 Found 819 hits with Last Name = 'maccioni' and Initial = 'e'
Found 819 hits with Last Name = 'maccioni' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50106422
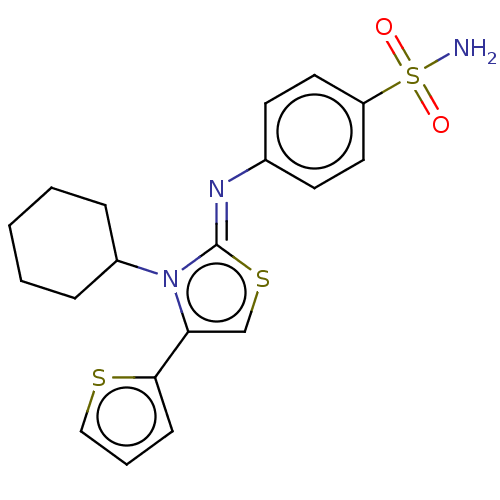
(CHEMBL3601892)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2cccs2)n1C1CCCCC1 Show InChI InChI=1S/C19H21N3O2S3/c20-27(23,24)16-10-8-14(9-11-16)21-19-22(15-5-2-1-3-6-15)17(13-26-19)18-7-4-12-25-18/h4,7-13,15H,1-3,5-6H2,(H2,20,23,24)/b21-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-2 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(MOUSE) | BDBM50180022
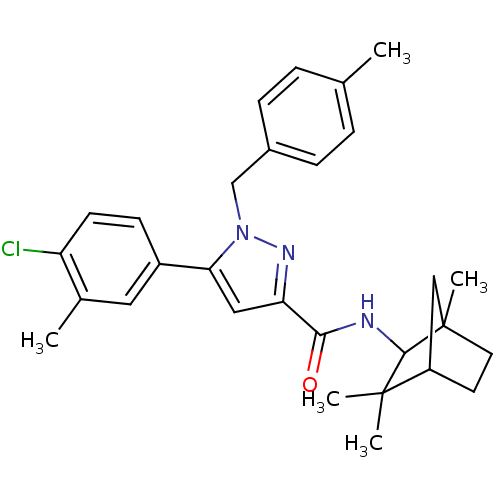
(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UOS of Cagliari
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from cannabinoid CB2 receptor in CD1 mouse spleen membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 21: 7074-82 (2013)
Article DOI: 10.1016/j.bmc.2013.09.017
BindingDB Entry DOI: 10.7270/Q25D8VTF |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106422

(CHEMBL3601892)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2cccs2)n1C1CCCCC1 Show InChI InChI=1S/C19H21N3O2S3/c20-27(23,24)16-10-8-14(9-11-16)21-19-22(15-5-2-1-3-6-15)17(13-26-19)18-7-4-12-25-18/h4,7-13,15H,1-3,5-6H2,(H2,20,23,24)/b21-19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50476106
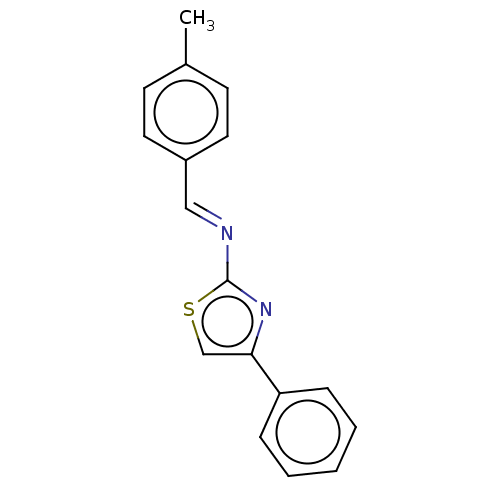
(CHEMBL385117)Show InChI InChI=1S/C17H14N2S/c1-13-7-9-14(10-8-13)11-18-17-19-16(12-20-17)15-5-3-2-4-6-15/h2-12H,1H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOB |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM11014
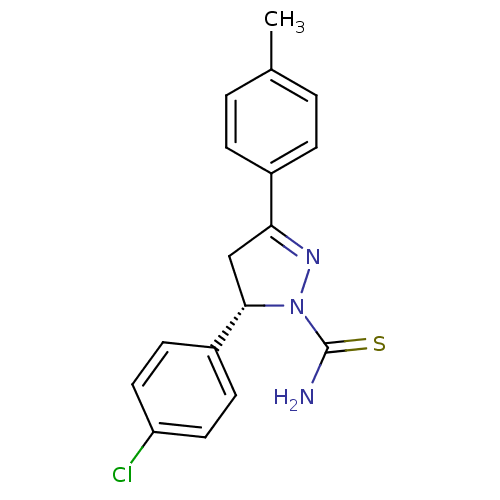
((-)-(S)1 | (5S)-5-(4-chlorophenyl)-3-(4-methylphen...)Show SMILES Cc1ccc(cc1)C1=NN([C@@H](C1)c1ccc(Cl)cc1)C(N)=S |r,t:8| Show InChI InChI=1S/C17H16ClN3S/c1-11-2-4-12(5-3-11)15-10-16(21(20-15)17(19)22)13-6-8-14(18)9-7-13/h2-9,16H,10H2,1H3,(H2,19,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UOS of Cagliari
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in CD1 mouse brain membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 21: 7074-82 (2013)
Article DOI: 10.1016/j.bmc.2013.09.017
BindingDB Entry DOI: 10.7270/Q25D8VTF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in CD1 mouse whole brain minus cerebellum membranes by liquid scintillation counting |
J Nat Prod 78: 69-76 (2015)
Article DOI: 10.1021/np500671v
BindingDB Entry DOI: 10.7270/Q2Q52R9R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50476100
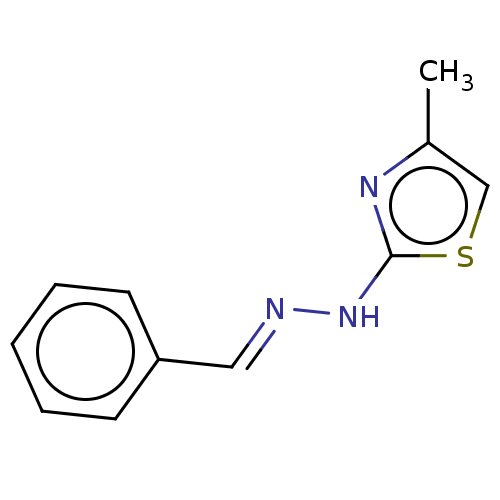
(CHEMBL220548)Show InChI InChI=1S/C11H11N3S/c1-9-8-15-11(13-9)14-12-7-10-5-3-2-4-6-10/h2-8H,1H3,(H,13,14)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOB |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM11004

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show SMILES Cc1ccc(cc1)C1=NN(C(C1)c1ccc(Cl)cc1)C(N)=S |t:8| Show InChI InChI=1S/C17H16ClN3S/c1-11-2-4-12(5-3-11)15-10-16(21(20-15)17(19)22)13-6-8-14(18)9-7-13/h2-9,16H,10H2,1H3,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50106422

(CHEMBL3601892)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2cccs2)n1C1CCCCC1 Show InChI InChI=1S/C19H21N3O2S3/c20-27(23,24)16-10-8-14(9-11-16)21-19-22(15-5-2-1-3-6-15)17(13-26-19)18-7-4-12-25-18/h4,7-13,15H,1-3,5-6H2,(H2,20,23,24)/b21-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-1 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50367288

(CHEMBL4175989)Show SMILES Cc1c(CC(=O)Nc2ccc(cc2)S(N)(=O)=O)c(=O)oc2cc3occ(-c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C26H19ClN2O6S/c1-14-19-10-21-22(15-2-4-16(27)5-3-15)13-34-23(21)12-24(19)35-26(31)20(14)11-25(30)29-17-6-8-18(9-7-17)36(28,32)33/h2-10,12-13H,11H2,1H3,(H,29,30)(H2,28,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM11015
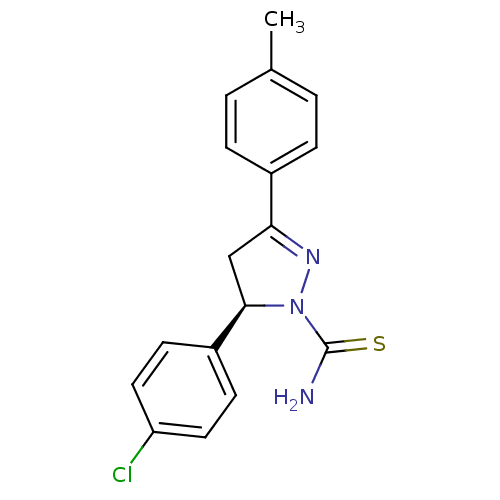
((+)-(R)1 | (5R)-5-(4-chlorophenyl)-3-(4-methylphen...)Show SMILES Cc1ccc(cc1)C1=NN([C@H](C1)c1ccc(Cl)cc1)C(N)=S |r,t:8| Show InChI InChI=1S/C17H16ClN3S/c1-11-2-4-12(5-3-11)15-10-16(21(20-15)17(19)22)13-6-8-14(18)9-7-13/h2-9,16H,10H2,1H3,(H2,19,22)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50367330
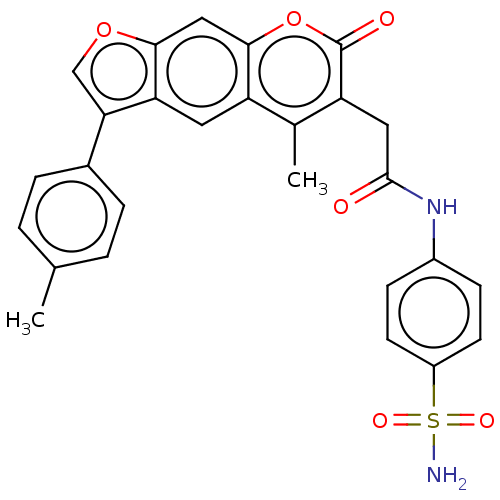
(CHEMBL4168142)Show SMILES Cc1ccc(cc1)-c1coc2cc3oc(=O)c(CC(=O)Nc4ccc(cc4)S(N)(=O)=O)c(C)c3cc12 Show InChI InChI=1S/C27H22N2O6S/c1-15-3-5-17(6-4-15)23-14-34-24-13-25-20(11-22(23)24)16(2)21(27(31)35-25)12-26(30)29-18-7-9-19(10-8-18)36(28,32)33/h3-11,13-14H,12H2,1-2H3,(H,29,30)(H2,28,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50367290
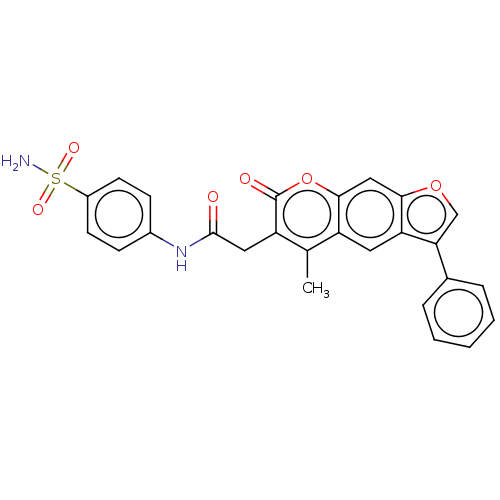
(CHEMBL4160871)Show SMILES Cc1c(CC(=O)Nc2ccc(cc2)S(N)(=O)=O)c(=O)oc2cc3occ(-c4ccccc4)c3cc12 Show InChI InChI=1S/C26H20N2O6S/c1-15-19-11-21-22(16-5-3-2-4-6-16)14-33-23(21)13-24(19)34-26(30)20(15)12-25(29)28-17-7-9-18(10-8-17)35(27,31)32/h2-11,13-14H,12H2,1H3,(H,28,29)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11006

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show InChI InChI=1S/C15H15N3OS/c1-10-4-6-11(7-5-10)12-9-13(14-3-2-8-19-14)18(17-12)15(16)20/h2-8,13H,9H2,1H3,(H2,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50476107

(CHEMBL375007)Show InChI InChI=1S/C12H13N3OS/c1-9-8-17-12(14-9)15-13-7-10-3-5-11(16-2)6-4-10/h3-8H,1-2H3,(H,14,15)/b13-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOB |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271176
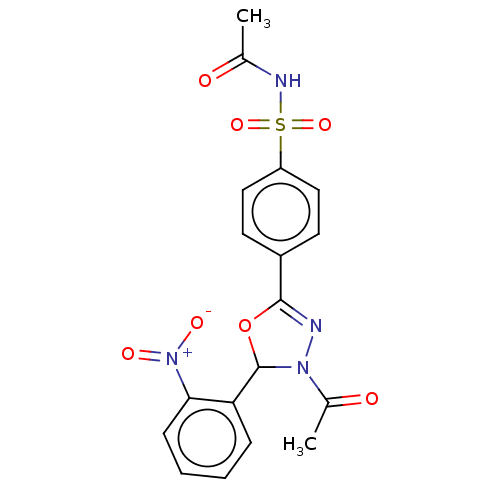
(CHEMBL4130085)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1ccccc1[N+]([O-])=O)C(C)=O |t:14| Show InChI InChI=1S/C18H16N4O7S/c1-11(23)20-30(27,28)14-9-7-13(8-10-14)17-19-21(12(2)24)18(29-17)15-5-3-4-6-16(15)22(25)26/h3-10,18H,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11016

((-)-(S)4 | (5S)-5-(4-chlorophenyl)-3-(4-fluorophen...)Show SMILES NC(=S)N1N=C(C[C@H]1c1ccc(Cl)cc1)c1ccc(F)cc1 |r,c:4| Show InChI InChI=1S/C16H13ClFN3S/c17-12-5-1-11(2-6-12)15-9-14(20-21(15)16(19)22)10-3-7-13(18)8-4-10/h1-8,15H,9H2,(H2,19,22)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271193
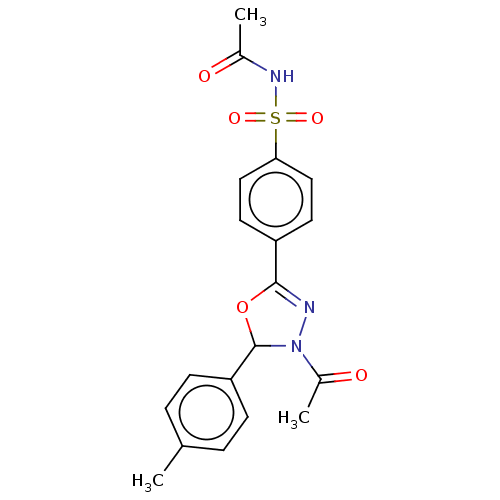
(CHEMBL4129969)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1ccc(C)cc1)C(C)=O |t:14| Show InChI InChI=1S/C19H19N3O5S/c1-12-4-6-16(7-5-12)19-22(14(3)24)20-18(27-19)15-8-10-17(11-9-15)28(25,26)21-13(2)23/h4-11,19H,1-3H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106418
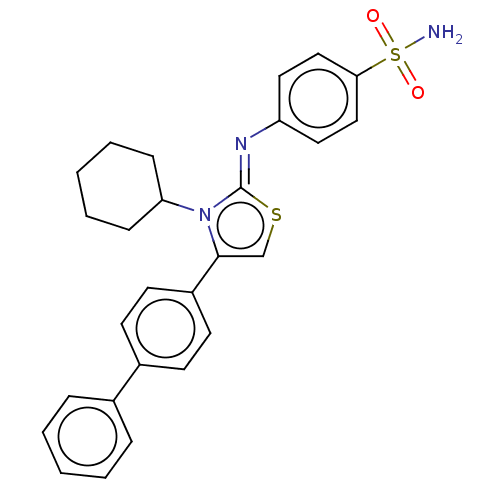
(CHEMBL3601889)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(cc2)-c2ccccc2)n1C1CCCCC1 Show InChI InChI=1S/C27H27N3O2S2/c28-34(31,32)25-17-15-23(16-18-25)29-27-30(24-9-5-2-6-10-24)26(19-33-27)22-13-11-21(12-14-22)20-7-3-1-4-8-20/h1,3-4,7-8,11-19,24H,2,5-6,9-10H2,(H2,28,31,32)/b29-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106416
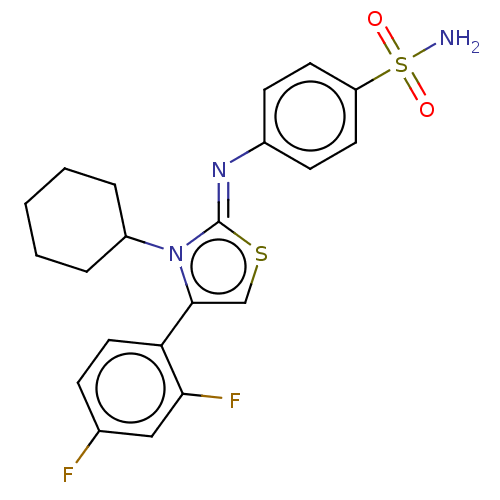
(CHEMBL3601887)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(F)cc2F)n1C1CCCCC1 Show InChI InChI=1S/C21H21F2N3O2S2/c22-14-6-11-18(19(23)12-14)20-13-29-21(26(20)16-4-2-1-3-5-16)25-15-7-9-17(10-8-15)30(24,27)28/h6-13,16H,1-5H2,(H2,24,27,28)/b25-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-fl... |
ACS Med Chem Lett 11: 852-856 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00644
BindingDB Entry DOI: 10.7270/Q25B0620 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106419
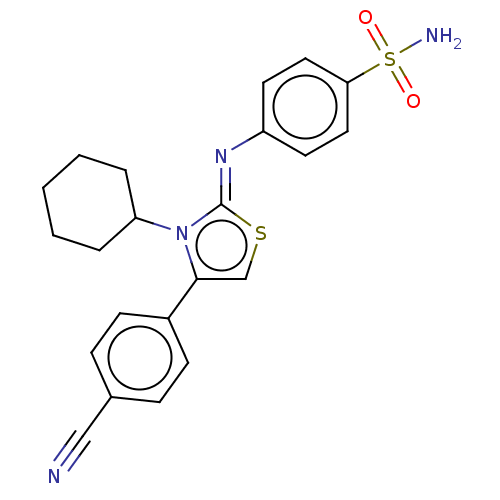
(CHEMBL3601886)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(cc2)C#N)n1C1CCCCC1 Show InChI InChI=1S/C22H22N4O2S2/c23-14-16-6-8-17(9-7-16)21-15-29-22(26(21)19-4-2-1-3-5-19)25-18-10-12-20(13-11-18)30(24,27)28/h6-13,15,19H,1-5H2,(H2,24,27,28)/b25-22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11007

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show SMILES NC(=S)N1N=C(CC1c1ccc(Cl)cc1)c1ccc(F)cc1 |c:4| Show InChI InChI=1S/C16H13ClFN3S/c17-12-5-1-11(2-6-12)15-9-14(20-21(15)16(19)22)10-3-7-13(18)8-4-10/h1-8,15H,9H2,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human CA12 by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 9: 1045-1050 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00352
BindingDB Entry DOI: 10.7270/Q21N83TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11005

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show SMILES Cc1ccc(cc1)C1CC(=NN1C(N)=S)c1ccc(C)cc1 |c:10| Show InChI InChI=1S/C18H19N3S/c1-12-3-7-14(8-4-12)16-11-17(21(20-16)18(19)22)15-9-5-13(2)6-10-15/h3-10,17H,11H2,1-2H3,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM11010

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show InChI InChI=1S/C14H12ClN3S2/c15-10-5-3-9(4-6-10)12-8-11(13-2-1-7-20-13)17-18(12)14(16)19/h1-7,12H,8H2,(H2,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106423
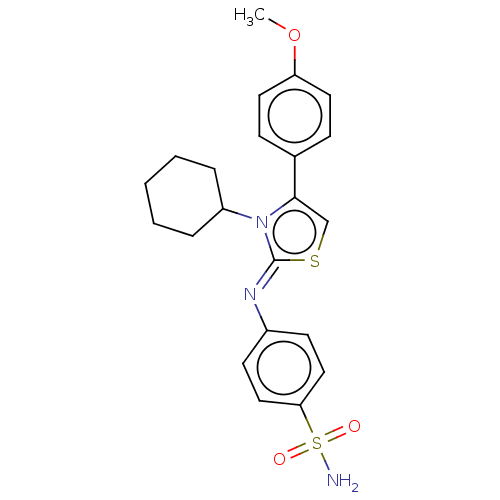
(CHEMBL3601891)Show SMILES COc1ccc(cc1)-c1cs\c(=N/c2ccc(cc2)S(N)(=O)=O)n1C1CCCCC1 Show InChI InChI=1S/C22H25N3O3S2/c1-28-19-11-7-16(8-12-19)21-15-29-22(25(21)18-5-3-2-4-6-18)24-17-9-13-20(14-10-17)30(23,26)27/h7-15,18H,2-6H2,1H3,(H2,23,26,27)/b24-22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106424
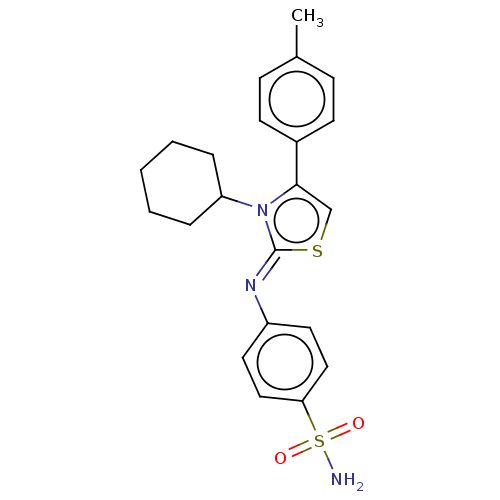
(CHEMBL3601890)Show SMILES Cc1ccc(cc1)-c1cs\c(=N/c2ccc(cc2)S(N)(=O)=O)n1C1CCCCC1 Show InChI InChI=1S/C22H25N3O2S2/c1-16-7-9-17(10-8-16)21-15-28-22(25(21)19-5-3-2-4-6-19)24-18-11-13-20(14-12-18)29(23,26)27/h7-15,19H,2-6H2,1H3,(H2,23,26,27)/b24-22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271194
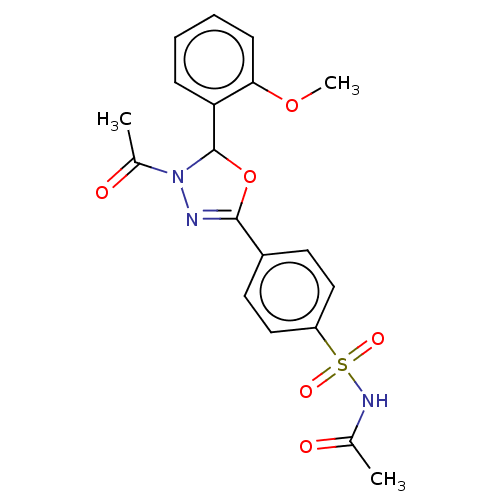
(CHEMBL4127617)Show SMILES COc1ccccc1C1OC(=NN1C(C)=O)c1ccc(cc1)S(=O)(=O)NC(C)=O |c:11| Show InChI InChI=1S/C19H19N3O6S/c1-12(23)21-29(25,26)15-10-8-14(9-11-15)18-20-22(13(2)24)19(28-18)16-6-4-5-7-17(16)27-3/h4-11,19H,1-3H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271182
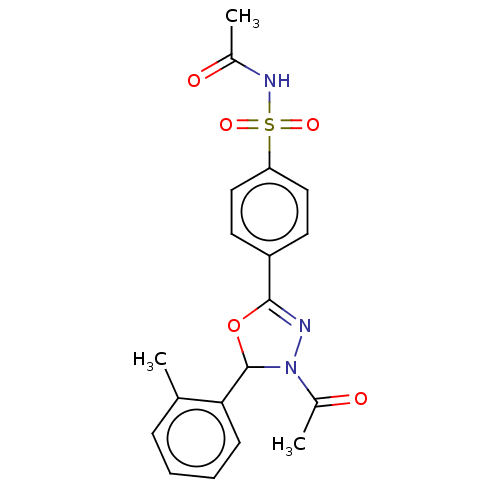
(CHEMBL4129897)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1ccccc1C)C(C)=O |t:14| Show InChI InChI=1S/C19H19N3O5S/c1-12-6-4-5-7-17(12)19-22(14(3)24)20-18(27-19)15-8-10-16(11-9-15)28(25,26)21-13(2)23/h4-11,19H,1-3H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
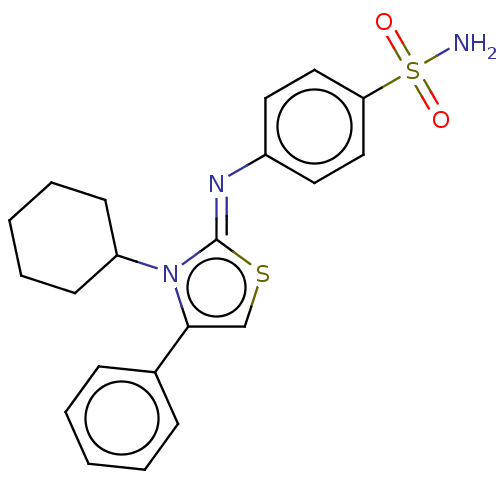
(Homo sapiens (Human)) | BDBM50106421
(CHEMBL3601893)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccccc2)n1C1CCCCC1 Show InChI InChI=1S/C21H23N3O2S2/c22-28(25,26)19-13-11-17(12-14-19)23-21-24(18-9-5-2-6-10-18)20(15-27-21)16-7-3-1-4-8-16/h1,3-4,7-8,11-15,18H,2,5-6,9-10H2,(H2,22,25,26)/b23-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
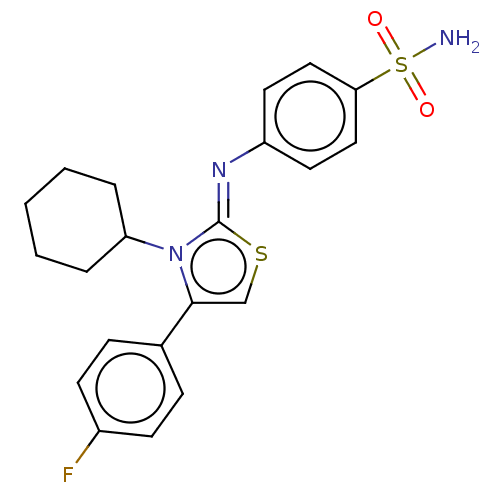
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106413
(CHEMBL3601883)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(F)cc2)n1C1CCCCC1 Show InChI InChI=1S/C21H22FN3O2S2/c22-16-8-6-15(7-9-16)20-14-28-21(25(20)18-4-2-1-3-5-18)24-17-10-12-19(13-11-17)29(23,26)27/h6-14,18H,1-5H2,(H2,23,26,27)/b24-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
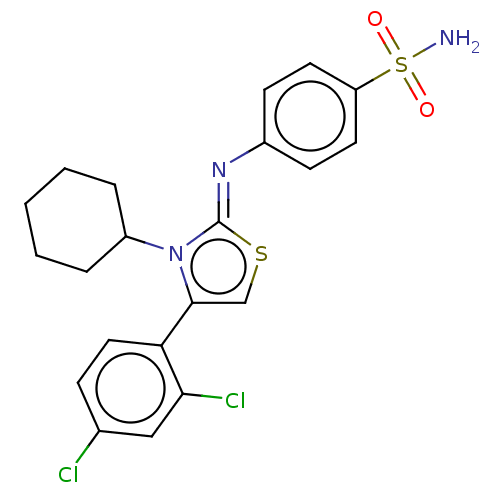
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106420
(CHEMBL3601885)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(Cl)cc2Cl)n1C1CCCCC1 Show InChI InChI=1S/C21H21Cl2N3O2S2/c22-14-6-11-18(19(23)12-14)20-13-29-21(26(20)16-4-2-1-3-5-16)25-15-7-9-17(10-8-15)30(24,27)28/h6-13,16H,1-5H2,(H2,24,27,28)/b25-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271198

(CHEMBL4126627)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1cccc(c1)[N+]([O-])=O)C(C)=O |t:14| Show InChI InChI=1S/C18H16N4O7S/c1-11(23)20-30(27,28)16-8-6-13(7-9-16)17-19-21(12(2)24)18(29-17)14-4-3-5-15(10-14)22(25)26/h3-10,18H,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271196

(CHEMBL4126396)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1ccc(F)cc1)C(C)=O |t:14| Show InChI InChI=1S/C18H16FN3O5S/c1-11(23)21-28(25,26)16-9-5-13(6-10-16)17-20-22(12(2)24)18(27-17)14-3-7-15(19)8-4-14/h3-10,18H,1-2H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50271202

(CHEMBL4126466)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=NN(C(O1)c1ccc(F)cc1F)C(C)=O |t:14| Show InChI InChI=1S/C18H15F2N3O5S/c1-10(24)22-29(26,27)14-6-3-12(4-7-14)17-21-23(11(2)25)18(28-17)15-8-5-13(19)9-16(15)20/h3-9,18H,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 assessed as reduction in CO2 hydration pretreated for 15 mins followed by CO2 addition measured... |
ACS Med Chem Lett 8: 792-796 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00205
BindingDB Entry DOI: 10.7270/Q2FN18PV |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11008
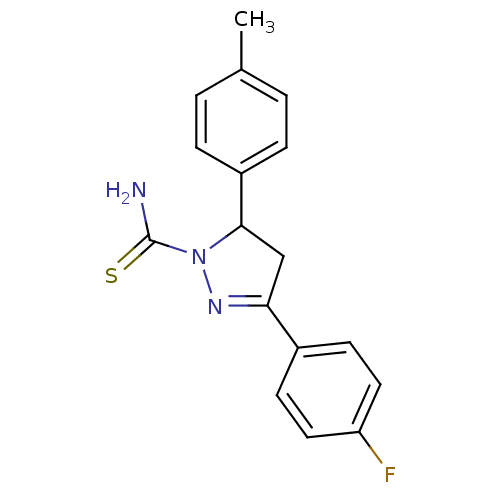
(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show SMILES Cc1ccc(cc1)C1CC(=NN1C(N)=S)c1ccc(F)cc1 |c:10| Show InChI InChI=1S/C17H16FN3S/c1-11-2-4-13(5-3-11)16-10-15(20-21(16)17(19)22)12-6-8-14(18)9-7-12/h2-9,16H,10H2,1H3,(H2,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106412
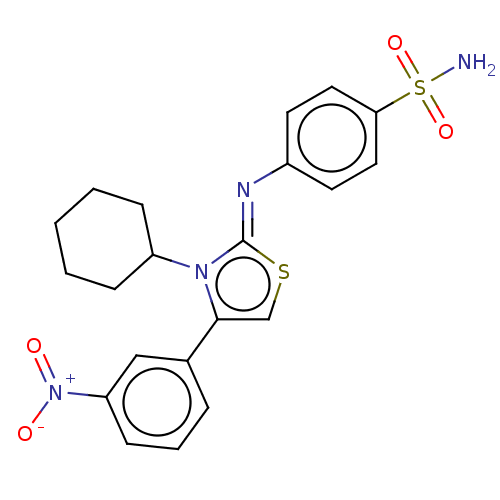
(CHEMBL3601884)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2cccc(c2)[N+]([O-])=O)n1C1CCCCC1 Show InChI InChI=1S/C21H22N4O4S2/c22-31(28,29)19-11-9-16(10-12-19)23-21-24(17-6-2-1-3-7-17)20(14-30-21)15-5-4-8-18(13-15)25(26)27/h4-5,8-14,17H,1-3,6-7H2,(H2,22,28,29)/b23-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50476114

(CHEMBL385124)Show InChI InChI=1S/C13H15N3O2S/c1-9-8-19-13(15-9)16-14-7-10-4-5-11(17-2)12(6-10)18-3/h4-8H,1-3H3,(H,15,16)/b14-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOB |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM50476100

(CHEMBL220548)Show InChI InChI=1S/C11H11N3S/c1-9-8-15-11(13-9)14-12-7-10-5-3-2-4-6-10/h2-8H,1H3,(H,13,14)/b12-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOA |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50367289
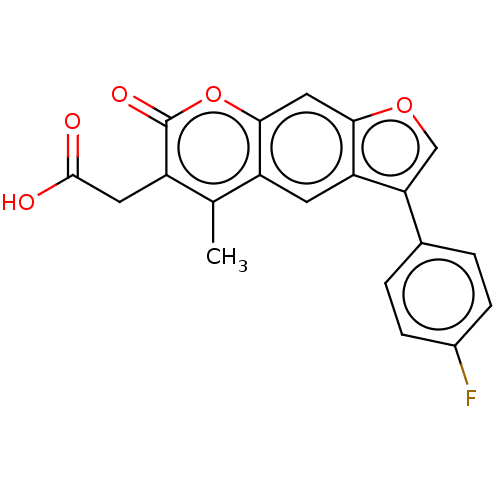
(CHEMBL1441824)Show SMILES Cc1c(CC(O)=O)c(=O)oc2cc3occ(-c4ccc(F)cc4)c3cc12 Show InChI InChI=1S/C20H13FO5/c1-10-13-6-15-16(11-2-4-12(21)5-3-11)9-25-17(15)8-18(13)26-20(24)14(10)7-19(22)23/h2-6,8-9H,7H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Bos taurus) | BDBM11009

(1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...)Show InChI InChI=1S/C14H12FN3OS/c15-10-5-3-9(4-6-10)11-8-12(13-2-1-7-19-13)18(17-11)14(16)20/h1-7,12H,8H2,(H2,16,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza
| Assay Description
MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... |
J Med Chem 48: 7113-22 (2005)
Article DOI: 10.1021/jm040903t
BindingDB Entry DOI: 10.7270/Q2JH3JDW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50540747

(CHEMBL4648617)Show SMILES CCn1c(cs\c1=N\c1ccc(cc1)S(N)(=O)=O)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C17H15Cl2N3O2S2/c1-2-22-16(14-8-3-11(18)9-15(14)19)10-25-17(22)21-12-4-6-13(7-5-12)26(20,23)24/h3-10H,2H2,1H3,(H2,20,23,24)/b21-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
ACS Med Chem Lett 11: 852-856 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00644
BindingDB Entry DOI: 10.7270/Q25B0620 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50106417
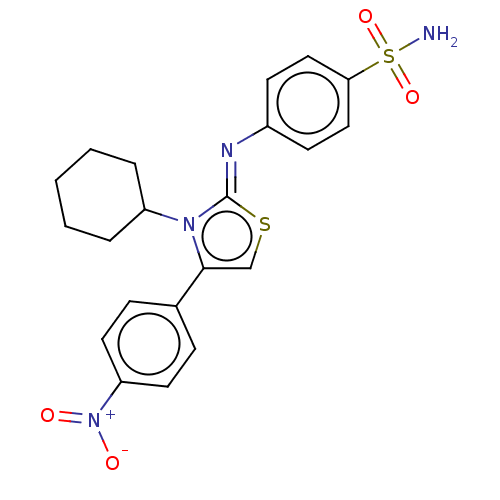
(CHEMBL3601888)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=c1/scc(-c2ccc(cc2)[N+]([O-])=O)n1C1CCCCC1 Show InChI InChI=1S/C21H22N4O4S2/c22-31(28,29)19-12-8-16(9-13-19)23-21-24(17-4-2-1-3-5-17)20(14-30-21)15-6-10-18(11-7-15)25(26)27/h6-14,17H,1-5H2,(H2,22,28,29)/b23-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-12 |
Bioorg Med Chem Lett 25: 3281-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.076
BindingDB Entry DOI: 10.7270/Q2RB76CZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50367287
(CHEMBL4168368)Show SMILES Cc1ccc(cc1)-c1coc2cc3oc(=O)c(CC(O)=O)c(C)c3cc12 Show InChI InChI=1S/C21H16O5/c1-11-3-5-13(6-4-11)17-10-25-18-9-19-14(7-16(17)18)12(2)15(8-20(22)23)21(24)26-19/h3-7,9-10H,8H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 12 by stopped flow CO2 hydration method |
ACS Med Chem Lett 9: 725-729 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00170
BindingDB Entry DOI: 10.7270/Q21Z46Z4 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Bos taurus) | BDBM50476111

(CHEMBL375807)Show InChI InChI=1S/C11H10ClN3S/c1-8-7-16-11(14-8)15-13-6-9-2-4-10(12)5-3-9/h2-7H,1H3,(H,14,15)/b13-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit?? degli Studi di Roma "La Sapienza"
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain mitochondria MAOB |
J Med Chem 50: 707-12 (2007)
Article DOI: 10.1021/jm060869d
BindingDB Entry DOI: 10.7270/Q23J3GQD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data