

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245474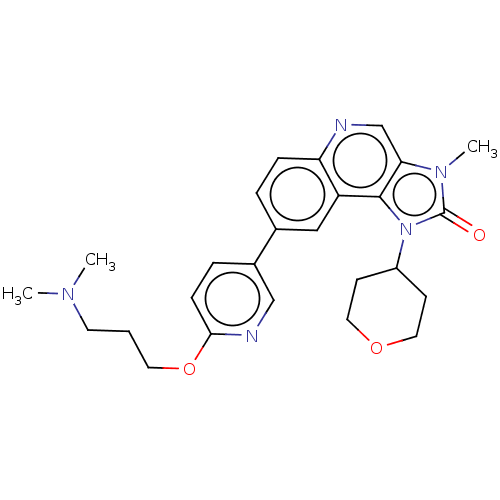 (US9428503, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate preincubated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF as... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50169743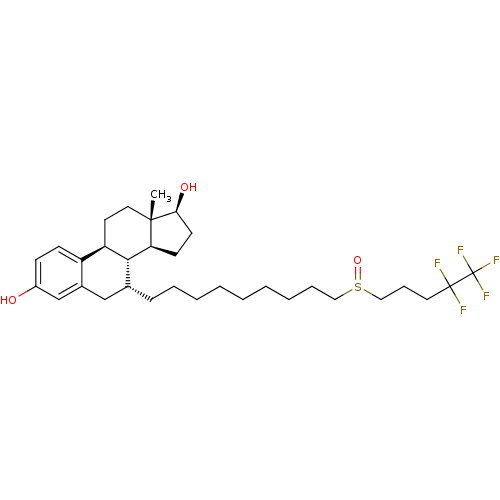 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha receptor in human MCF7 cells | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha receptor in human MCF7 cells | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084948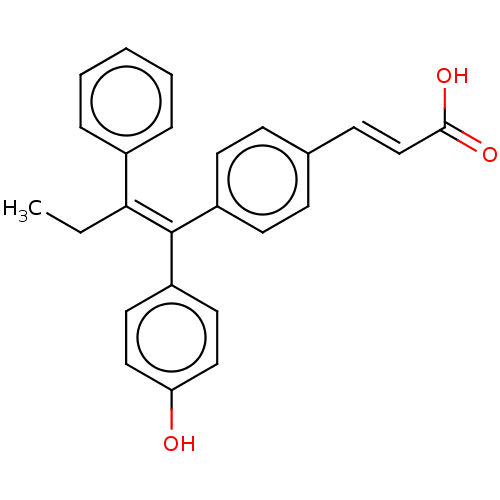 (CHEMBL195515 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084948 (CHEMBL195515 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245500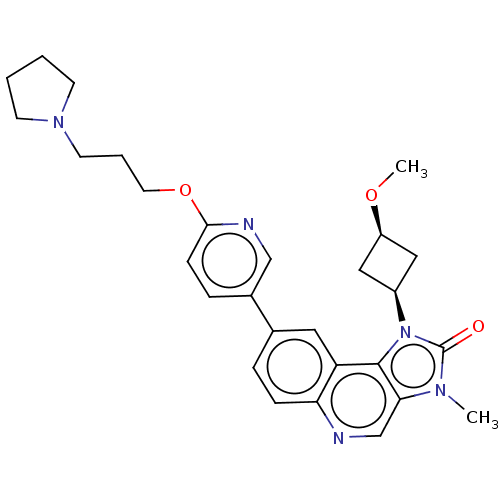 (US9428503, 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245505 (US9428503, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha receptor in human MCF7 cells in presence of 0.25 uM tamoxifen | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245478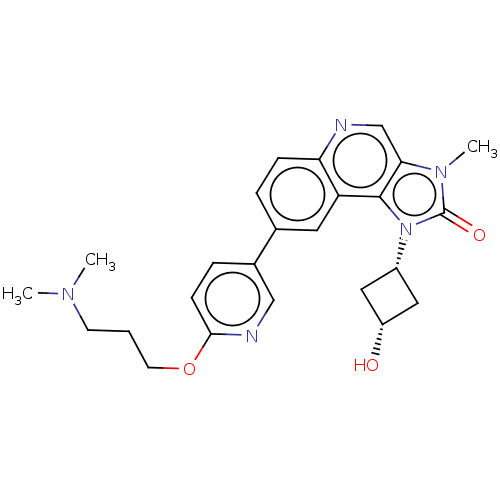 (US9428503, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245475 (US9428503, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245514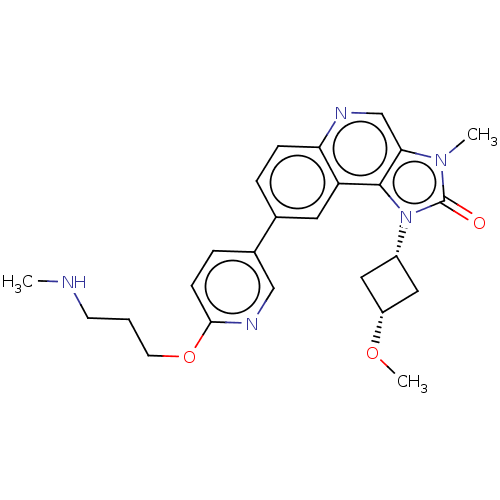 (US9428503, 42) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153694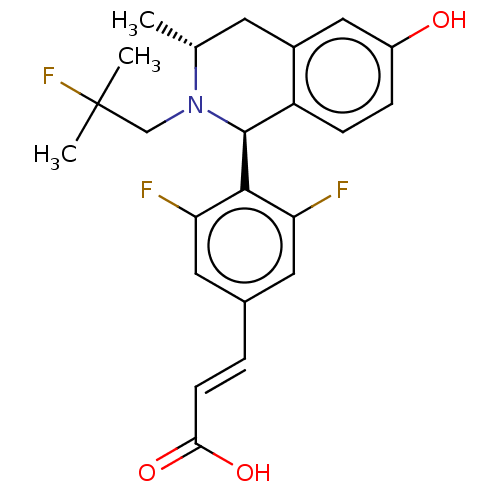 (CHEMBL3774584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459004 (CHEMBL4217549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate pretreated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF assa... | ACS Med Chem Lett 9: 809-814 (2018) Article DOI: 10.1021/acsmedchemlett.8b00200 BindingDB Entry DOI: 10.7270/Q27D2XRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245510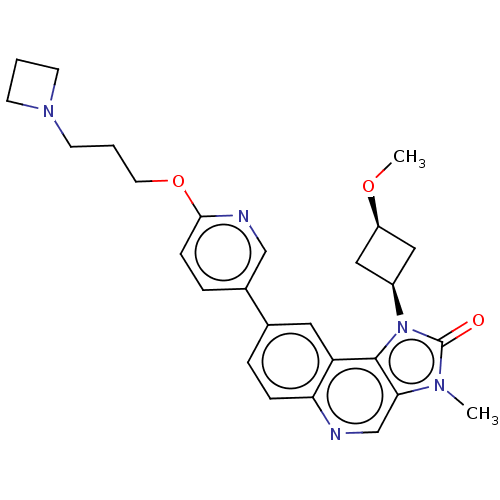 (US9428503, 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608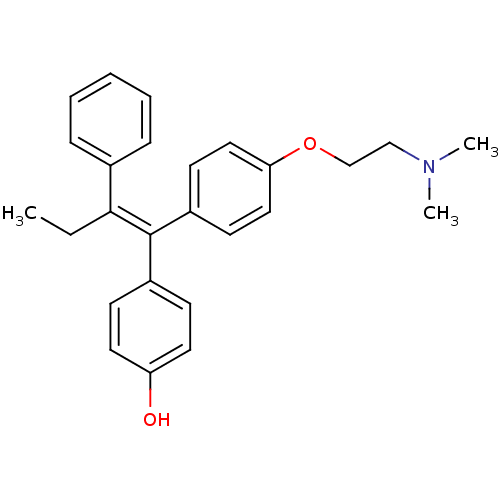 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.309 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245490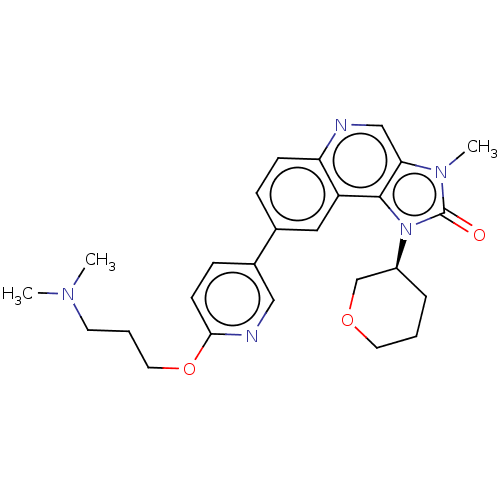 (US9428503, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153695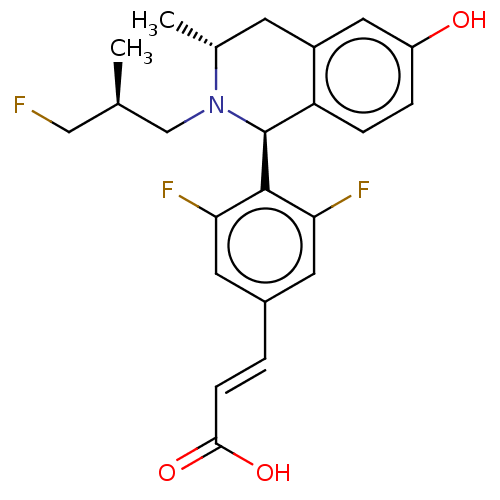 (CHEMBL3775908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084973 (CHEMBL3427401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153696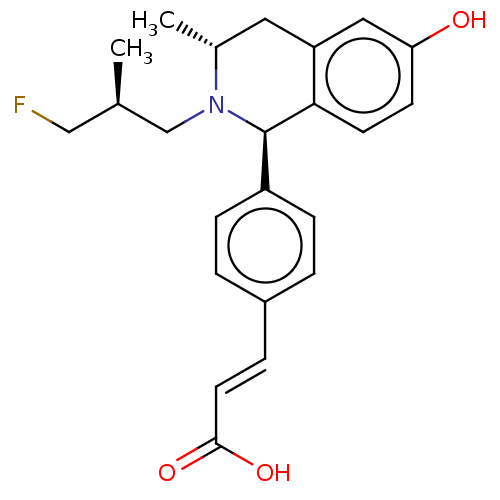 (CHEMBL3774690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Downregulation of ERalpha in human MCF7 cells incubated for 18 to 22 hrs by immunofluorescence assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125055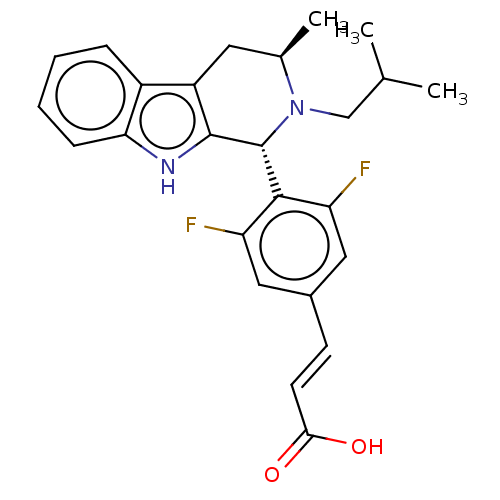 (CHEMBL3623002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha receptor in human MCF7 cells | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084974 (CHEMBL3427402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459016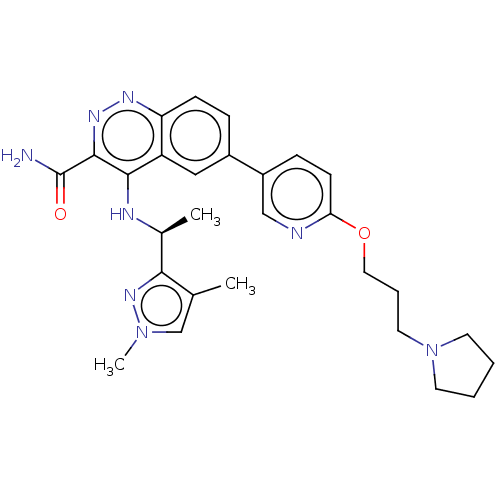 (CHEMBL4210045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay | ACS Med Chem Lett 9: 809-814 (2018) Article DOI: 10.1021/acsmedchemlett.8b00200 BindingDB Entry DOI: 10.7270/Q27D2XRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245474 (US9428503, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153661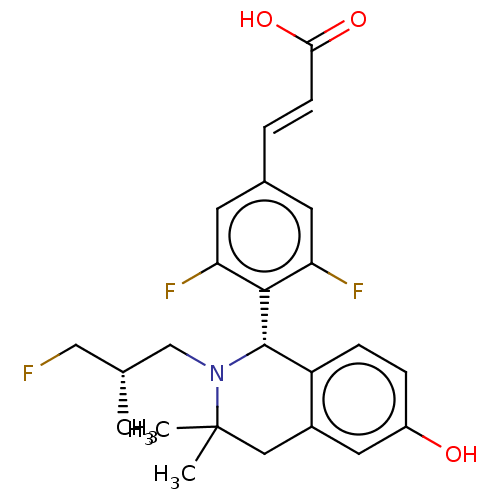 (CHEMBL3774510) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459003 (CHEMBL4218854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM (unknown origin) using p53 as substrate pretreated for 30 mins followed by substrate addition and measured after 2 hrs by HTRF assa... | ACS Med Chem Lett 9: 809-814 (2018) Article DOI: 10.1021/acsmedchemlett.8b00200 BindingDB Entry DOI: 10.7270/Q27D2XRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125055 (CHEMBL3623002) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245491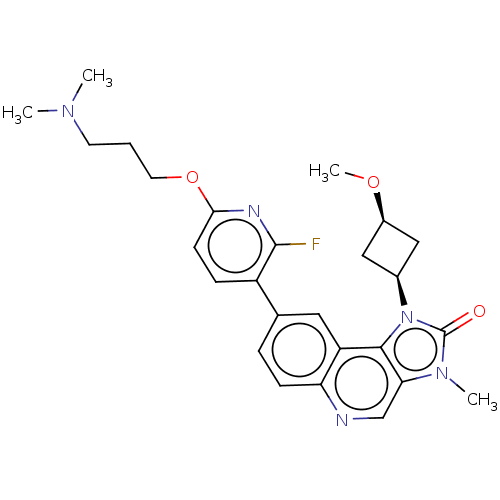 (US9428503, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245481 (US9428503, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Canis familiaris) | BDBM50395256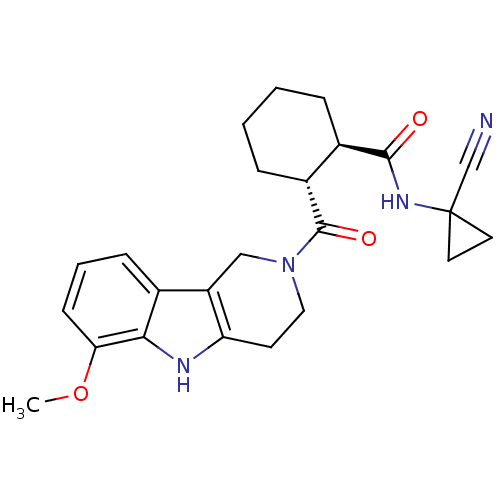 (CHEMBL2163587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of dog recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125052 (CHEMBL3623004 | US10130617, Example 1 | US20240043...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50125055 (CHEMBL3623002) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human MCF cells assessed as estradiol-induced receptor response | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125054 (CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at ERalpha receptor in human MCF7 cells | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM50459014 (CHEMBL4212961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 residue in human HT-29 cells measured after 1 hr by Hoechst staining based fluorescence assay | ACS Med Chem Lett 9: 809-814 (2018) Article DOI: 10.1021/acsmedchemlett.8b00200 BindingDB Entry DOI: 10.7270/Q27D2XRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245489 (US9428503, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153708 (CHEMBL3774777) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50153694 (CHEMBL3774584) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from GST-tagged recombinant human ERalpha LBD after 1 hr by Lanthascreen TR-FRET assay | ACS Med Chem Lett 7: 94-9 (2016) Article DOI: 10.1021/acsmedchemlett.5b00413 BindingDB Entry DOI: 10.7270/Q2HD7XHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395254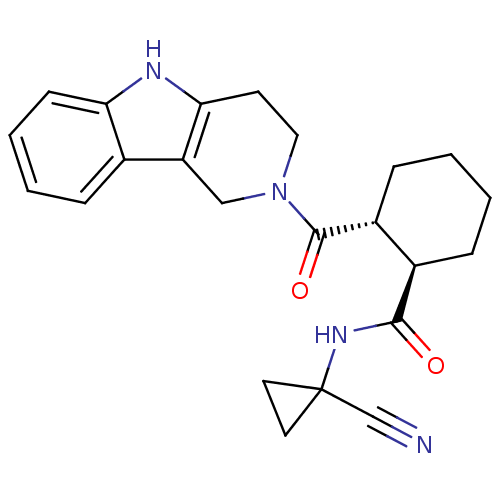 (CHEMBL2164682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50084971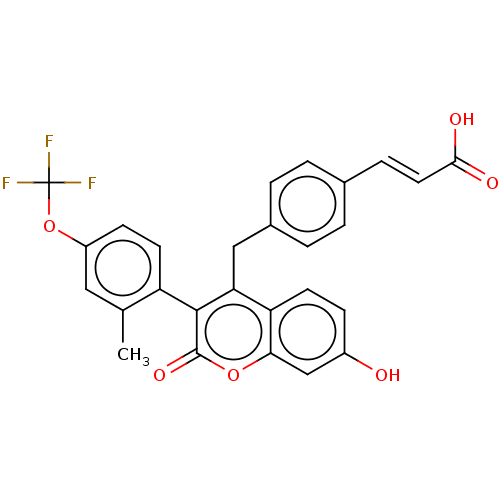 (CHEMBL3427399) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ER alpha (unknown origin) by LanthaScreen TR-FRET competitive binding assay | J Med Chem 58: 3522-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00066 BindingDB Entry DOI: 10.7270/Q2M32XGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50125054 (CHEMBL3623003 | US10130617, Example 2 | WO-2014/19...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor (unknown origin) | J Med Chem 58: 8128-40 (2015) Article DOI: 10.1021/acs.jmedchem.5b00984 BindingDB Entry DOI: 10.7270/Q2F76FCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395236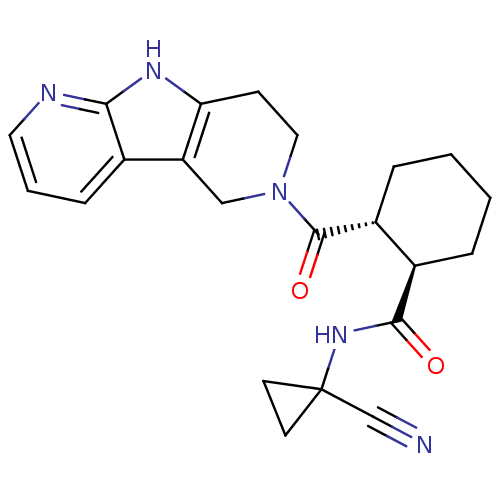 (CHEMBL2163360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50395235 (CHEMBL2164670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant CatK assessed as suppression of enzyme-mediated Z-Phe-Arg-AMC cleavage incubated for 1 hrs by QFRET assay | J Med Chem 55: 6363-74 (2012) Article DOI: 10.1021/jm3007257 BindingDB Entry DOI: 10.7270/Q2833T5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50420842 (CHEMBL2086684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... | Bioorg Med Chem Lett 23: 3175-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.006 BindingDB Entry DOI: 10.7270/Q25Q4XGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-dependent insulinotropic receptor (Homo sapiens (Human)) | BDBM50433856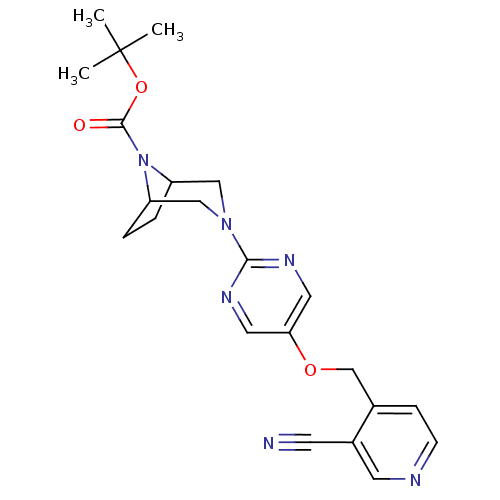 (CHEMBL2382410) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... | Bioorg Med Chem Lett 23: 3175-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.006 BindingDB Entry DOI: 10.7270/Q25Q4XGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245519 (US9428503, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine-protein kinase ATM (Homo sapiens (Human)) | BDBM245479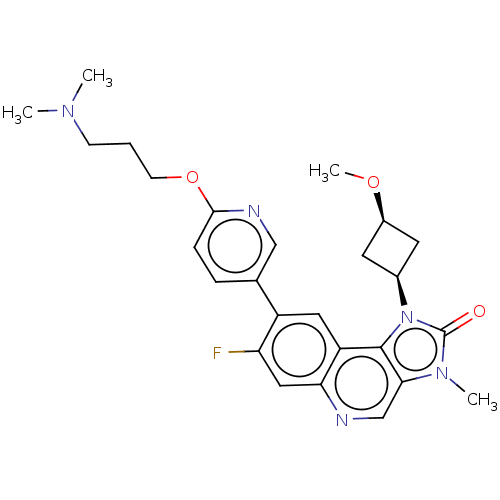 (US9428503, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATM autophosphorylation at Ser1981 in human HT29 cells preincubated for 1 hr followed by X ray irradiation and measured after 1 hr by H... | J Med Chem 61: 3823-3841 (2018) Article DOI: 10.1021/acs.jmedchem.7b01896 BindingDB Entry DOI: 10.7270/Q2RB775P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1310 total ) | Next | Last >> |