 Found 97 hits with Last Name = 'möller' and Initial = 'g'
Found 97 hits with Last Name = 'möller' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from human ERalpha expressed in SF9 cells |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311679
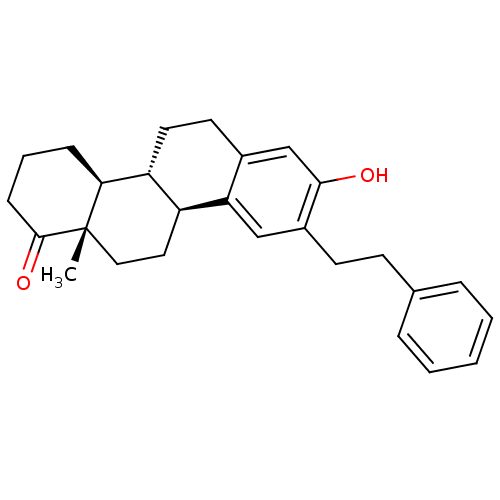
((4bR,10bS,12aS)-8-hydroxy-12a-methyl-9-phenethyl-2...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(CCc5ccccc5)cc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C27H32O2/c1-27-15-14-21-22(24(27)8-5-9-26(27)29)13-12-19-17-25(28)20(16-23(19)21)11-10-18-6-3-2-4-7-18/h2-4,6-7,16-17,21-22,24,28H,5,8-15H2,1H3/t21-,22+,24-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357463
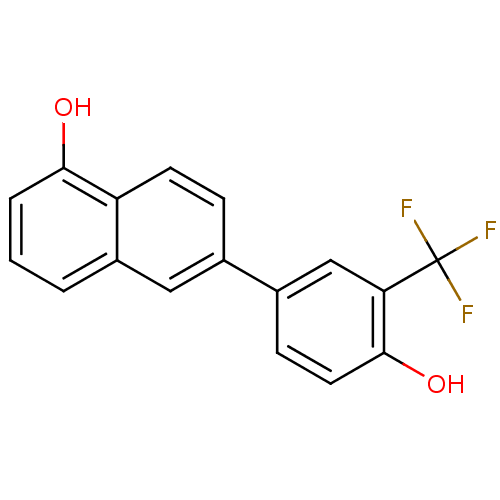
(CHEMBL1917897)Show InChI InChI=1S/C17H11F3O2/c18-17(19,20)14-9-11(5-7-16(14)22)10-4-6-13-12(8-10)2-1-3-15(13)21/h1-9,21-22H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta-HSD2 using [3H]E2 as substrate by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311682
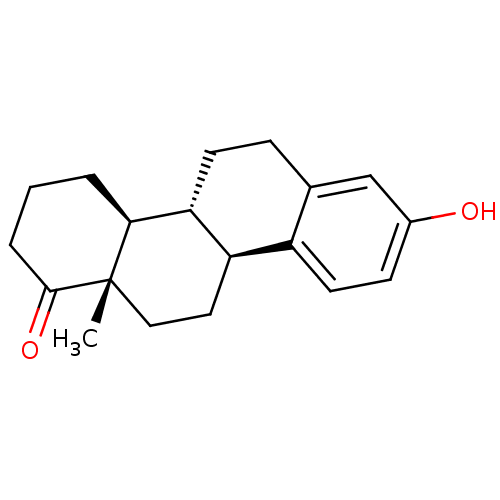
((4bR,10bS,12aS)-8-hydroxy-12a-methyl-2,3,4,4a,5,6,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C19H24O2/c1-19-10-9-15-14-8-6-13(20)11-12(14)5-7-16(15)17(19)3-2-4-18(19)21/h6,8,11,15-17,20H,2-5,7,9-10H2,1H3/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357463

(CHEMBL1917897)Show InChI InChI=1S/C17H11F3O2/c18-17(19,20)14-9-11(5-7-16(14)22)10-4-6-13-12(8-10)2-1-3-15(13)21/h1-9,21-22H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human MDA-MB-231 cells after 3.5 hrs by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311678
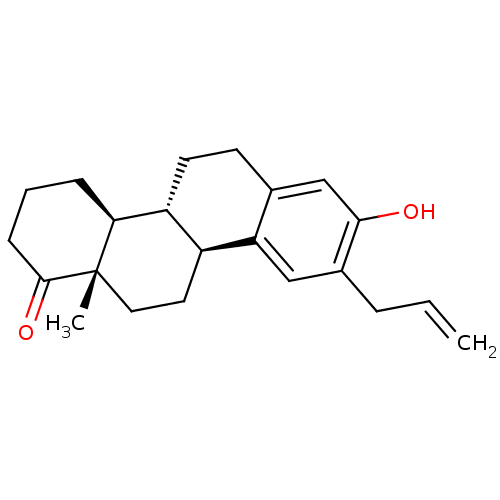
((4bR,10bS,12aS)-9-allyl-8-hydroxy-12a-methyl-2,3,4...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(CC=C)cc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C22H28O2/c1-3-5-15-12-18-14(13-20(15)23)8-9-17-16(18)10-11-22(2)19(17)6-4-7-21(22)24/h3,12-13,16-17,19,23H,1,4-11H2,2H3/t16-,17+,19-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50267362
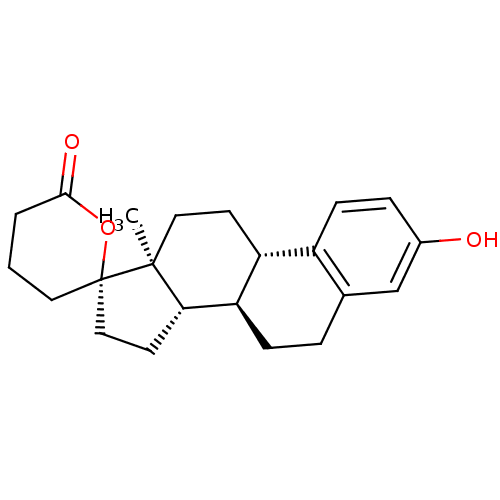
((2'S,8R,9S,13S,14S)-3-hydroxy-13-methyl-4',5',6,7,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]21CCCC(=O)O1 |r| Show InChI InChI=1S/C22H28O3/c1-21-11-8-17-16-7-5-15(23)13-14(16)4-6-18(17)19(21)9-12-22(21)10-2-3-20(24)25-22/h5,7,13,17-19,23H,2-4,6,8-12H2,1H3/t17-,18-,19+,21+,22+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 (unknown origin) |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311673

((8R,9S,13S,14S,16S)-2-chloro-16-fluoro-3-hydroxy-1...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1C[C@H](F)C2=O |r| Show InChI InChI=1S/C18H20ClFO2/c1-18-5-4-10-11(13(18)8-15(20)17(18)22)3-2-9-6-16(21)14(19)7-12(9)10/h6-7,10-11,13,15,21H,2-5,8H2,1H3/t10-,11+,13-,15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311667

((8R,9S,13S,14S)-3-hydroxy-13-methyl-2-phenethyl-7,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(CCc5ccccc5)cc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C26H30O2/c1-26-14-13-20-21(23(26)11-12-25(26)28)10-9-18-16-24(27)19(15-22(18)20)8-7-17-5-3-2-4-6-17/h2-6,15-16,20-21,23,27H,7-14H2,1H3/t20-,21+,23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50358116
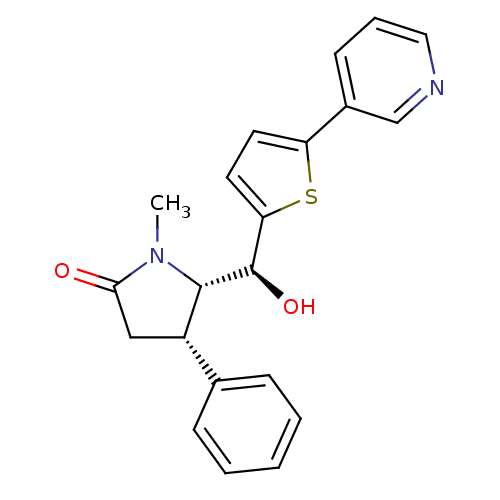
(CHEMBL1915968)Show SMILES CN1[C@H]([C@H](O)c2ccc(s2)-c2cccnc2)[C@@H](CC1=O)c1ccccc1 |r| Show InChI InChI=1S/C21H20N2O2S/c1-23-19(24)12-16(14-6-3-2-4-7-14)20(23)21(25)18-10-9-17(26-18)15-8-5-11-22-13-15/h2-11,13,16,20-21,25H,12H2,1H3/t16-,20-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 (unknown origin) |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311666

((8R,9S,13S,14S)-3-hydroxy-13-methyl-2-(phenylethyn...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(cc34)C#Cc3ccccc3)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C26H26O2/c1-26-14-13-20-21(23(26)11-12-25(26)28)10-9-18-16-24(27)19(15-22(18)20)8-7-17-5-3-2-4-6-17/h2-6,15-16,20-21,23,27H,9-14H2,1H3/t20-,21+,23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426580
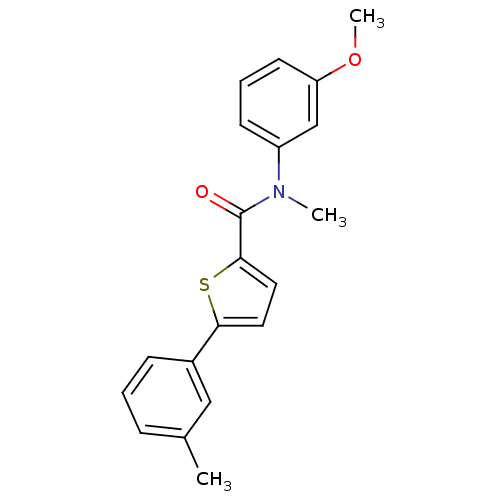
(CHEMBL2324690)Show InChI InChI=1S/C20H19NO2S/c1-14-6-4-7-15(12-14)18-10-11-19(24-18)20(22)21(2)16-8-5-9-17(13-16)23-3/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50358118

(CHEMBL1915964)Show SMILES COc1cccc(-c2ccc(s2)C(=O)N(C)Cc2cccc(O)c2)c1F Show InChI InChI=1S/C20H18FNO3S/c1-22(12-13-5-3-6-14(23)11-13)20(24)18-10-9-17(26-18)15-7-4-8-16(25-2)19(15)21/h3-11,23H,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426579

(CHEMBL2324360)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(OC)c1F Show InChI InChI=1S/C20H18FNO3S/c1-22(13-6-4-7-14(12-13)24-2)20(23)18-11-10-17(26-18)15-8-5-9-16(25-3)19(15)21/h4-12H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426587

(CHEMBL2324361)Show SMILES COc1cccc(-c2ccc(s2)C(=O)N(C)c2cccc(C)c2)c1F Show InChI InChI=1S/C20H18FNO2S/c1-13-6-4-7-14(12-13)22(2)20(23)18-11-10-17(25-18)15-8-5-9-16(24-3)19(15)21/h4-12H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426581
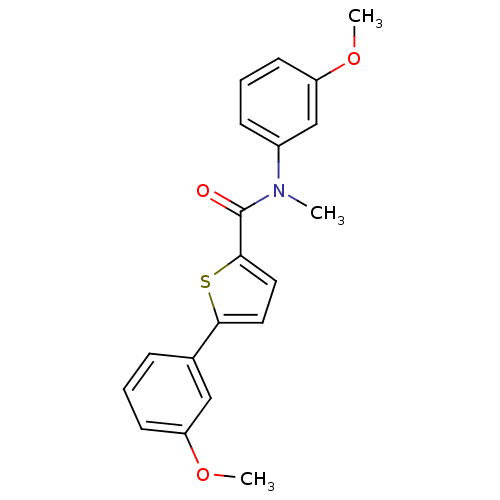
(CHEMBL2324679)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(OC)c1 Show InChI InChI=1S/C20H19NO3S/c1-21(15-7-5-9-17(13-15)24-3)20(22)19-11-10-18(25-19)14-6-4-8-16(12-14)23-2/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426580

(CHEMBL2324690)Show InChI InChI=1S/C20H19NO2S/c1-14-6-4-7-15(12-14)18-10-11-19(24-18)20(22)21(2)16-8-5-9-17(13-16)23-3/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311675
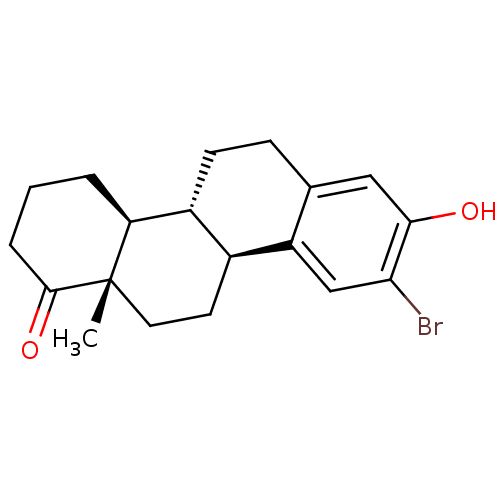
((4bR,10bS,12aS)-9-bromo-8-hydroxy-12a-methyl-2,3,4...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Br)cc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C19H23BrO2/c1-19-8-7-12-13(15(19)3-2-4-18(19)22)6-5-11-9-17(21)16(20)10-14(11)12/h9-10,12-13,15,21H,2-8H2,1H3/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311674
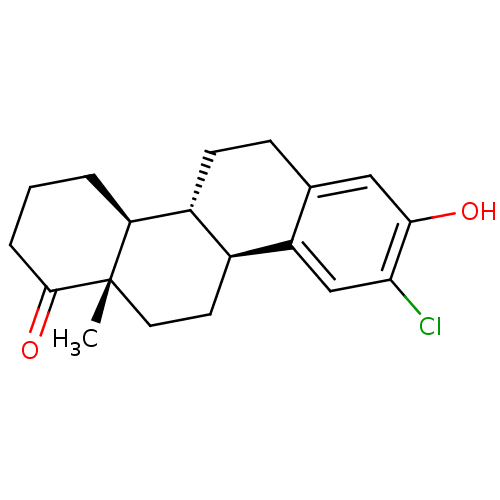
((4bR,10bS,12aS)-9-chloro-8-hydroxy-12a-methyl-2,3,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C19H23ClO2/c1-19-8-7-12-13(15(19)3-2-4-18(19)22)6-5-11-9-17(21)16(20)10-14(11)12/h9-10,12-13,15,21H,2-8H2,1H3/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311677

((4bR,10bS,12aS)-8-hydroxy-9-methoxy-12a-methyl-2,3...)Show SMILES COc1cc2[C@H]3CC[C@@]4(C)[C@@H](CCCC4=O)[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C20H26O3/c1-20-9-8-13-14(16(20)4-3-5-19(20)22)7-6-12-10-17(21)18(23-2)11-15(12)13/h10-11,13-14,16,21H,3-9H2,1-2H3/t13-,14+,16-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311680
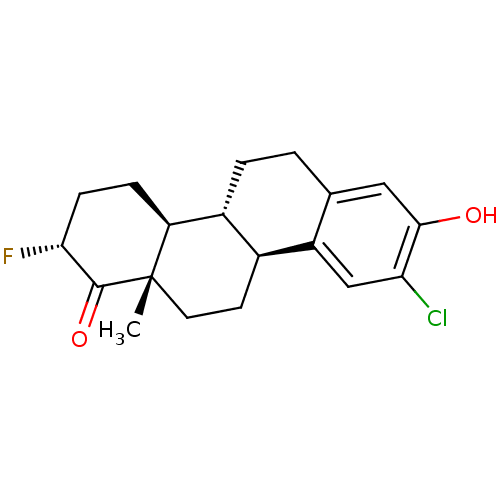
((2R,4bR,10bS,12aS)-9-chloro-2-fluoro-8-hydroxy-12a...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1CC[C@@H](F)C2=O |r| Show InChI InChI=1S/C19H22ClFO2/c1-19-7-6-11-12(14(19)4-5-16(21)18(19)23)3-2-10-8-17(22)15(20)9-13(10)11/h8-9,11-12,14,16,22H,2-7H2,1H3/t11-,12+,14-,16+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311661
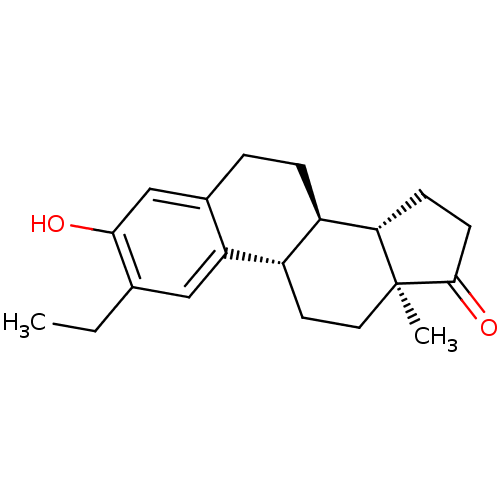
((8R,9S,13S,14S)-2-Ethyl-3-hydroxy-13-methyl-6,7,8,...)Show SMILES CCc1cc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C20H26O2/c1-3-12-10-16-13(11-18(12)21)4-5-15-14(16)8-9-20(2)17(15)6-7-19(20)22/h10-11,14-15,17,21H,3-9H2,1-2H3/t14-,15+,17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17289

((1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from human ERalpha expressed in SF9 cells |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311672
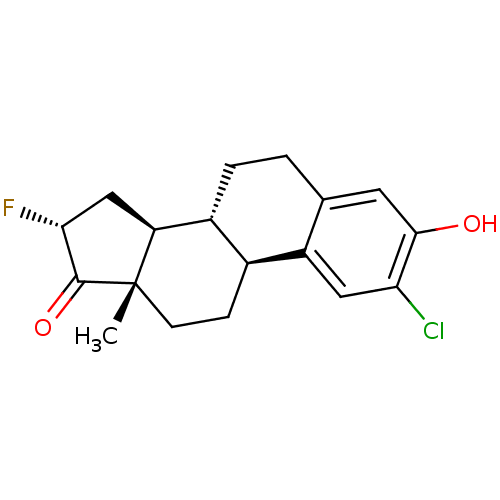
((8R,9S,13S,14S,16R)-2-chloro-16-fluoro-3-hydroxy-1...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1C[C@@H](F)C2=O |r| Show InChI InChI=1S/C18H20ClFO2/c1-18-5-4-10-11(13(18)8-15(20)17(18)22)3-2-9-6-16(21)14(19)7-12(9)10/h6-7,10-11,13,15,21H,2-5,8H2,1H3/t10-,11+,13-,15+,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426579

(CHEMBL2324360)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(OC)c1F Show InChI InChI=1S/C20H18FNO3S/c1-22(13-6-4-7-14(12-13)24-2)20(23)18-11-10-17(26-18)15-8-5-9-16(25-3)19(15)21/h4-12H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM17289

((1S,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311663

((8R,9S,13S,14S)-2-allyl-3-hydroxy-13-methyl-7,8,9,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(CC=C)cc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C21H26O2/c1-3-4-14-11-17-13(12-19(14)22)5-6-16-15(17)9-10-21(2)18(16)7-8-20(21)23/h3,11-12,15-16,18,22H,1,4-10H2,2H3/t15-,16+,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426581

(CHEMBL2324679)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(OC)c1 Show InChI InChI=1S/C20H19NO3S/c1-21(15-7-5-9-17(13-15)24-3)20(22)19-11-10-18(25-19)14-6-4-8-16(12-14)23-2/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human MDA-MB-231 cells using [3H]E2 as substrate after 6 hrs by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311671

((8R,9S,13S,14S)-2-chloro-13-ethyl-3-hydroxy-7,8,9,...)Show SMILES CC[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23ClO2/c1-2-19-8-7-12-13(15(19)5-6-18(19)22)4-3-11-9-17(21)16(20)10-14(11)12/h9-10,12-13,15,21H,2-8H2,1H3/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311676
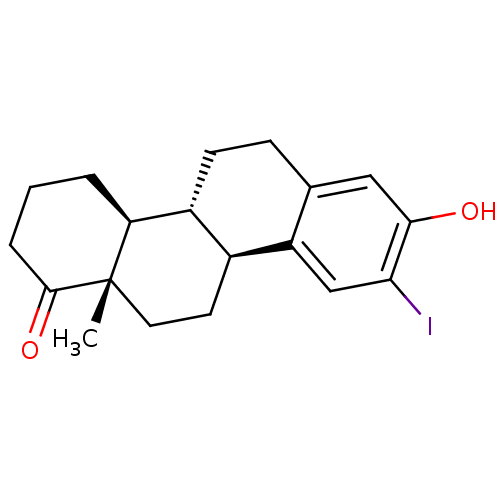
((4bR,10bS,12aS)-8-hydroxy-9-iodo-12a-methyl-2,3,4,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(I)cc34)[C@@H]1CCCC2=O |r| Show InChI InChI=1S/C19H23IO2/c1-19-8-7-12-13(15(19)3-2-4-18(19)22)6-5-11-9-17(21)16(20)10-14(11)12/h9-10,12-13,15,21H,2-8H2,1H3/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311681
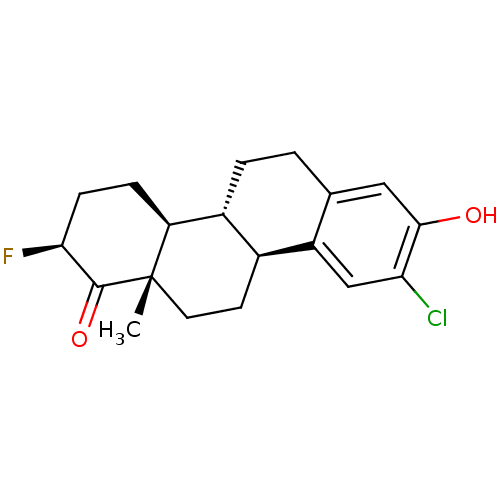
((2S,4bR,10bS,12aS)-9-chloro-2-fluoro-8-hydroxy-12a...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1CC[C@H](F)C2=O |r| Show InChI InChI=1S/C19H22ClFO2/c1-19-7-6-11-12(14(19)4-5-16(21)18(19)23)3-2-10-8-17(22)15(20)9-13(10)11/h8-9,11-12,14,16,22H,2-7H2,1H3/t11-,12+,14-,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426586
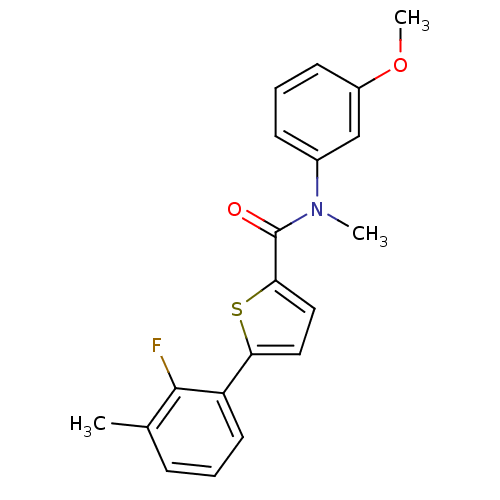
(CHEMBL2324365)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(C)c1F Show InChI InChI=1S/C20H18FNO2S/c1-13-6-4-9-16(19(13)21)17-10-11-18(25-17)20(23)22(2)14-7-5-8-15(12-14)24-3/h4-12H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357467
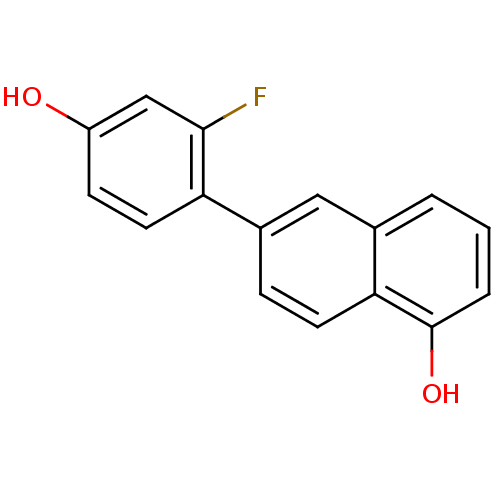
(CHEMBL1917889)Show InChI InChI=1S/C16H11FO2/c17-15-9-12(18)5-7-13(15)11-4-6-14-10(8-11)2-1-3-16(14)19/h1-9,18-19H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta-HSD2 using [3H]E2 as substrate by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426588

(CHEMBL2324673)Show SMILES COc1cccc(c1)-c1ccc(s1)C(=O)N(C)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C25H21NO2S/c1-26(21-12-6-10-19(16-21)18-8-4-3-5-9-18)25(27)24-15-14-23(29-24)20-11-7-13-22(17-20)28-2/h3-17H,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311658
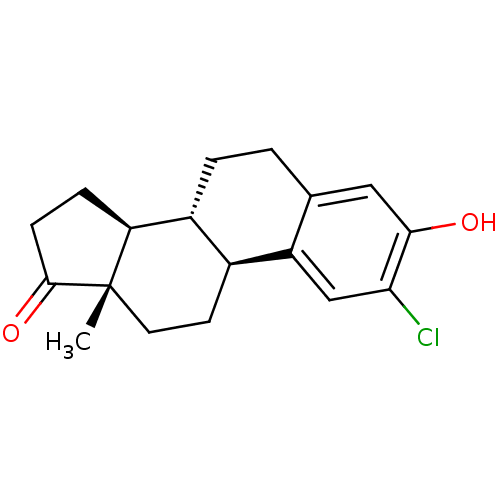
((8R,9S,13S,14S)-2-chloro-3-hydroxy-13-methyl-7,8,9...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Cl)cc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C18H21ClO2/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-16(20)15(19)9-13(10)11/h8-9,11-12,14,20H,2-7H2,1H3/t11-,12+,14-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311660

((8R,9S,13S,14S)-3-hydroxy-13-methyl-17-oxo-7,8,9,1...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(cc34)C#N)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H21NO2/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-9,13-14,16,21H,2-7H2,1H3/t13-,14+,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426582
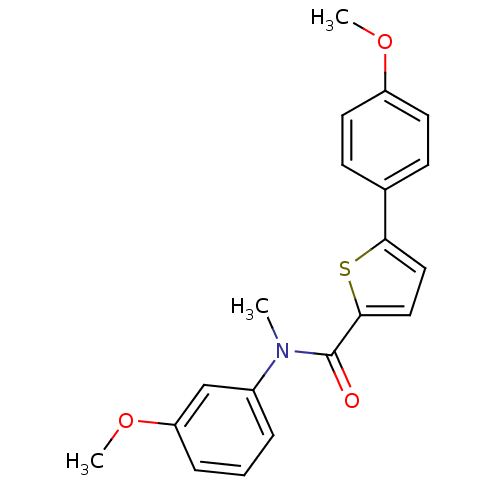
(CHEMBL2324678)Show SMILES COc1ccc(cc1)-c1ccc(s1)C(=O)N(C)c1cccc(OC)c1 Show InChI InChI=1S/C20H19NO3S/c1-21(15-5-4-6-17(13-15)24-3)20(22)19-12-11-18(25-19)14-7-9-16(23-2)10-8-14/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357464
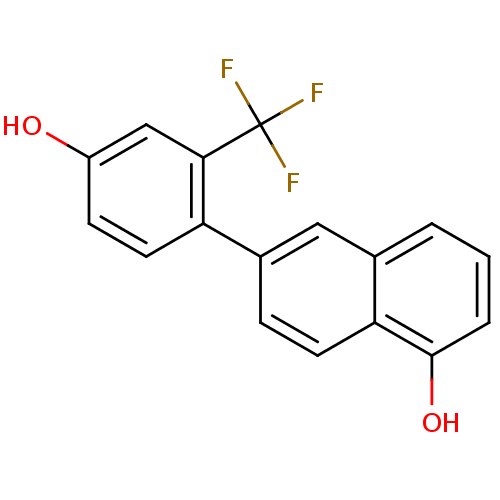
(CHEMBL1917895)Show InChI InChI=1S/C17H11F3O2/c18-17(19,20)15-9-12(21)5-7-13(15)11-4-6-14-10(8-11)2-1-3-16(14)22/h1-9,21-22H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta-HSD2 using [3H]E2 as substrate by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426593
(CHEMBL2324691)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(c1)N(C)C Show InChI InChI=1S/C21H22N2O2S/c1-22(2)16-8-5-7-15(13-16)19-11-12-20(26-19)21(24)23(3)17-9-6-10-18(14-17)25-4/h5-14H,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357464

(CHEMBL1917895)Show InChI InChI=1S/C17H11F3O2/c18-17(19,20)15-9-12(21)5-7-13(15)11-4-6-14-10(8-11)2-1-3-16(14)22/h1-9,21-22H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human MDA-MB-231 cells after 3.5 hrs by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426585

(CHEMBL2324366)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1c(F)ccc(OC)c1F Show InChI InChI=1S/C20H17F2NO3S/c1-23(12-5-4-6-13(11-12)25-2)20(24)17-10-9-16(27-17)18-14(21)7-8-15(26-3)19(18)22/h4-11H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311669

((13S,14S,15S)-3-Hydroxy-2-methoxy-13-methyl-7,11,1...)Show SMILES COc1cc2C3=C(CCc2cc1O)[C@]12C[C@H]1CC(=O)[C@@]2(C)CC3 |r,c:5| Show InChI InChI=1S/C20H22O3/c1-19-6-5-13-14-9-17(23-2)16(21)7-11(14)3-4-15(13)20(19)10-12(20)8-18(19)22/h7,9,12,21H,3-6,8,10H2,1-2H3/t12-,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 193 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311662
((8R,9S,13S,14S)-3-Hydroxy-2-methoxy-13-methyl-6,7,...)Show SMILES COc1cc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C19H24O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,20H,3-8H2,1-2H3/t12-,13+,15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426591
(CHEMBL2324693)Show InChI InChI=1S/C20H19NO2S/c1-14-6-4-8-16(12-14)21(2)20(22)19-11-10-18(24-19)15-7-5-9-17(13-15)23-3/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426594
(CHEMBL2324683)Show InChI InChI=1S/C19H17NO2S/c1-20(15-9-6-10-16(13-15)22-2)19(21)18-12-11-17(23-18)14-7-4-3-5-8-14/h3-13H,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311665
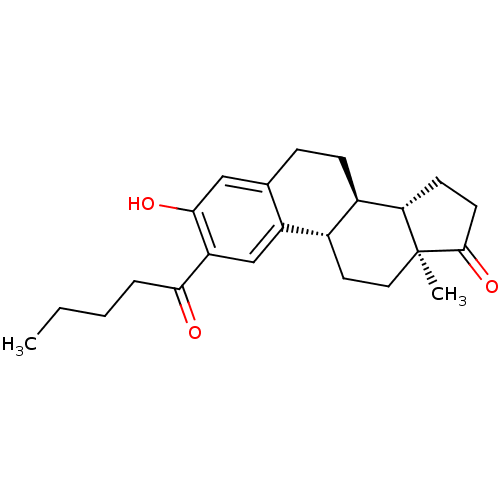
((8R,9S,13S,14S)-3-hydroxy-13-methyl-2-pentanoyl-7,...)Show SMILES CCCCC(=O)c1cc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2cc1O |r| Show InChI InChI=1S/C23H30O3/c1-3-4-5-20(24)18-13-17-14(12-21(18)25)6-7-16-15(17)10-11-23(2)19(16)8-9-22(23)26/h12-13,15-16,19,25H,3-11H2,1-2H3/t15-,16+,19-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50311659
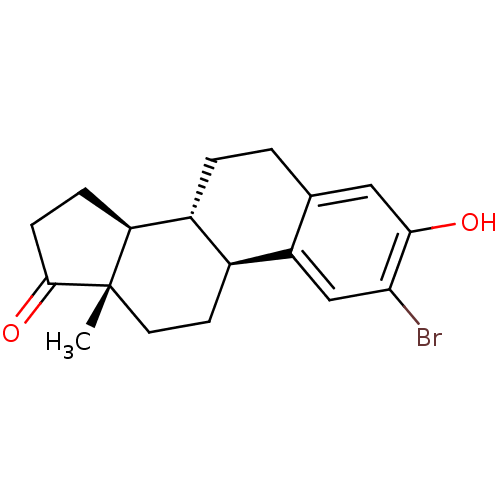
((8R,9S,13S,14S)-2-Bromo-3-hydroxy-13-methyl-6,7,8,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(Br)cc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C18H21BrO2/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-16(20)15(19)9-13(10)11/h8-9,11-12,14,20H,2-7H2,1H3/t11-,12+,14-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Genetics
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human 17beta-HSD1 expressed in Escherichia coli by scintillation counting |
Bioorg Med Chem Lett 19: 6740-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.113
BindingDB Entry DOI: 10.7270/Q20K29HP |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50357465
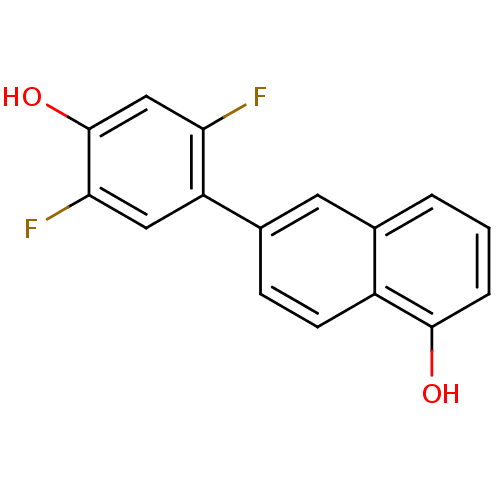
(CHEMBL1917893)Show InChI InChI=1S/C16H10F2O2/c17-13-8-16(20)14(18)7-12(13)10-4-5-11-9(6-10)2-1-3-15(11)19/h1-8,19-20H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta-HSD2 using [3H]E2 as substrate by HPLC analysis |
J Med Chem 54: 7547-57 (2011)
Article DOI: 10.1021/jm2008453
BindingDB Entry DOI: 10.7270/Q2M045V0 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426592
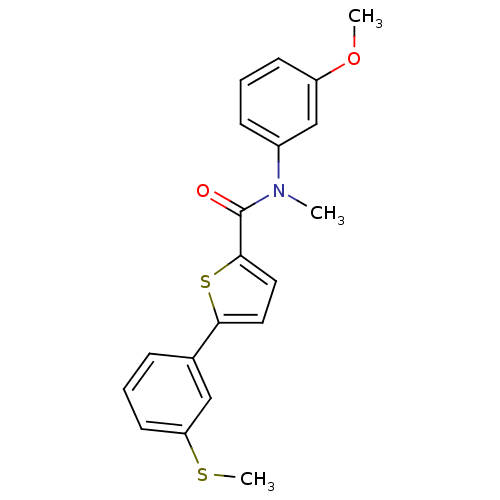
(CHEMBL2324692)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1cccc(SC)c1 Show InChI InChI=1S/C20H19NO2S2/c1-21(15-7-5-8-16(13-15)23-2)20(22)19-11-10-18(25-19)14-6-4-9-17(12-14)24-3/h4-13H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50426584

(CHEMBL2324367)Show SMILES COc1cccc(c1)N(C)C(=O)c1ccc(s1)-c1ccc(OC)c(F)c1 Show InChI InChI=1S/C20H18FNO3S/c1-22(14-5-4-6-15(12-14)24-2)20(23)19-10-9-18(26-19)13-7-8-17(25-3)16(21)11-13/h4-12H,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD2 in human placental microsomal fraction using [3H]E2 as substrate assessed as formation of E1 by HPLC analysis |
J Med Chem 56: 167-81 (2013)
Article DOI: 10.1021/jm3014053
BindingDB Entry DOI: 10.7270/Q2ZK5J0P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data