 Found 14013 hits with Last Name = 'lu' and Initial = 'b'
Found 14013 hits with Last Name = 'lu' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50292202
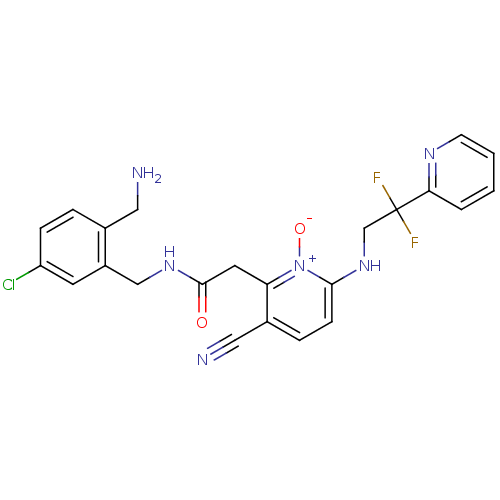
(CHEMBL382542 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1c(ccc(NCC(F)(F)c2ccccn2)[n+]1[O-])C#N Show InChI InChI=1S/C23H21ClF2N6O2/c24-18-6-4-15(11-27)17(9-18)13-30-22(33)10-19-16(12-28)5-7-21(32(19)34)31-14-23(25,26)20-3-1-2-8-29-20/h1-9,31H,10-11,13-14,27H2,(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147818
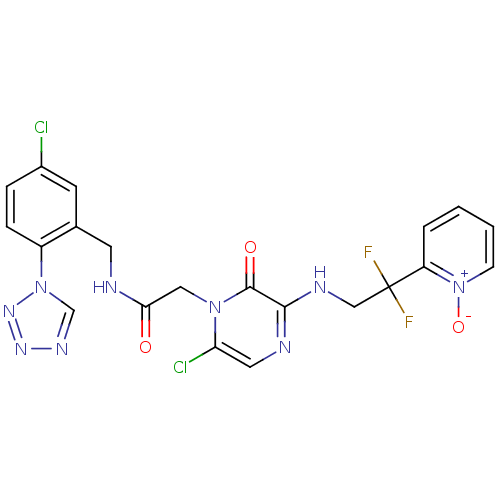
((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H17Cl2F2N9O3/c22-14-4-5-15(33-12-29-30-31-33)13(7-14)8-26-18(35)10-32-17(23)9-27-19(20(32)36)28-11-21(24,25)16-3-1-2-6-34(16)37/h1-7,9,12H,8,10-11H2,(H,26,35)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50147824
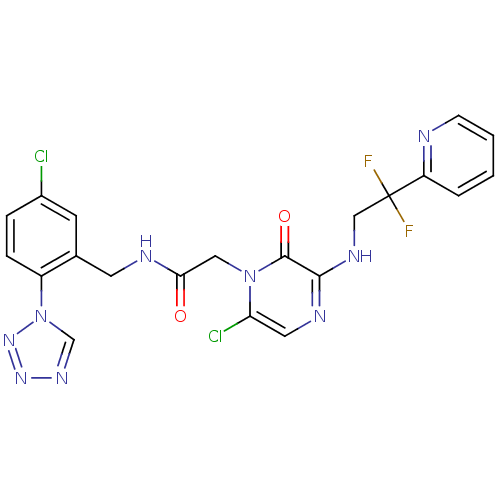
(2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES FC(F)(CNc1ncc(Cl)n(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H17Cl2F2N9O2/c22-14-4-5-15(34-12-30-31-32-34)13(7-14)8-27-18(35)10-33-17(23)9-28-19(20(33)36)29-11-21(24,25)16-3-1-2-6-26-16/h1-7,9,12H,8,10-11H2,(H,27,35)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292203
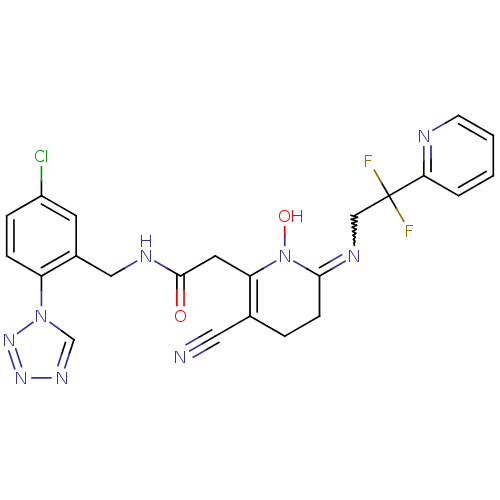
(CHEMBL196030 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES ON1C(CCC(C#N)=C1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1)=NCC(F)(F)c1ccccn1 |w:26.29,c:7| Show InChI InChI=1S/C23H20ClF2N9O2/c24-17-5-6-18(34-14-31-32-33-34)16(9-17)12-29-22(36)10-19-15(11-27)4-7-21(35(19)37)30-13-23(25,26)20-3-1-2-8-28-20/h1-3,5-6,8-9,14,37H,4,7,10,12-13H2,(H,29,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133524
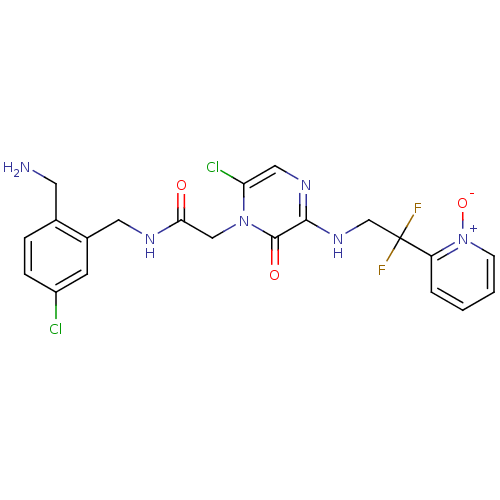
(CHEMBL419773 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cn1c(Cl)cnc(NCC(F)(F)c2cccc[n+]2[O-])c1=O Show InChI InChI=1S/C21H20Cl2F2N6O3/c22-15-5-4-13(8-26)14(7-15)9-27-18(32)11-30-17(23)10-28-19(20(30)33)29-12-21(24,25)16-3-1-2-6-31(16)34/h1-7,10H,8-9,11-12,26H2,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of GCP2 by top scintillation counter in presence of 30 nM NAA[3]G |
Bioorg Med Chem Lett 20: 7222-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.109
BindingDB Entry DOI: 10.7270/Q2GB24B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147793
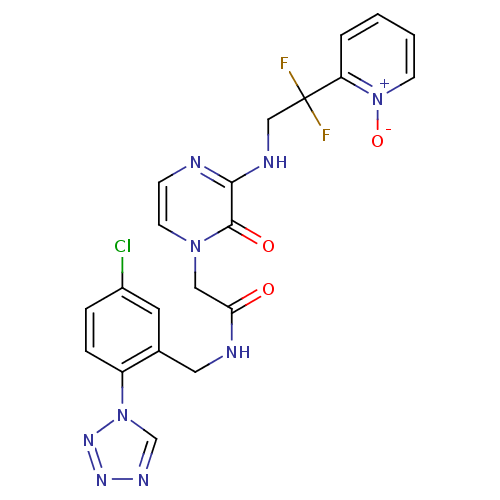
(CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-15-4-5-16(32-13-28-29-30-32)14(9-15)10-26-18(34)11-31-8-6-25-19(20(31)35)27-12-21(23,24)17-3-1-2-7-33(17)36/h1-9,13H,10-12H2,(H,25,27)(H,26,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50337452
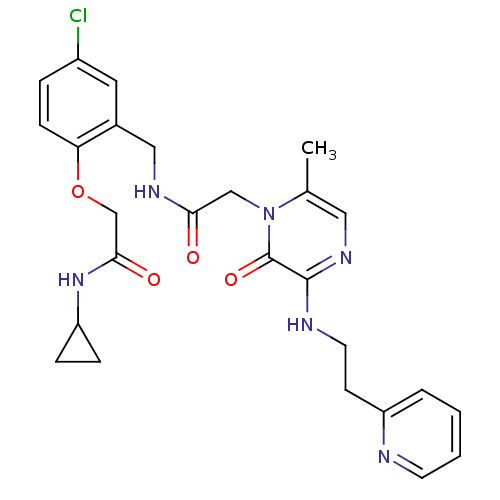
(2-(4-chloro-2-((2-(6-methyl-2-oxo-3-(2-(pyridin-2-...)Show SMILES Cc1cnc(NCCc2ccccn2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1OCC(=O)NC1CC1 Show InChI InChI=1S/C26H29ClN6O4/c1-17-13-31-25(29-11-9-20-4-2-3-10-28-20)26(36)33(17)15-23(34)30-14-18-12-19(27)5-8-22(18)37-16-24(35)32-21-6-7-21/h2-5,8,10,12-13,21H,6-7,9,11,14-16H2,1H3,(H,29,31)(H,30,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 21: 1532-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.108
BindingDB Entry DOI: 10.7270/Q2ZS2WS2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147788

(CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1-n1cnnn1 Show InChI InChI=1S/C23H22ClN7O4S/c1-16-7-9-20(27-36(34,35)14-17-5-3-2-4-6-17)23(33)30(16)13-22(32)25-12-18-11-19(24)8-10-21(18)31-15-26-28-29-31/h2-11,15,27H,12-14H2,1H3,(H,25,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131480
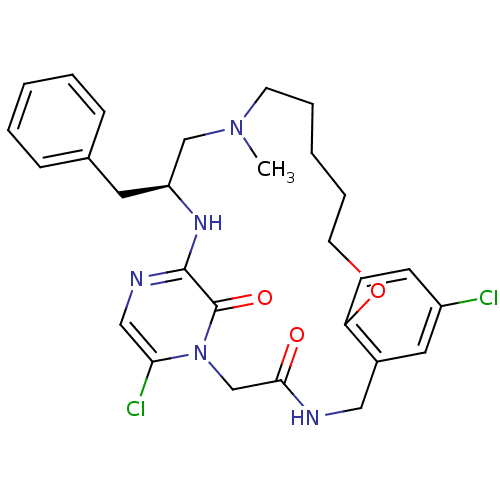
((S)-20-Benzyl-8,25-dichloro-18-methyl-12-oxa-1,4,1...)Show SMILES CN1CCCCCOc2ccc(Cl)cc2CNC(=O)Cn2c(Cl)cnc(N[C@@H](Cc3ccccc3)C1)c2=O Show InChI InChI=1S/C28H33Cl2N5O3/c1-34-12-6-3-7-13-38-24-11-10-22(29)15-21(24)16-31-26(36)19-35-25(30)17-32-27(28(35)37)33-23(18-34)14-20-8-4-2-5-9-20/h2,4-5,8-11,15,17,23H,3,6-7,12-14,16,18-19H2,1H3,(H,31,36)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Dog) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UnWersity of Misscuri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 241: 875-81 (1987)
BindingDB Entry DOI: 10.7270/Q29885JF |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 865-75 (2003)
Article DOI: 10.1124/mol.64.4.865
BindingDB Entry DOI: 10.7270/Q2RX99PV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2C adrenergic receptor
(OK) | BDBM81811
(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147809
(CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...)Show SMILES FC(F)(CNc1nccn(CC(=O)NCc2cc(Cl)ccc2-n2cnnn2)c1=O)c1ccccn1 Show InChI InChI=1S/C21H18ClF2N9O2/c22-15-4-5-16(33-13-29-30-31-33)14(9-15)10-27-18(34)11-32-8-7-26-19(20(32)35)28-12-21(23,24)17-3-1-2-6-25-17/h1-9,13H,10-12H2,(H,26,28)(H,27,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172842
(CHEMBL198820 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Cc1cc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc2c(NCC(F)(F)c2ccccn2)n1 Show InChI InChI=1S/C23H18ClF2N7O/c1-14-8-18-21(22(31-14)29-11-23(25,26)19-4-2-3-7-28-19)32-20(34-18)10-15-9-16(24)5-6-17(15)33-13-27-12-30-33/h2-9,12-13H,10-11H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 244: 571-8 (1988)
BindingDB Entry DOI: 10.7270/Q25H7DS8 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 244: 571-8 (1988)
BindingDB Entry DOI: 10.7270/Q25H7DS8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172826
(CHEMBL426101 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES Clc1ccc(c(Cc2nc3c(NCCC4CCCCN4)nccc3o2)c1)-n1cncn1 Show InChI InChI=1S/C22H24ClN7O/c23-16-4-5-18(30-14-24-13-28-30)15(11-16)12-20-29-21-19(31-20)7-10-27-22(21)26-9-6-17-3-1-2-8-25-17/h4-5,7,10-11,13-14,17,25H,1-3,6,8-9,12H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50122190
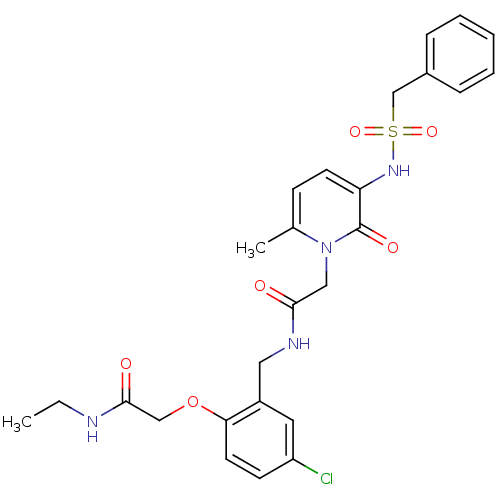
(CHEMBL296737 | N-(5-chloro-2-ethylcarbamoylmethoxy...)Show SMILES CCNC(=O)COc1ccc(Cl)cc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C26H29ClN4O6S/c1-3-28-25(33)16-37-23-12-10-21(27)13-20(23)14-29-24(32)15-31-18(2)9-11-22(26(31)34)30-38(35,36)17-19-7-5-4-6-8-19/h4-13,30H,3,14-17H2,1-2H3,(H,28,33)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 18: 2062-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.098
BindingDB Entry DOI: 10.7270/Q2P27005 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50122190

(CHEMBL296737 | N-(5-chloro-2-ethylcarbamoylmethoxy...)Show SMILES CCNC(=O)COc1ccc(Cl)cc1CNC(=O)Cn1c(C)ccc(NS(=O)(=O)Cc2ccccc2)c1=O Show InChI InChI=1S/C26H29ClN4O6S/c1-3-28-25(33)16-37-23-12-10-21(27)13-20(23)14-29-24(32)15-31-18(2)9-11-22(26(31)34)30-38(35,36)17-19-7-5-4-6-8-19/h4-13,30H,3,14-17H2,1-2H3,(H,28,33)(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thrombin in human plasma |
Bioorg Med Chem Lett 13: 161-4 (2002)
BindingDB Entry DOI: 10.7270/Q2348JQ0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292196
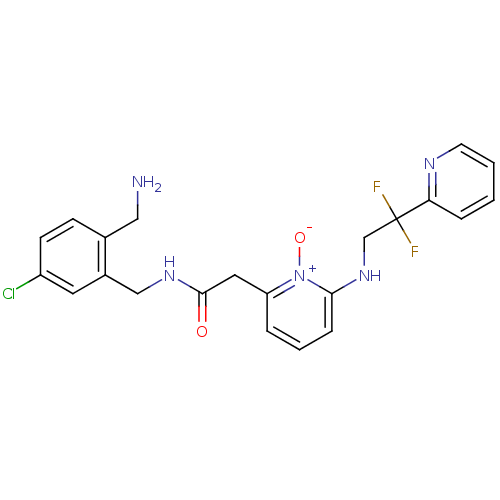
(CHEMBL195366 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES NCc1ccc(Cl)cc1CNC(=O)Cc1cccc(NCC(F)(F)c2ccccn2)[n+]1[O-] Show InChI InChI=1S/C22H22ClF2N5O2/c23-17-8-7-15(12-26)16(10-17)13-28-21(31)11-18-4-3-6-20(30(18)32)29-14-22(24,25)19-5-1-2-9-27-19/h1-10,29H,11-14,26H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
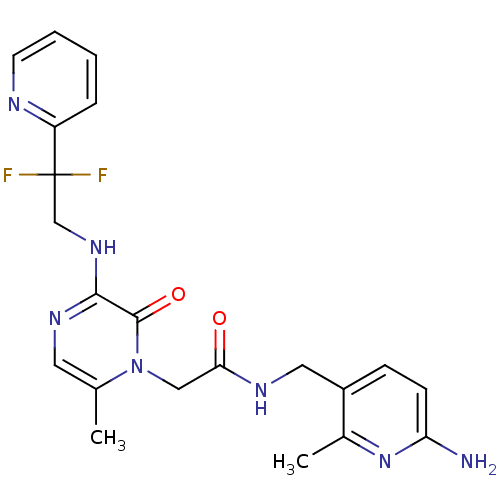
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 865-75 (2003)
Article DOI: 10.1124/mol.64.4.865
BindingDB Entry DOI: 10.7270/Q2RX99PV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133529
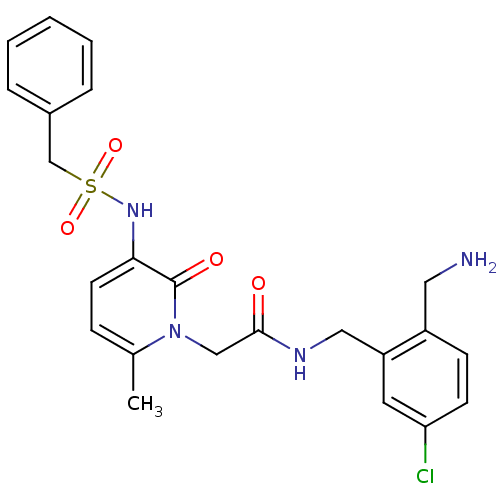
(CHEMBL420682 | N-(2-Aminomethyl-5-chloro-benzyl)-2...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CN Show InChI InChI=1S/C23H25ClN4O4S/c1-16-7-10-21(27-33(31,32)15-17-5-3-2-4-6-17)23(30)28(16)14-22(29)26-13-19-11-20(24)9-8-18(19)12-25/h2-11,27H,12-15,25H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81806
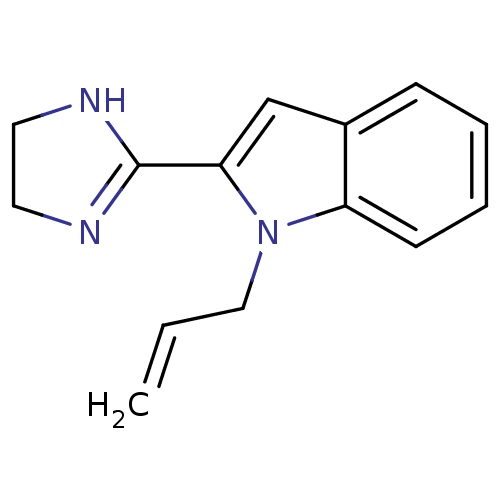
(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50133521
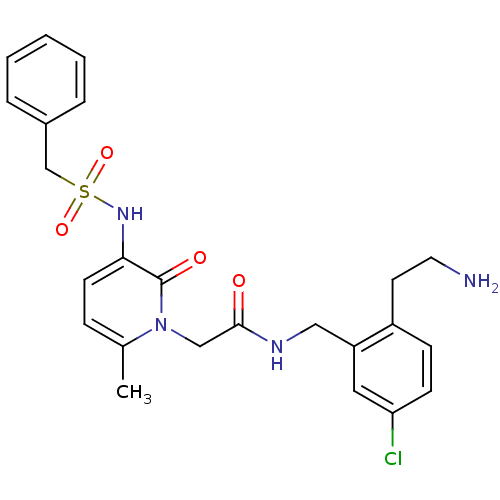
(CHEMBL116202 | N-[2-(2-Amino-ethyl)-5-chloro-benzy...)Show SMILES Cc1ccc(NS(=O)(=O)Cc2ccccc2)c(=O)n1CC(=O)NCc1cc(Cl)ccc1CCN Show InChI InChI=1S/C24H27ClN4O4S/c1-17-7-10-22(28-34(32,33)16-18-5-3-2-4-6-18)24(31)29(17)15-23(30)27-14-20-13-21(25)9-8-19(20)11-12-26/h2-10,13,28H,11-12,14-16,26H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant evaluated against thrombin (Factor IIa) |
Bioorg Med Chem Lett 13: 3477-82 (2003)
BindingDB Entry DOI: 10.7270/Q29P3119 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005
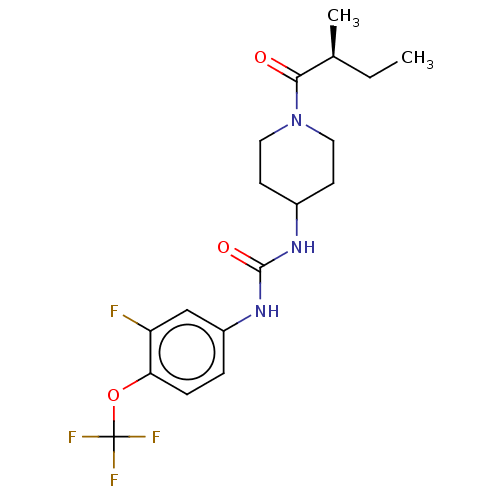
(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131464
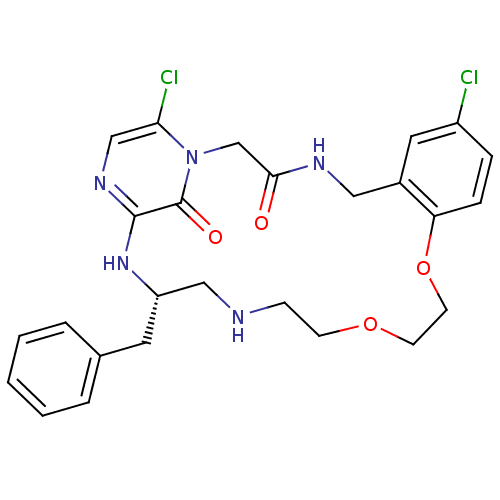
((S)-20-Benzyl-8,25-dichloro-12,15-dioxa-1,4,18,21,...)Show SMILES Clc1ccc2OCCOCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C26H29Cl2N5O4/c27-20-6-7-22-19(13-20)14-30-24(34)17-33-23(28)16-31-25(26(33)35)32-21(12-18-4-2-1-3-5-18)15-29-8-9-36-10-11-37-22/h1-7,13,16,21,29H,8-12,14-15,17H2,(H,30,34)(H,31,32)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131460
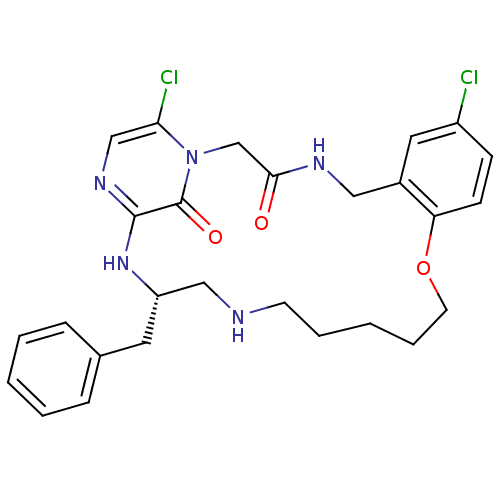
((S)-20-Benzyl-8,25-dichloro-12-oxa-1,4,18,21,23-pe...)Show SMILES Clc1ccc2OCCCCCNC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O Show InChI InChI=1S/C27H31Cl2N5O3/c28-21-9-10-23-20(14-21)15-31-25(35)18-34-24(29)17-32-26(27(34)36)33-22(13-19-7-3-1-4-8-19)16-30-11-5-2-6-12-37-23/h1,3-4,7-10,14,17,22,30H,2,5-6,11-13,15-16,18H2,(H,31,35)(H,32,33)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409005

(US10377744, Compound No. 26 | US11123311, Compound...)Show SMILES CC[C@H](C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |r| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409007
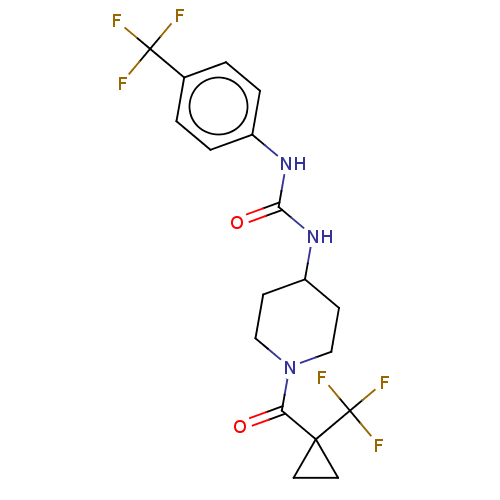
(US10377744, Compound No. 28 | US11723929, Compound...)Show SMILES FC(F)(F)c1ccc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)cc1 Show InChI InChI=1S/C18H19F6N3O2/c19-17(20,21)11-1-3-12(4-2-11)25-15(29)26-13-5-9-27(10-6-13)14(28)16(7-8-16)18(22,23)24/h1-4,13H,5-10H2,(H2,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409009
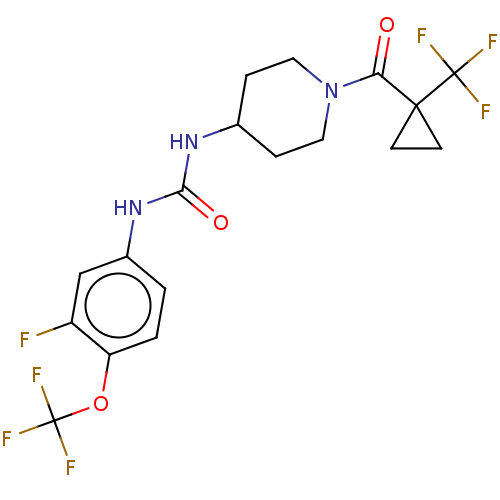
(US10377744, Compound No. 30 | US11123311, Compound...)Show SMILES Fc1cc(NC(=O)NC2CCN(CC2)C(=O)C2(CC2)C(F)(F)F)ccc1OC(F)(F)F Show InChI InChI=1S/C18H18F7N3O3/c19-12-9-11(1-2-13(12)31-18(23,24)25)27-15(30)26-10-3-7-28(8-4-10)14(29)16(5-6-16)17(20,21)22/h1-2,9-10H,3-8H2,(H2,26,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409020
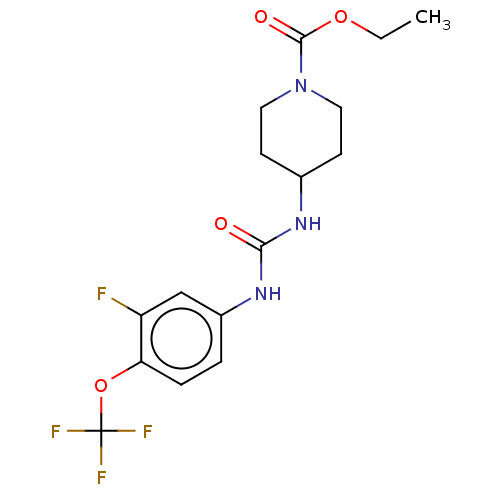
(US10377744, Compound No. 40 | US11123311, Compound...)Show SMILES CCOC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 Show InChI InChI=1S/C16H19F4N3O4/c1-2-26-15(25)23-7-5-10(6-8-23)21-14(24)22-11-3-4-13(12(17)9-11)27-16(18,19)20/h3-4,9-10H,2,5-8H2,1H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50292200
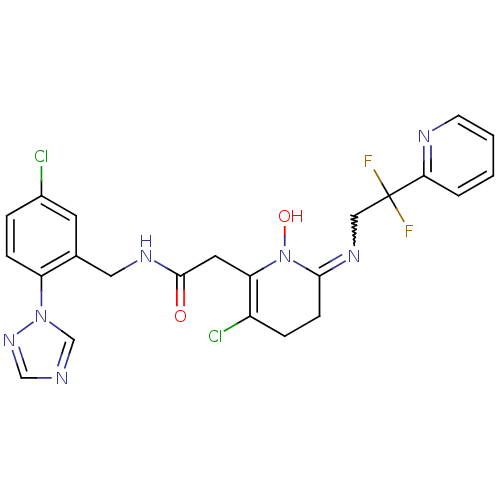
(2-[3-Chloro-6-(2,2-difluoro-2-pyridin-2-yl-ethylam...)Show SMILES ON1C(CCC(Cl)=C1CC(=O)NCc1cc(Cl)ccc1-n1cncn1)=NCC(F)(F)c1ccccn1 |w:25.28,c:6| Show InChI InChI=1S/C23H21Cl2F2N7O2/c24-16-4-6-18(33-14-28-13-32-33)15(9-16)11-30-22(35)10-19-17(25)5-7-21(34(19)36)31-12-23(26,27)20-3-1-2-8-29-20/h1-4,6,8-9,13-14,36H,5,7,10-12H2,(H,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against thrombin in human plasma |
Bioorg Med Chem Lett 15: 2771-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.110
BindingDB Entry DOI: 10.7270/Q2PN96C6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50147812
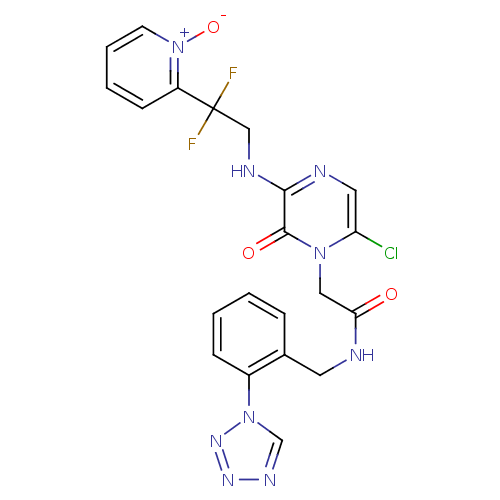
(2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...)Show SMILES [O-][n+]1ccccc1C(F)(F)CNc1ncc(Cl)n(CC(=O)NCc2ccccc2-n2cnnn2)c1=O Show InChI InChI=1S/C21H18ClF2N9O3/c22-17-10-26-19(27-12-21(23,24)16-7-3-4-8-33(16)36)20(35)31(17)11-18(34)25-9-14-5-1-2-6-15(14)32-13-28-29-30-32/h1-8,10,13H,9,11-12H2,(H,25,34)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human thrombin |
J Med Chem 47: 2995-3008 (2004)
Article DOI: 10.1021/jm030303e
BindingDB Entry DOI: 10.7270/Q270826B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50057826

((S)-1-((R)-2-Amino-3,3-dicyclohexyl-propionyl)-pyr...)Show SMILES N[C@H](C(C1CCCCC1)C1CCCCC1)C(=O)N1CCC[C@H]1C(=O)NCC1CCC(N)CC1 |wU:21.24,wD:1.0,(8.57,-5.29,;9.89,-4.53,;9.89,-2.99,;11.24,-2.22,;12.55,-2.99,;13.9,-2.22,;13.9,-.68,;12.58,.09,;11.23,-.68,;8.57,-2.21,;7.23,-2.98,;5.9,-2.21,;5.9,-.67,;7.23,.1,;8.57,-.67,;11.24,-5.3,;11.24,-6.84,;12.72,-4.89,;13.26,-3.44,;14.8,-3.51,;15.22,-5,;13.92,-5.85,;13.86,-7.39,;12.49,-8.1,;15.15,-8.21,;16.52,-7.51,;17.82,-8.32,;17.78,-9.66,;18.86,-11.05,;17.78,-12.17,;18.86,-13.25,;17.85,-10.92,;16.76,-9.44,)| Show InChI InChI=1S/C27H48N4O2/c28-22-15-13-19(14-16-22)18-30-26(32)23-12-7-17-31(23)27(33)25(29)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h19-25H,1-18,28-29H2,(H,30,32)/t19?,22?,23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thrombin |
J Med Chem 40: 1565-9 (1997)
Article DOI: 10.1021/jm970140s
BindingDB Entry DOI: 10.7270/Q2PR7V29 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81804
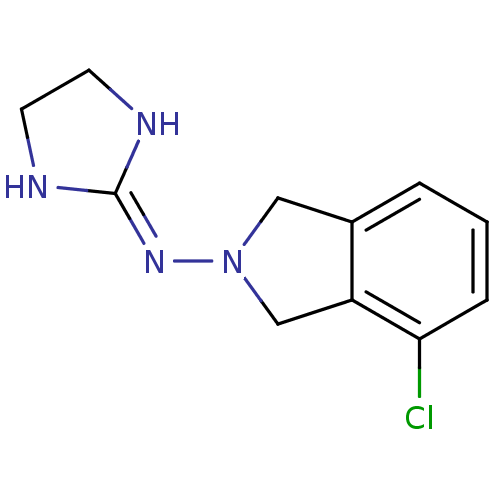
(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM409030
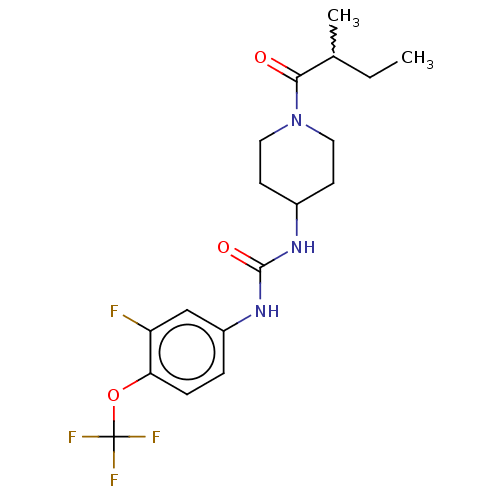
(US10377744, Compound No. 51 | US11123311, Compound...)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)c(F)c1 |w:2.2| Show InChI InChI=1S/C18H23F4N3O3/c1-3-11(2)16(26)25-8-6-12(7-9-25)23-17(27)24-13-4-5-15(14(19)10-13)28-18(20,21)22/h4-5,10-12H,3,6-9H2,1-2H3,(H2,23,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eicosis, LLC
US Patent
| Assay Description
FRET assays to determine Ki for the compounds of Table I were carried out as described previously (Lee et al. Analytical Biochemistry 434 (2013) 259-... |
US Patent US10377744 (2019)
BindingDB Entry DOI: 10.7270/Q2N3009K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172829
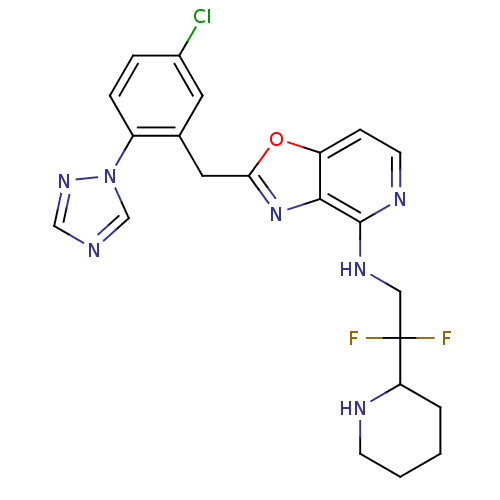
(CHEMBL198735 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES FC(F)(CNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12)C1CCCCN1 Show InChI InChI=1S/C22H22ClF2N7O/c23-15-4-5-16(32-13-26-12-30-32)14(9-15)10-19-31-20-17(33-19)6-8-28-21(20)29-11-22(24,25)18-3-1-2-7-27-18/h4-6,8-9,12-13,18,27H,1-3,7,10-11H2,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50131471
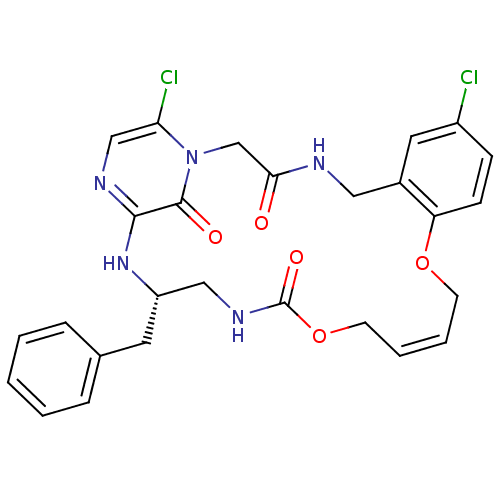
((14Z,21S)-21-benzyl-8,26-dichloro-12,17-dioxa-1,4,...)Show SMILES Clc1ccc2OC\C=C/COC(=O)NC[C@H](Cc3ccccc3)Nc3ncc(Cl)n(CC(=O)NCc2c1)c3=O |c:7| Show InChI InChI=1S/C27H27Cl2N5O5/c28-20-8-9-22-19(13-20)14-30-24(35)17-34-23(29)16-31-25(26(34)36)33-21(12-18-6-2-1-3-7-18)15-32-27(37)39-11-5-4-10-38-22/h1-9,13,16,21H,10-12,14-15,17H2,(H,30,35)(H,31,33)(H,32,37)/b5-4-/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin IIa |
Bioorg Med Chem Lett 13: 2781-4 (2003)
BindingDB Entry DOI: 10.7270/Q2GF0V2P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50122183
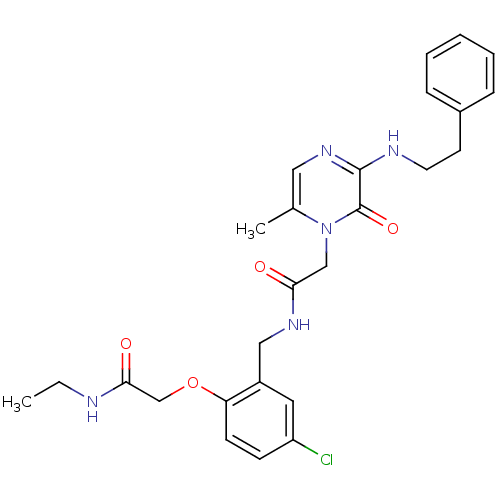
(CHEMBL431524 | N-(5-Chloro-2-ethylcarbamoylmethoxy...)Show SMILES CCNC(=O)COc1ccc(Cl)cc1CNC(=O)Cn1c(C)cnc(NCCc2ccccc2)c1=O Show InChI InChI=1S/C26H30ClN5O4/c1-3-28-24(34)17-36-22-10-9-21(27)13-20(22)15-30-23(33)16-32-18(2)14-31-25(26(32)35)29-12-11-19-7-5-4-6-8-19/h4-10,13-14H,3,11-12,15-17H2,1-2H3,(H,28,34)(H,29,31)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of thrombin in human plasma |
Bioorg Med Chem Lett 13: 161-4 (2002)
BindingDB Entry DOI: 10.7270/Q2348JQ0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172841
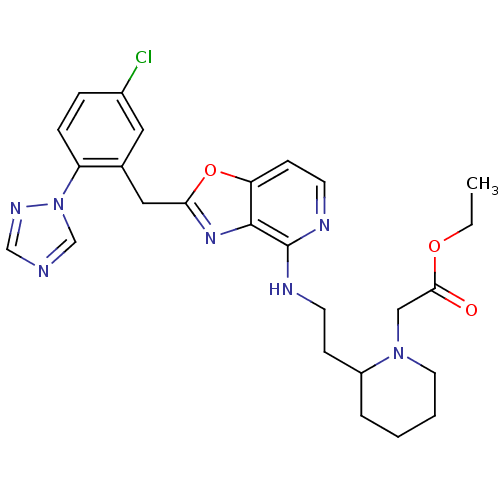
((2-{2-[2-(5-Chloro-2-[1,2,4]triazol-1-yl-benzyl)-o...)Show SMILES CCOC(=O)CN1CCCCC1CCNc1nccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12 Show InChI InChI=1S/C26H30ClN7O3/c1-2-36-24(35)15-33-12-4-3-5-20(33)8-10-29-26-25-22(9-11-30-26)37-23(32-25)14-18-13-19(27)6-7-21(18)34-17-28-16-31-34/h6-7,9,11,13,16-17,20H,2-5,8,10,12,14-15H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences
Curated by PDSP Ki Database
| |
Mol Pharmacol 64: 865-75 (2003)
Article DOI: 10.1124/mol.64.4.865
BindingDB Entry DOI: 10.7270/Q2RX99PV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50172839
(CHEMBL197668 | [2-(5-Chloro-2-[1,2,4]triazol-1-yl-...)Show SMILES FC(F)(CNc1cccc2oc(Cc3cc(Cl)ccc3-n3cncn3)nc12)c1ccccn1 Show InChI InChI=1S/C23H17ClF2N6O/c24-16-7-8-18(32-14-27-13-30-32)15(10-16)11-21-31-22-17(4-3-5-19(22)33-21)29-12-23(25,26)20-6-1-2-9-28-20/h1-10,13-14,29H,11-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
Bioorg Med Chem Lett 15: 4411-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.022
BindingDB Entry DOI: 10.7270/Q22Z153P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data