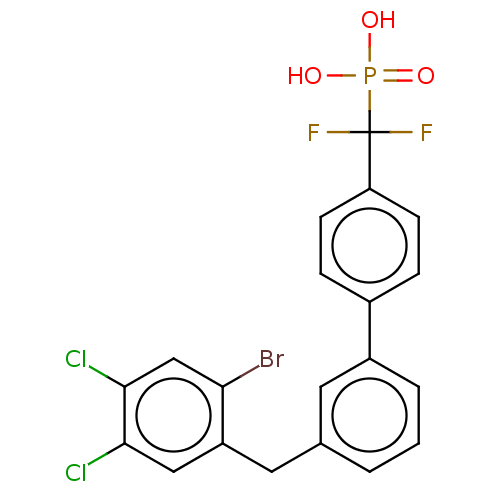
(CHEMBL4096664)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Br)c1 Show InChI InChI=1S/C20H14BrCl2F2O3P/c21-17-11-19(23)18(22)10-15(17)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250596

(CHEMBL4073186)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1 Show InChI InChI=1S/C20H14Cl3F2O3P/c21-17-11-19(23)18(22)10-15(17)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrop... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250595
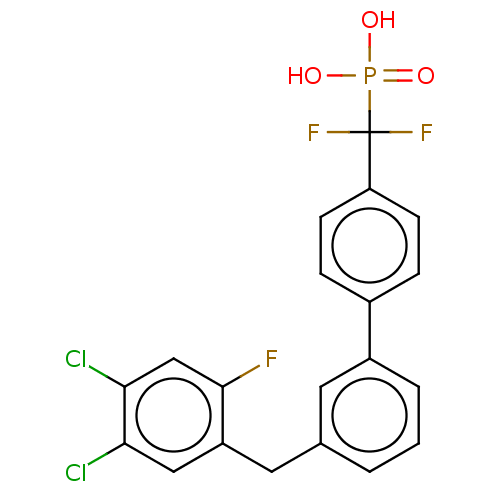
(CHEMBL4100037)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2F)c1 Show InChI InChI=1S/C20H14Cl2F3O3P/c21-17-10-15(19(23)11-18(17)22)9-12-2-1-3-14(8-12)13-4-6-16(7-5-13)20(24,25)29(26,27)28/h1-8,10-11H,9H2,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectroph... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Low molecular weight phosphotyrosine protein phosphatase
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of LMW-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectro... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250605

(CHEMBL4075995)Show SMILES CNC(=O)C(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O Show InChI InChI=1S/C22H18Cl3FNO4P/c1-27-22(28)21(32(29,30)31)13-7-5-12(6-8-13)16-4-2-3-14(20(16)26)9-15-10-18(24)19(25)11-17(15)23/h2-8,10-11,21H,9H2,1H3,(H,27,28)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 10
(Homo sapiens (Human)) | BDBM50250606

(CHEMBL4077342)Show SMILES OP(O)(=O)C(F)(c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)S(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C24H21Cl3F2NO6PS/c25-20-14-22(27)21(26)13-17(20)12-16-2-1-3-19(23(16)28)15-4-6-18(7-5-15)24(29,37(31,32)33)38(34,35)30-8-10-36-11-9-30/h1-7,13-14H,8-12H2,(H2,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of MKP5 (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441296
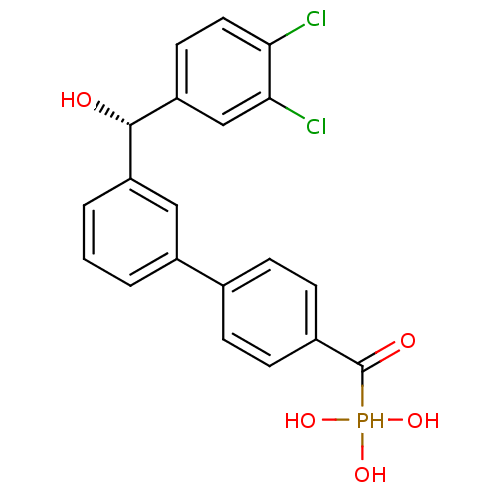
(CHEMBL2431665 | CHEMBL2431667)Show SMILES O[C@@H](c1cccc(c1)-c1ccc(cc1)C(=O)P(O)(O)O)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H17Cl2O5P/c21-17-9-8-16(11-18(17)22)19(23)15-3-1-2-14(10-15)12-4-6-13(7-5-12)20(24)28(25,26)27/h1-11,19,23,25-28H/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441302

(CHEMBL2431688)Show SMILES OP(O)(O)C(=O)c1ccc(cc1)-c1cccc(Cc2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C20H17Cl2O4P/c21-18-9-4-14(12-19(18)22)10-13-2-1-3-17(11-13)15-5-7-16(8-6-15)20(23)27(24,25)26/h1-9,11-12,24-27H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441297
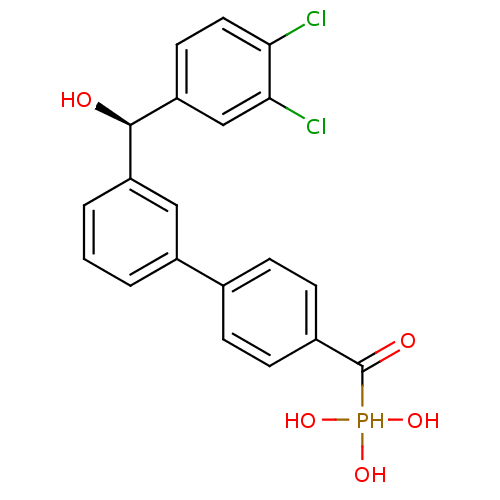
(CHEMBL2431664 | CHEMBL2431666)Show SMILES O[C@H](c1cccc(c1)-c1ccc(cc1)C(=O)P(O)(O)O)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H17Cl2O5P/c21-17-9-8-16(11-18(17)22)19(23)15-3-1-2-14(10-15)12-4-6-13(7-5-12)20(24)28(25,26)27/h1-11,19,23,25-28H/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441304

(CHEMBL2431686)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C20H15Cl2F2O3P/c21-18-9-4-14(12-19(18)22)10-13-2-1-3-16(11-13)15-5-7-17(8-6-15)20(23,24)28(25,26)27/h1-9,11-12H,10H2,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441304

(CHEMBL2431686)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C20H15Cl2F2O3P/c21-18-9-4-14(12-19(18)22)10-13-2-1-3-16(11-13)15-5-7-17(8-6-15)20(23,24)28(25,26)27/h1-9,11-12H,10H2,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250594
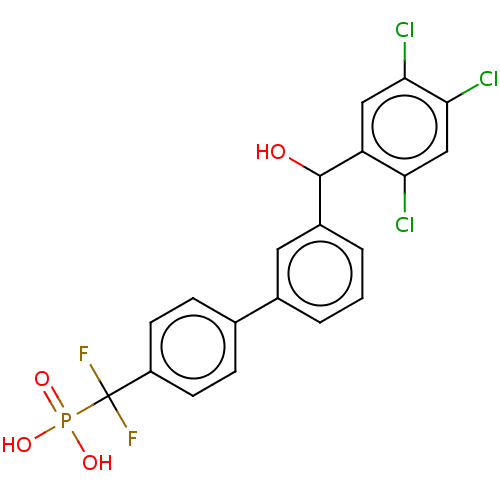
(CHEMBL4092285)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1cc(Cl)c(Cl)cc1Cl Show InChI InChI=1S/C20H14Cl3F2O4P/c21-16-10-18(23)17(22)9-15(16)19(26)13-3-1-2-12(8-13)11-4-6-14(7-5-11)20(24,25)30(27,28)29/h1-10,19,26H,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250593
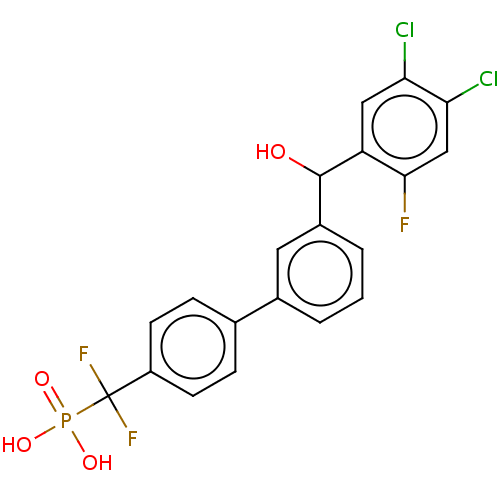
(CHEMBL4091376)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1cc(Cl)c(Cl)cc1F Show InChI InChI=1S/C20H14Cl2F3O4P/c21-16-9-15(18(23)10-17(16)22)19(26)13-3-1-2-12(8-13)11-4-6-14(7-5-11)20(24,25)30(27,28)29/h1-10,19,26H,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50250598
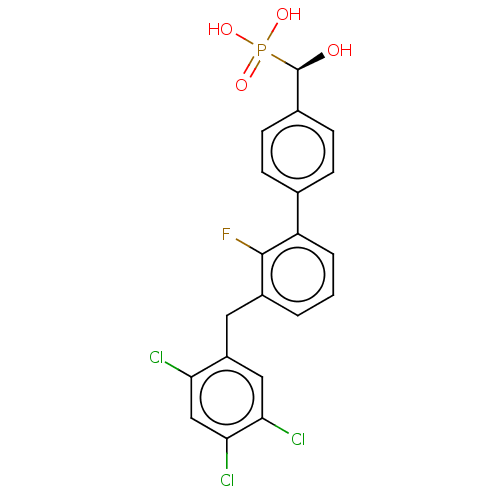
(CHEMBL4066498)Show SMILES O[C@@H](c1ccc(cc1)-c1cccc(Cc2cc(Cl)c(Cl)cc2Cl)c1F)P(O)(O)=O |r| Show InChI InChI=1S/C20H15Cl3FO4P/c21-16-10-18(23)17(22)9-14(16)8-13-2-1-3-15(19(13)24)11-4-6-12(7-5-11)20(25)29(26,27)28/h1-7,9-10,20,25H,8H2,(H2,26,27,28)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441303
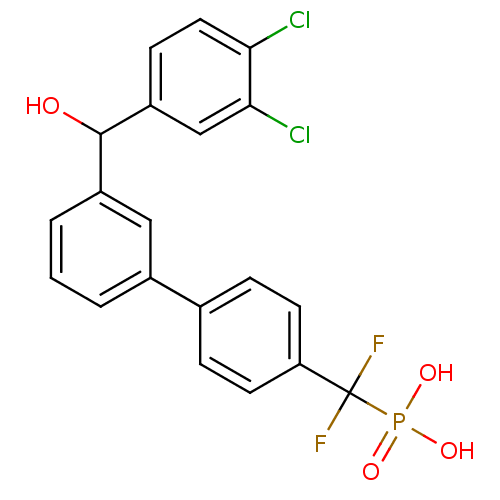
(CHEMBL2431687)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H15Cl2F2O4P/c21-17-9-6-15(11-18(17)22)19(25)14-3-1-2-13(10-14)12-4-7-16(8-5-12)20(23,24)29(26,27)28/h1-11,19,25H,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90... |
J Med Chem 60: 9299-9319 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01292
BindingDB Entry DOI: 10.7270/Q2NZ8B27 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441303

(CHEMBL2431687)Show SMILES OC(c1cccc(c1)-c1ccc(cc1)C(F)(F)P(O)(O)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H15Cl2F2O4P/c21-17-9-6-15(11-18(17)22)19(25)14-3-1-2-13(10-14)12-4-7-16(8-5-12)20(23,24)29(26,27)28/h1-11,19,25H,(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441305
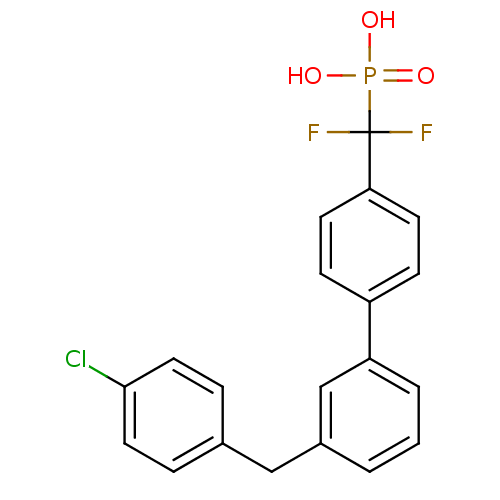
(CHEMBL2431685)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2ccc(Cl)cc2)c1 Show InChI InChI=1S/C20H16ClF2O3P/c21-19-10-4-14(5-11-19)12-15-2-1-3-17(13-15)16-6-8-18(9-7-16)20(22,23)27(24,25)26/h1-11,13H,12H2,(H2,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441298
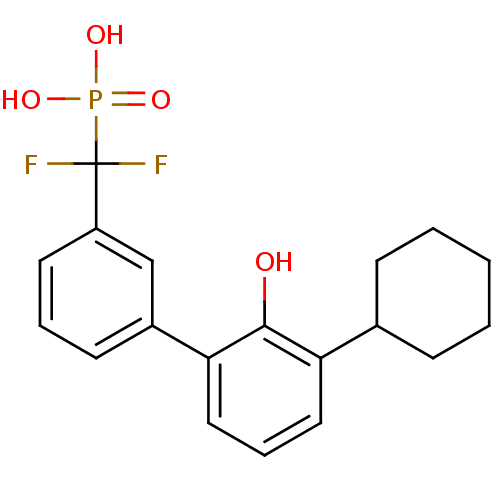
(CHEMBL2431708)Show SMILES Oc1c(cccc1-c1cccc(c1)C(F)(F)P(O)(O)=O)C1CCCCC1 Show InChI InChI=1S/C19H21F2O4P/c20-19(21,26(23,24)25)15-9-4-8-14(12-15)17-11-5-10-16(18(17)22)13-6-2-1-3-7-13/h4-5,8-13,22H,1-3,6-7H2,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441322
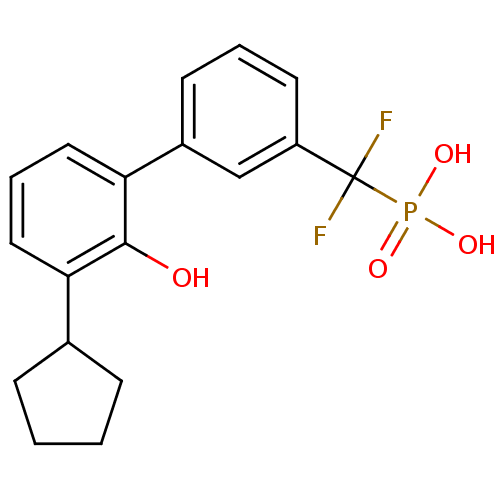
(CHEMBL2431707)Show SMILES Oc1c(cccc1-c1cccc(c1)C(F)(F)P(O)(O)=O)C1CCCC1 Show InChI InChI=1S/C18H19F2O4P/c19-18(20,25(22,23)24)14-8-3-7-13(11-14)16-10-4-9-15(17(16)21)12-5-1-2-6-12/h3-4,7-12,21H,1-2,5-6H2,(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441306
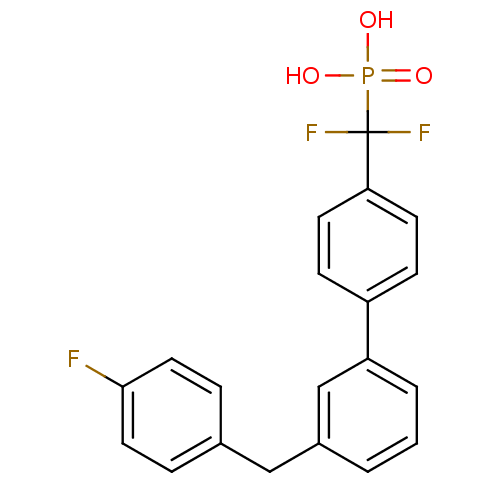
(CHEMBL2431684)Show SMILES OP(O)(=O)C(F)(F)c1ccc(cc1)-c1cccc(Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C20H16F3O3P/c21-19-10-4-14(5-11-19)12-15-2-1-3-17(13-15)16-6-8-18(9-7-16)20(22,23)27(24,25)26/h1-11,13H,12H2,(H2,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
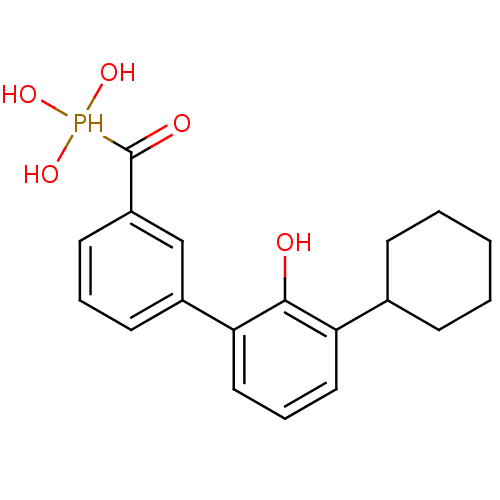
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441320
(CHEMBL2431670)Show SMILES Oc1c(cccc1-c1cccc(c1)C(=O)P(O)(O)O)C1CCCCC1 Show InChI InChI=1S/C19H23O5P/c20-18-16(13-6-2-1-3-7-13)10-5-11-17(18)14-8-4-9-15(12-14)19(21)25(22,23)24/h4-5,8-13,20,22-25H,1-3,6-7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441323

(CHEMBL2431706)Show SMILES Oc1c(cccc1-c1cccc(c1)C(F)(F)P(O)(O)=O)C1CCC1 Show InChI InChI=1S/C17H17F2O4P/c18-17(19,24(21,22)23)13-7-2-6-12(10-13)15-9-3-8-14(16(15)20)11-4-1-5-11/h2-3,6-11,20H,1,4-5H2,(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
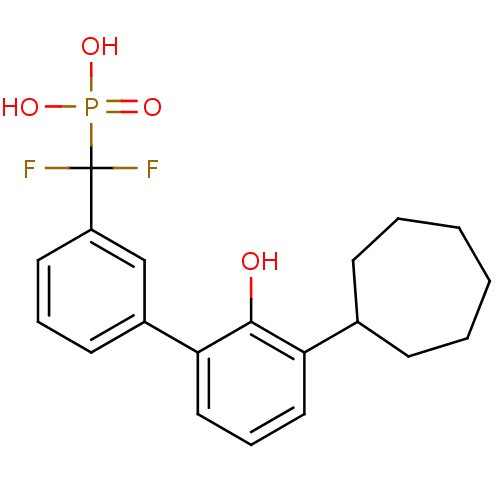
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441321
(CHEMBL2431669)Show SMILES Oc1c(cccc1-c1cccc(c1)C(F)(F)P(O)(O)=O)C1CCCCCC1 Show InChI InChI=1S/C20H23F2O4P/c21-20(22,27(24,25)26)16-10-5-9-15(13-16)18-12-6-11-17(19(18)23)14-7-3-1-2-4-8-14/h5-6,9-14,23H,1-4,7-8H2,(H2,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441324

(CHEMBL2431705)Show SMILES CC(C)(C)c1cccc(c1O)-c1cccc(c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C17H19F2O4P/c1-16(2,3)14-9-5-8-13(15(14)20)11-6-4-7-12(10-11)17(18,19)24(21,22)23/h4-10,20H,1-3H3,(H2,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |
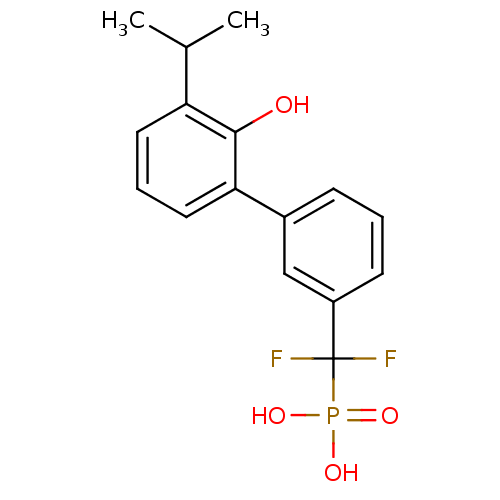
Tyrosine-protein phosphatase non-receptor type 5
(Homo sapiens (Human)) | BDBM50441327
(CHEMBL2431702)Show SMILES CC(C)c1cccc(c1O)-c1cccc(c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C16H17F2O4P/c1-10(2)13-7-4-8-14(15(13)19)11-5-3-6-12(9-11)16(17,18)23(20,21)22/h3-10,19H,1-2H3,(H2,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human STEP using pNPP as substrate after 5 mins by spectrophotometric plate reader analysis |
J Med Chem 56: 7636-50 (2013)
Article DOI: 10.1021/jm401037h
BindingDB Entry DOI: 10.7270/Q2HH6MHW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data