

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-1 (Homo sapiens (Human)) | BDBM10355 ((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283345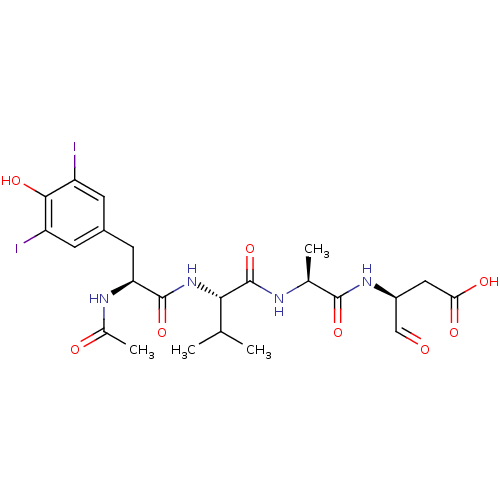 ((S)-3-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50290014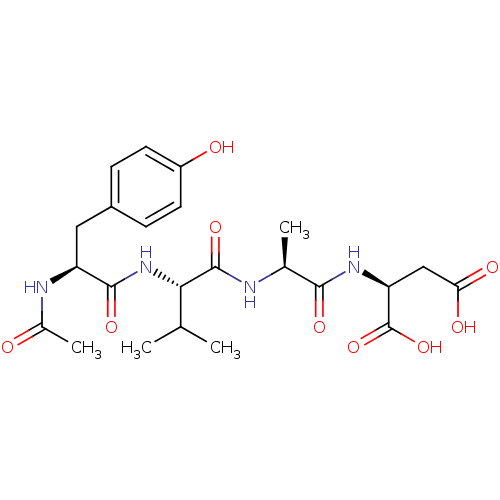 ((S)-2-((S)-2-{(S)-2-[(S)-2-Acetylamino-3-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143464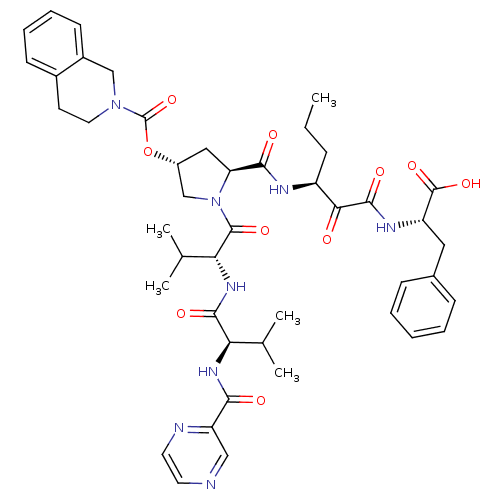 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (3R,...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283348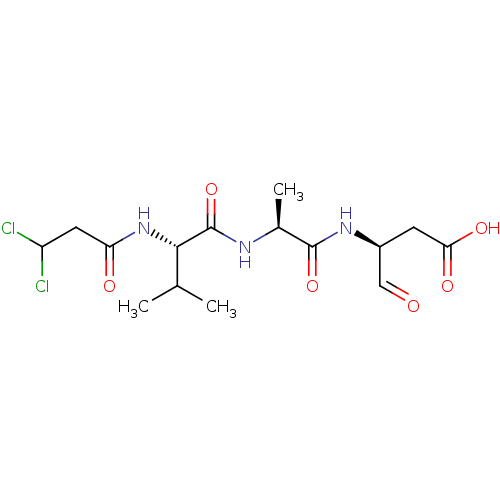 ((S)-3-{(S)-2-[(S)-2-(3,3-Dichloro-propionylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50486212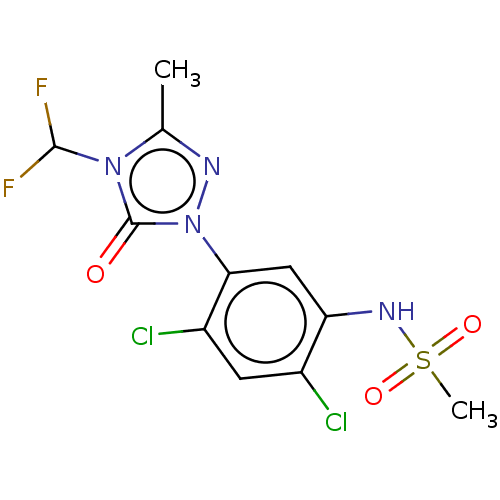 (CHEBI:9339 | SULFENTRAZONE) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283341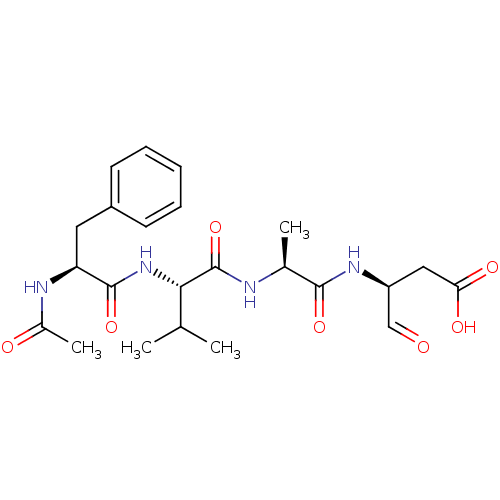 ((S)-3-{(S)-2-[(S)-2-((S)-2-Acetylamino-3-phenyl-pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50290012 ((S)-2-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50290009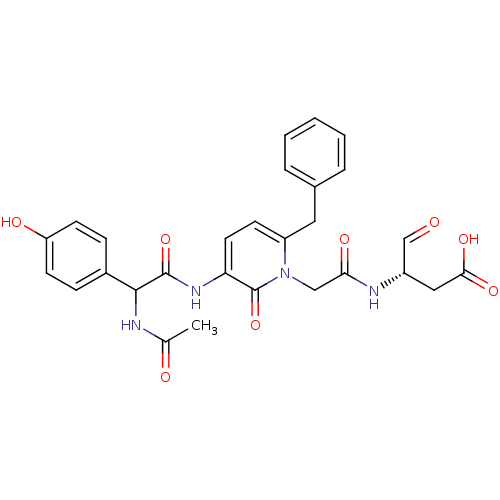 ((S)-3-(2-{3-[2-Acetylamino-2-(4-hydroxy-phenyl)-ac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50058529 ((S)-2-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283346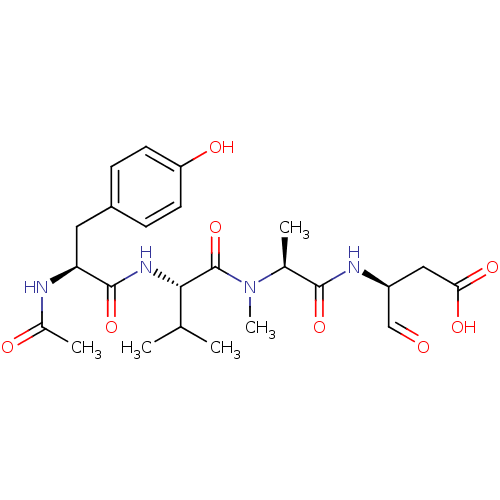 ((S)-3-[(S)-2-({(S)-2-[(S)-2-Acetylamino-3-(4-hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488401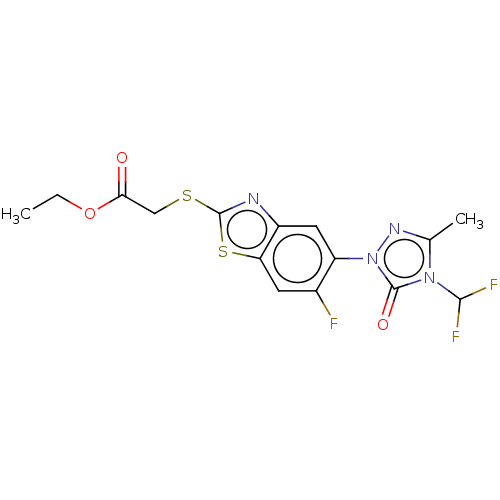 (CHEMBL2286233) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143458 ((4S,5R)-1-((R)-2-{2-Cyclohexyl-2-[(hydroxy-pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143472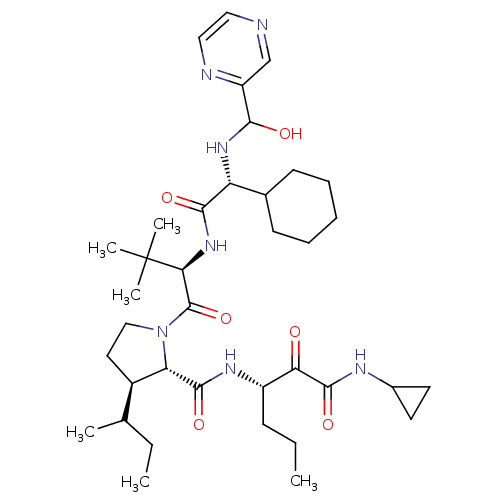 ((4S,5R)-3-sec-Butyl-1-((R)-2-{2-cyclohexyl-2-[(hyd...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143459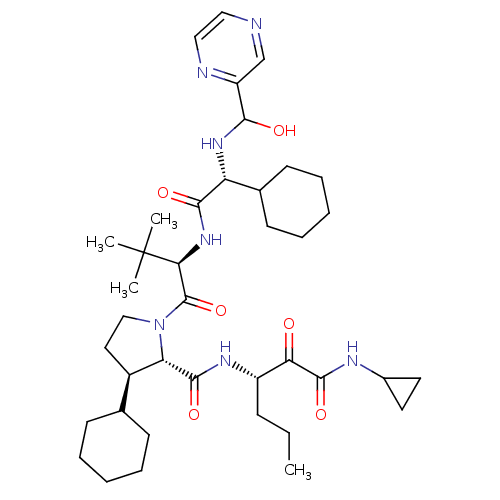 ((4S,5R)-3-Cyclohexyl-1-((R)-2-{2-cyclohexyl-2-[(hy...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50135430 (CHEMBL434033 | Naphthalene-2-carboxylic acid (3R,5...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. | Bioorg Med Chem Lett 13: 4059-63 (2003) BindingDB Entry DOI: 10.7270/Q2028QX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143470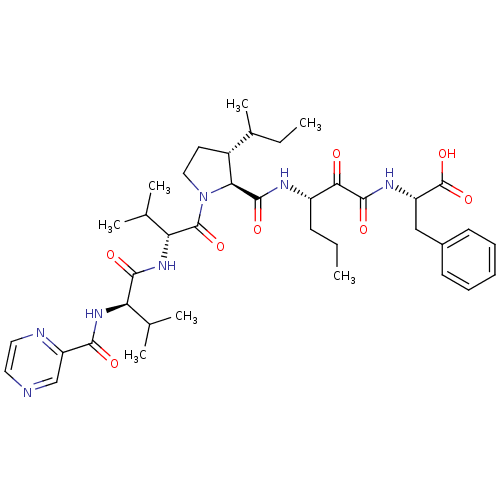 (2-((S)-3-{[(4S,5R)-3-sec-Butyl-1-((R)-3-methyl-2-{...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283338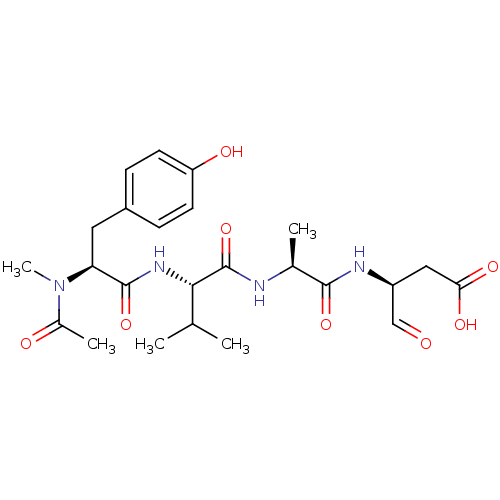 ((S)-3-((S)-2-{(S)-2-[(S)-2-(Acetyl-methyl-amino)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143469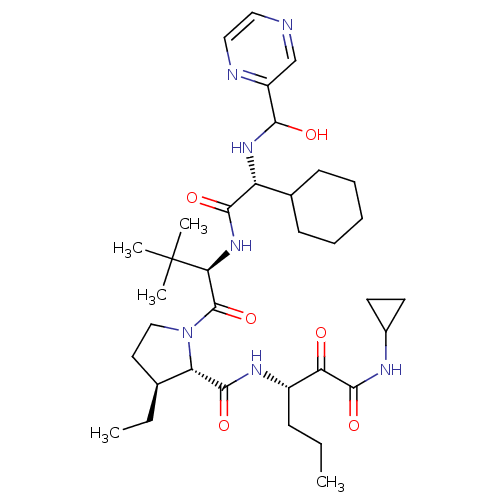 ((4S,5S)-1-((R)-2-{2-Cyclohexyl-2-[(hydroxy-pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50290006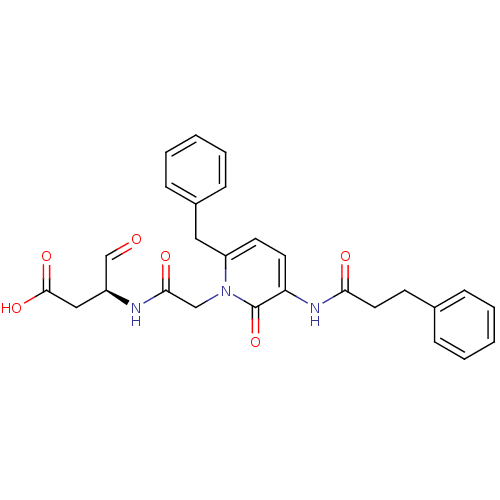 ((S)-3-{2-[6-Benzyl-2-oxo-3-(3-phenyl-propionylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50290015 ((S)-4-Oxo-3-{(S)-2-[2-oxo-3-(3-phenyl-propionylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of IL-1 beta converting enzyme | Bioorg Med Chem Lett 7: 2181-2186 (1997) Article DOI: 10.1016/S0960-894X(97)00394-6 BindingDB Entry DOI: 10.7270/Q2VH5NT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143471 ((4S,5S)-2-[1-((S)-(S)-1-Carboxy-2-phenyl-ethylamin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488398 (CHEMBL2286231) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141194 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.03) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141198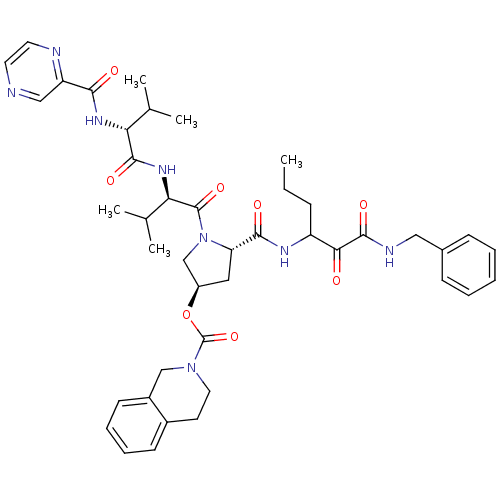 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141206 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.10) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141210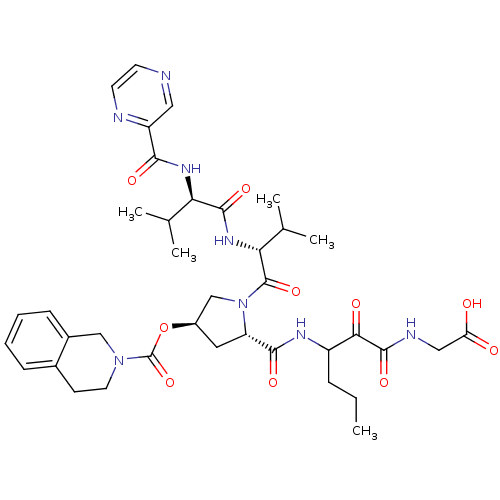 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141196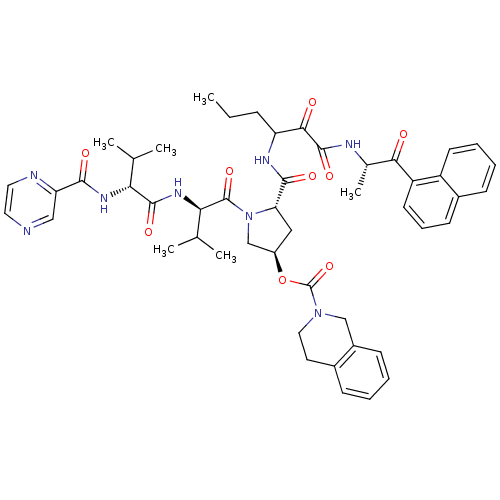 (1-[3-methyl-2-[2-methyl-1-(2-pyrazinylcarboxamido)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.18) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141199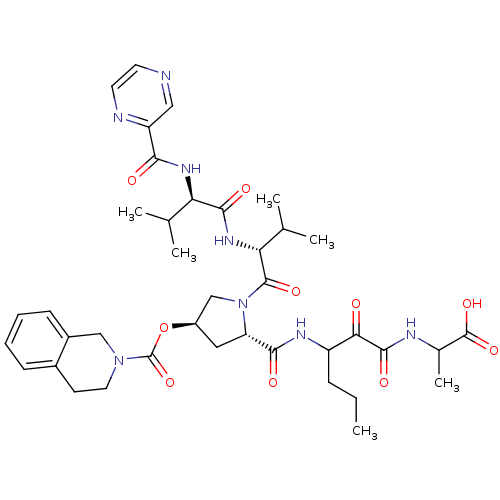 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid 5-[(...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.18) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141203 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488385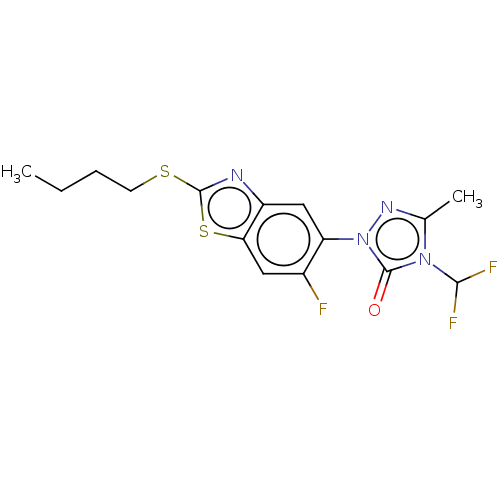 (CHEMBL2286232) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143473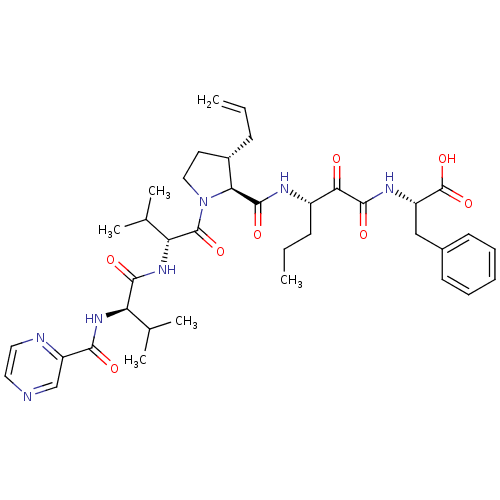 (2-((S)-3-{[(4S,5S)-3-Allyl-1-((R)-3-methyl-2-{(R)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50141202 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA); <0.2 (0.15) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143462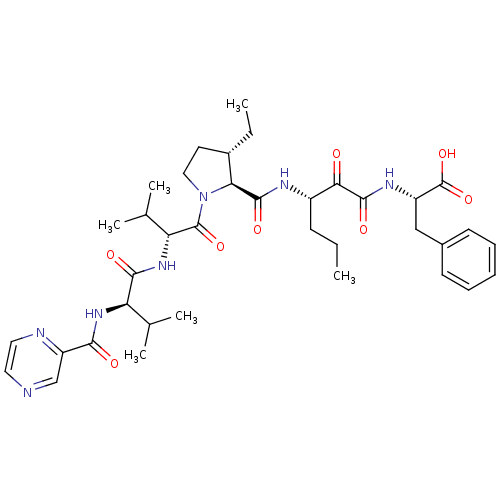 (2-((S)-3-{[(4S,5S)-3-Ethyl-1-((R)-3-methyl-2-{(R)-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488397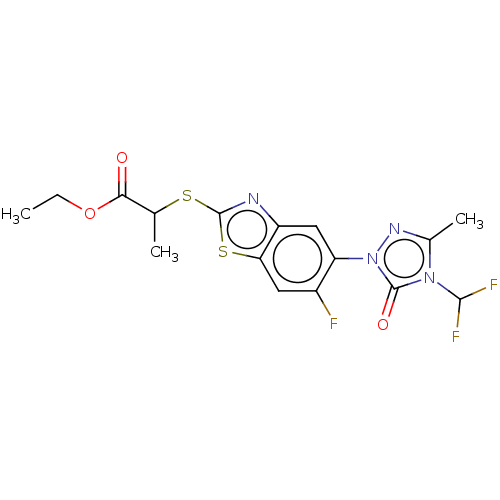 (CHEMBL2286234) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143467 ((4S,5R)-1-((R)-2-{2-Cyclohexyl-2-[(hydroxy-pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488382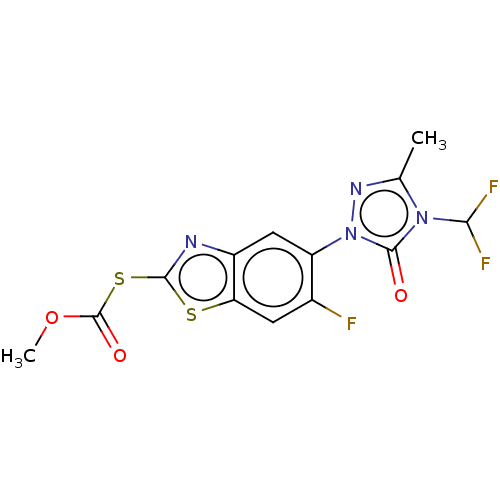 (CHEMBL2286235) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141186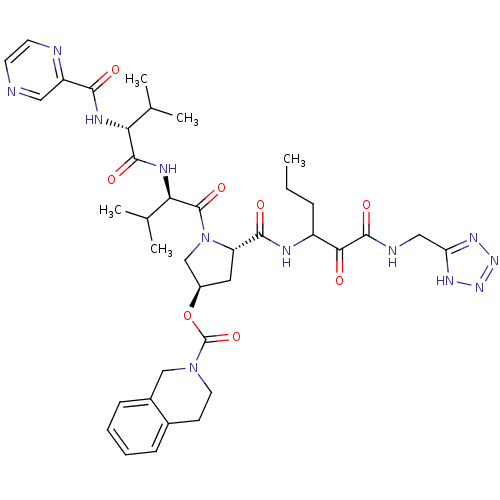 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488396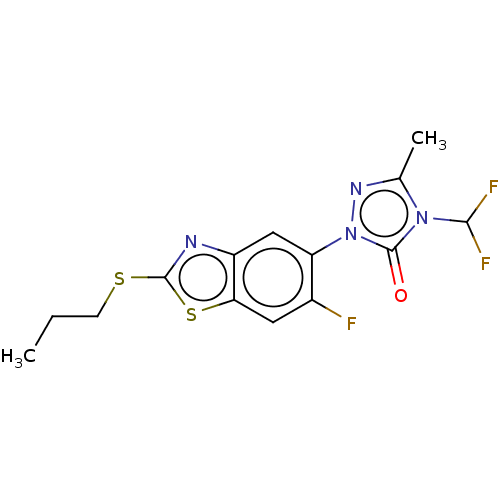 (CHEMBL2286230) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143466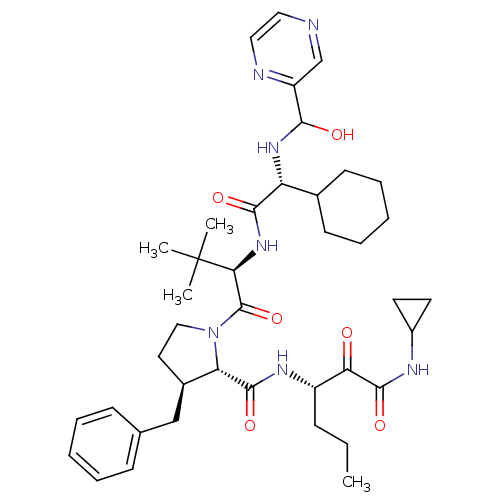 ((4S,5S)-3-Benzyl-1-((R)-2-{2-cyclohexyl-2-[(hydrox...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143468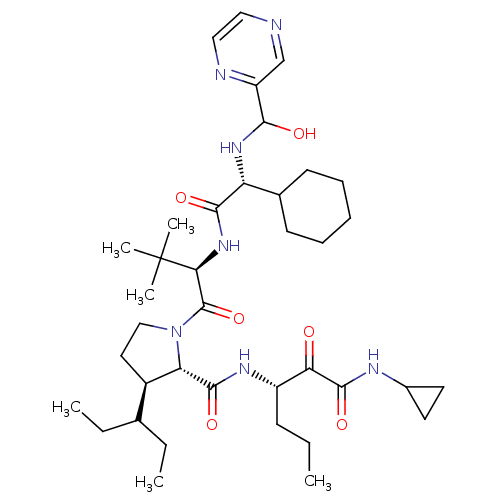 ((4S,5R)-1-((R)-2-{2-Cyclohexyl-2-[(hydroxy-pyrazin...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50135432 (CHEMBL340890 | Naphthalene-2-carboxylic acid (3R,5...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. | Bioorg Med Chem Lett 13: 4059-63 (2003) BindingDB Entry DOI: 10.7270/Q2028QX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141207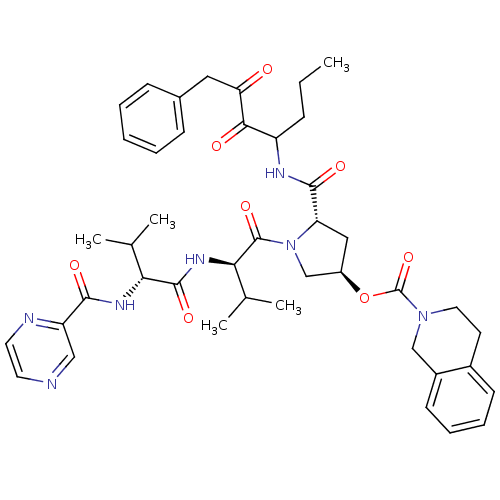 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50143460 ((2S,3R)-3-tert-Butyl-1-((R)-2-{(R)-2-cyclohexyl-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3.4A protease | Bioorg Med Chem Lett 14: 1939-42 (2004) Article DOI: 10.1016/j.bmcl.2004.01.078 BindingDB Entry DOI: 10.7270/Q2862FVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50283343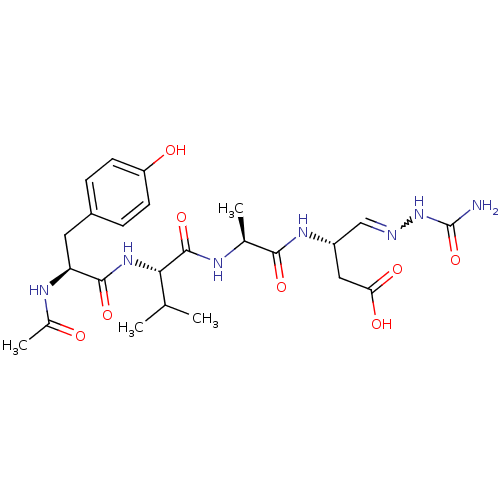 (3-((S)-2-{(S)-2-[(S)-2-((S)-Acetylamino)-3-(4-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards IL-1 beta converting enzyme (ICE) | Bioorg Med Chem Lett 4: 2359-2364 (1994) Article DOI: 10.1016/0960-894X(94)85040-2 BindingDB Entry DOI: 10.7270/Q2NG4QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488395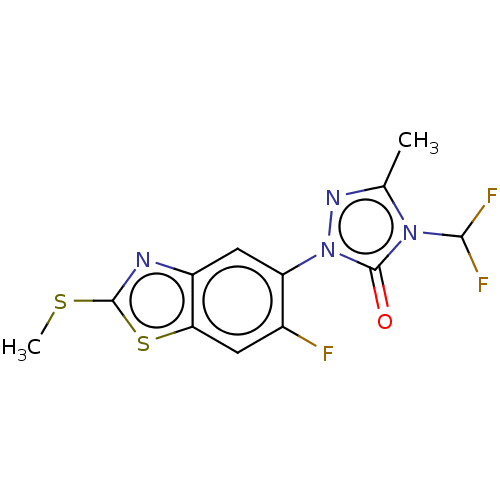 (CHEMBL2286229) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141193 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141212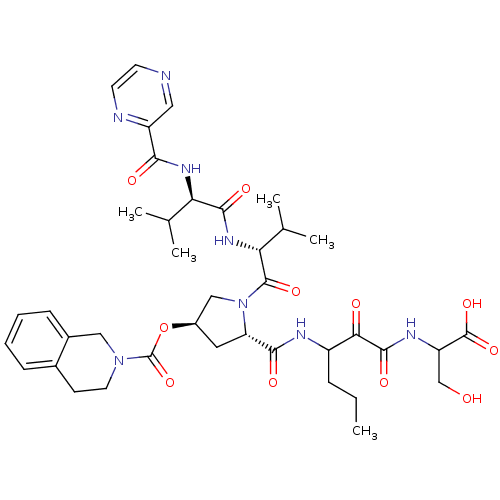 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protoporphyrinogen oxidase, chloroplastic (Nicotiana tabacum) | BDBM50488402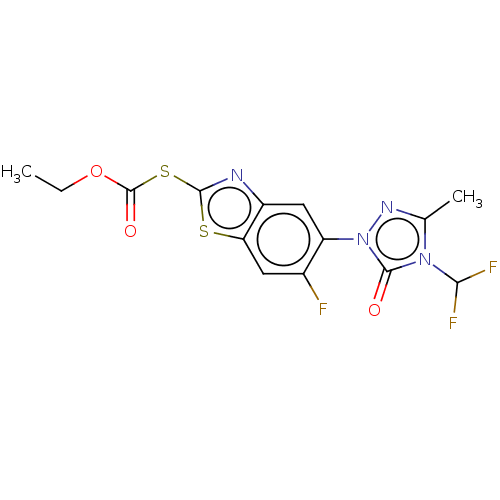 (CHEMBL2286467) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central China Normal University Curated by ChEMBL | Assay Description Inhibition of Nicotiana tabacum (tobacco) recombinant PPO assessed as protoporphyrinogen IX formation at room temperature by fluorimetric assay | Bioorg Med Chem 21: 3245-55 (2013) Article DOI: 10.1016/j.bmc.2013.03.056 BindingDB Entry DOI: 10.7270/Q2ZW1PTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50141188 (3,4-Dihydro-1H-isoquinoline-2-carboxylic acid (S)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding inhibition of hepatitis C virus NS3.4A protease 2 using p-nitroaniline assay (pNA) | Bioorg Med Chem Lett 14: 1441-6 (2004) Article DOI: 10.1016/j.bmcl.2004.01.022 BindingDB Entry DOI: 10.7270/Q24Q7TD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |