

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93049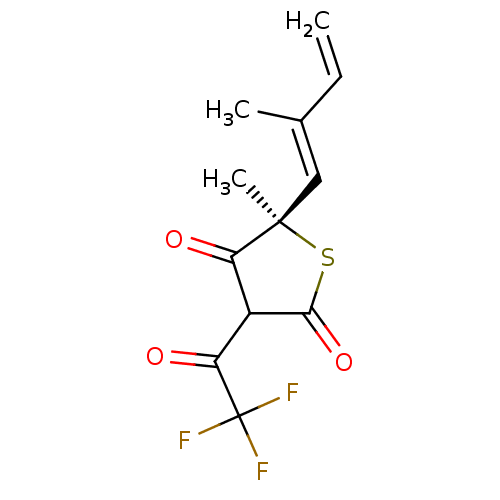 (TLM analog, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93048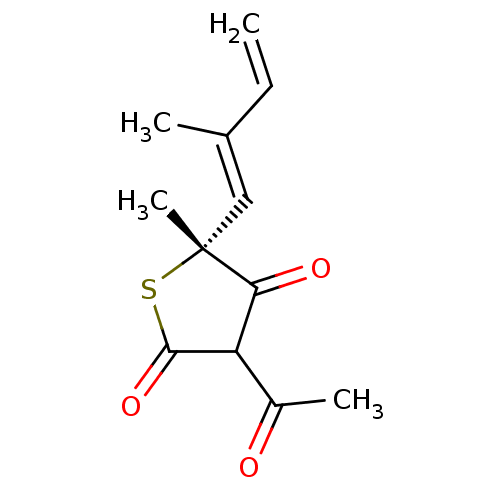 (TLM analog, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM50241313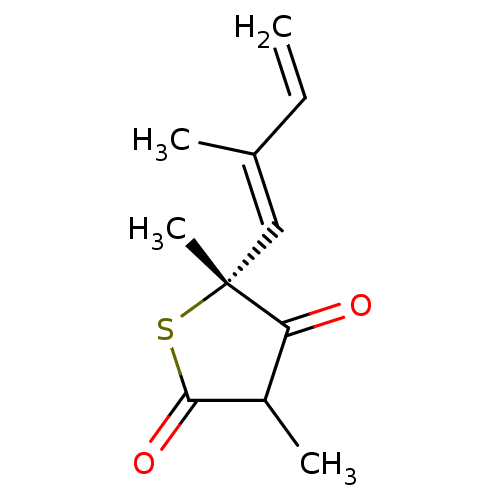 ((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93046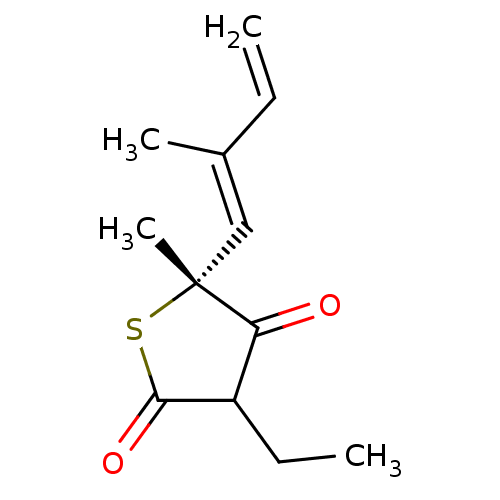 (TLM analog, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93054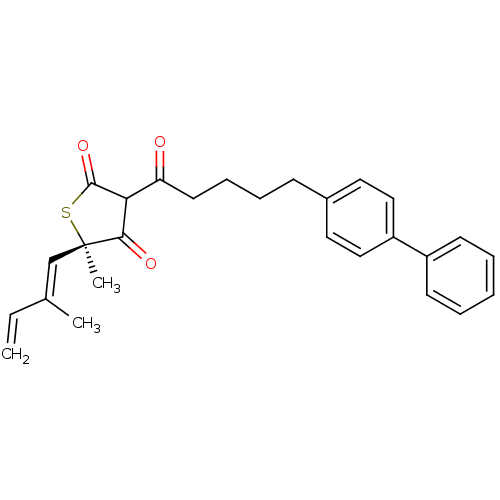 (TLM analog, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93048 (TLM analog, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93049 (TLM analog, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93059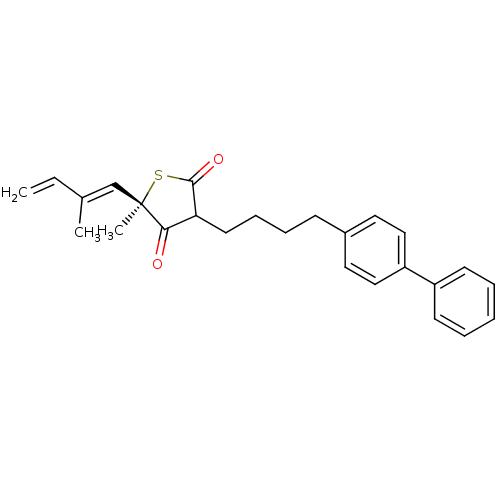 (TLM analog, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93047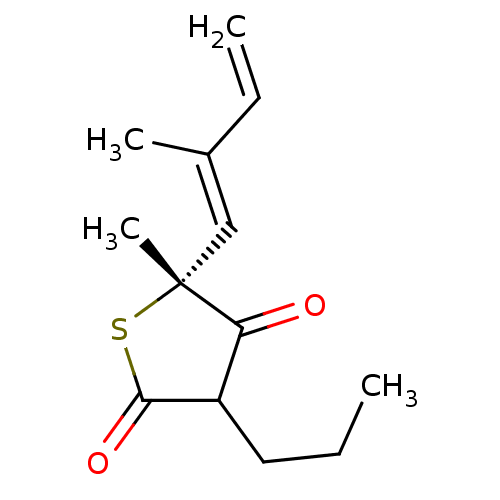 (TLM analog, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93051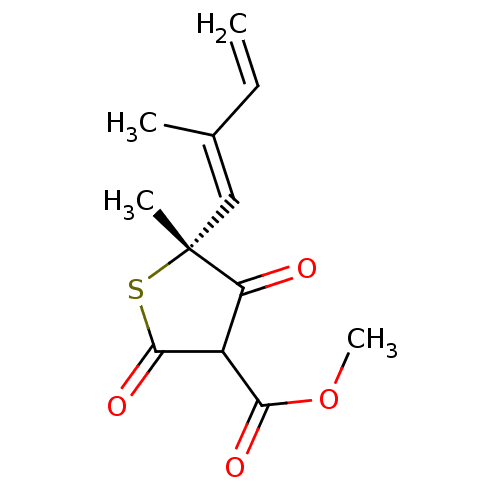 (TLM analog, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93060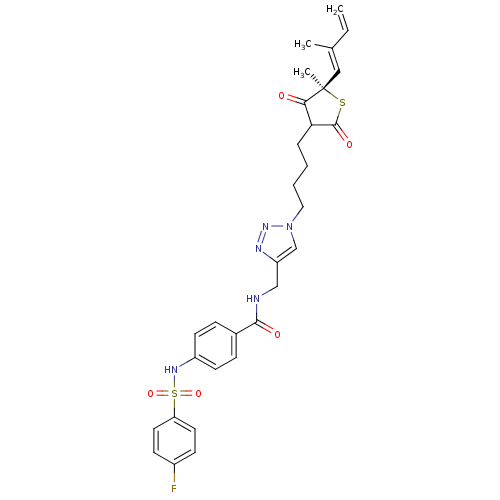 (TLM analog, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93045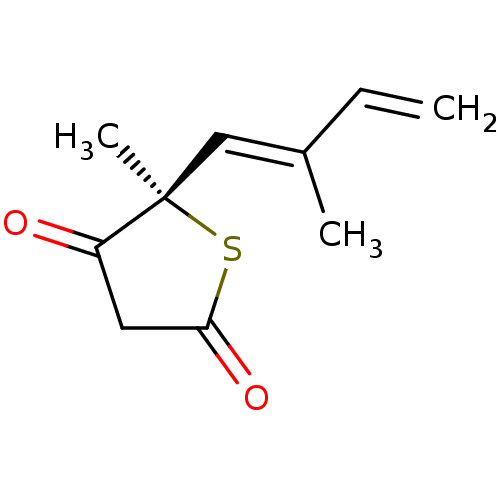 (TLM analog, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93050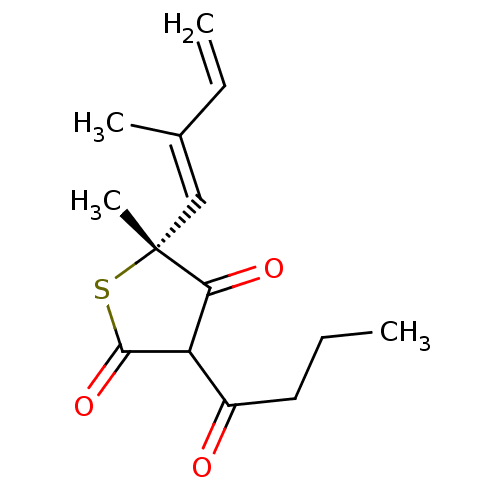 (TLM analog, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93057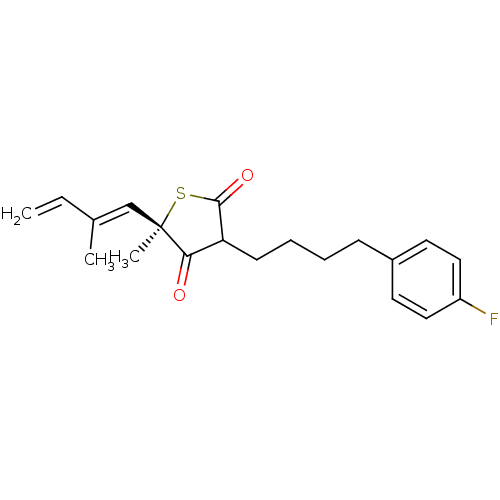 (TLM analog, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93058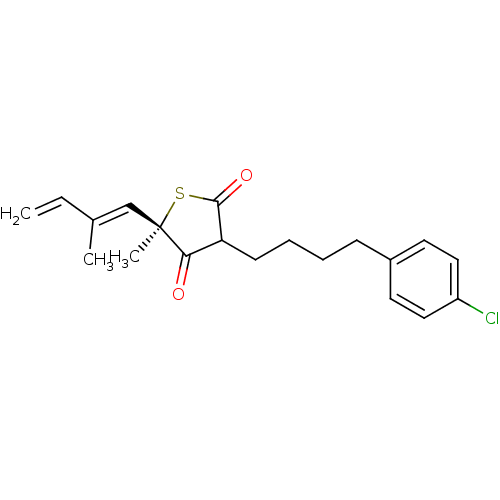 (TLM analog, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93056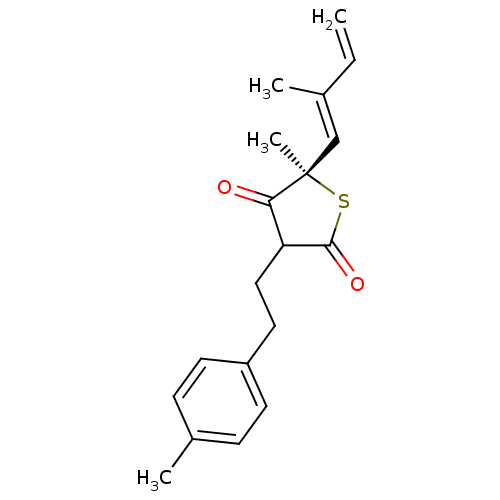 (TLM analog, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93053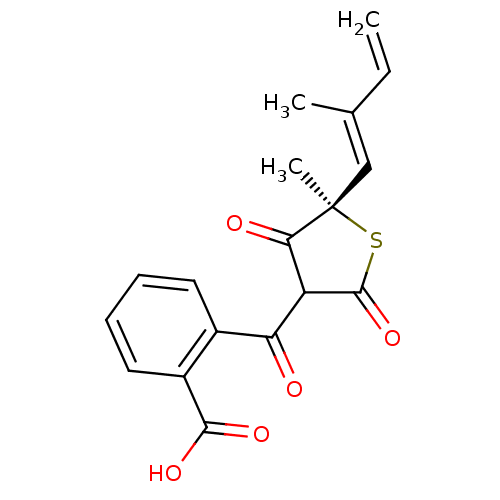 (TLM analog, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93052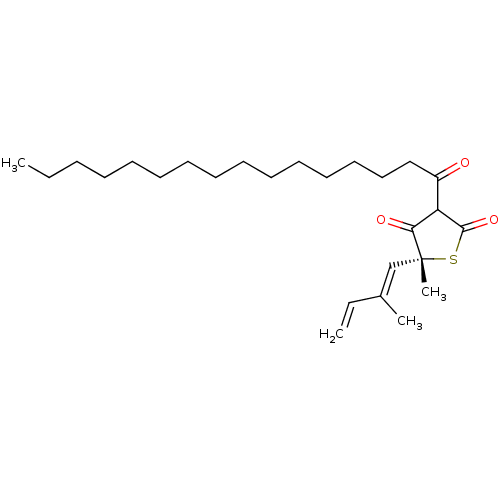 (TLM analog, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM50241313 ((5R)-4-hydroxy-3,5-dimethyl-5-[(1E)-2-methylbuta-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93047 (TLM analog, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93046 (TLM analog, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Similars | Article PubMed | 3.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 (Mycobacterium tuberculosis) | BDBM93055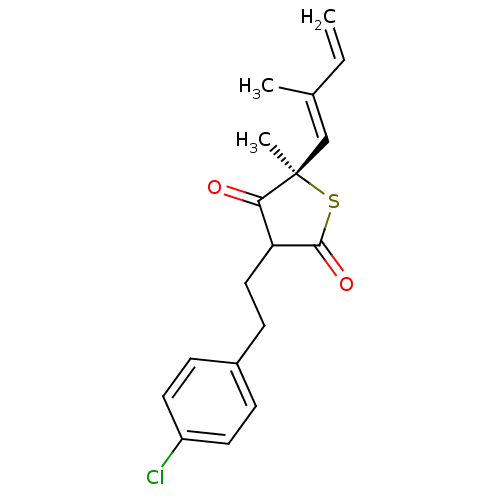 (TLM analog, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
Institute for Chemical Biology & Drug Discovery | Assay Description Binding of TLM and the TLM analogs to KasA was quantified by monitoring changes in the intrinsic tryptophan fluroescence of the enzyme using 280-nm e... | J Biol Chem 288: 6045-52 (2013) Article DOI: 10.1074/jbc.M112.414516 BindingDB Entry DOI: 10.7270/Q2KP80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400781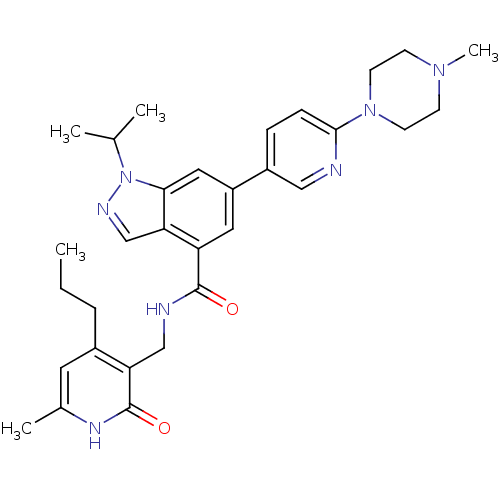 (CHEMBL2204997) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400778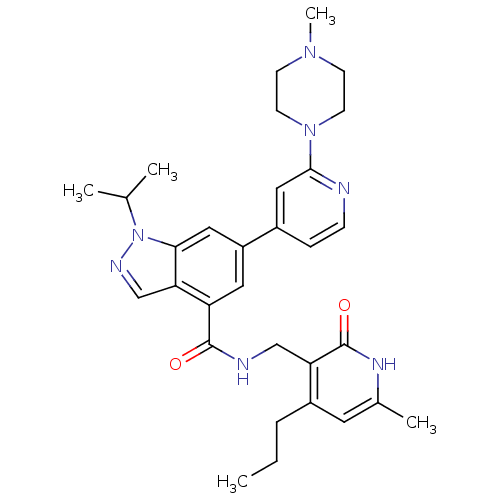 (CHEMBL2204995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400779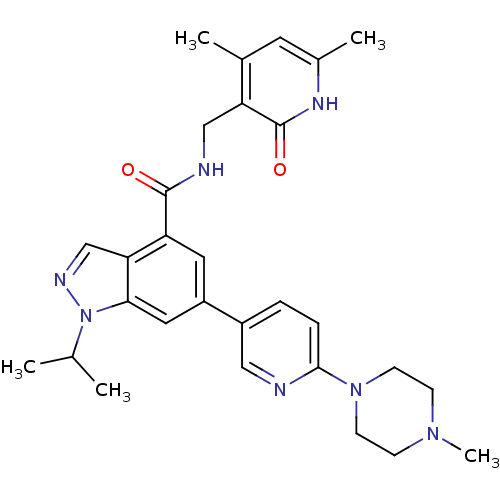 (CHEMBL2204998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400780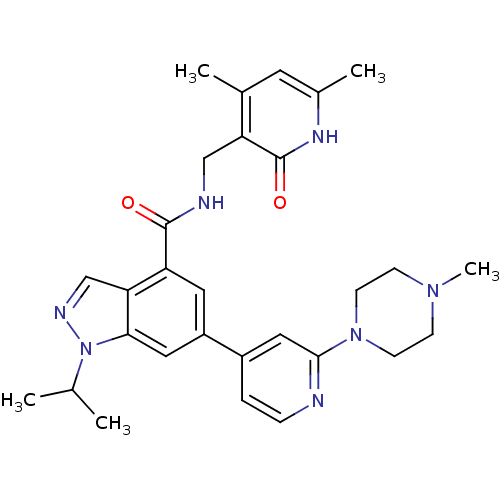 (CHEMBL2204996) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400782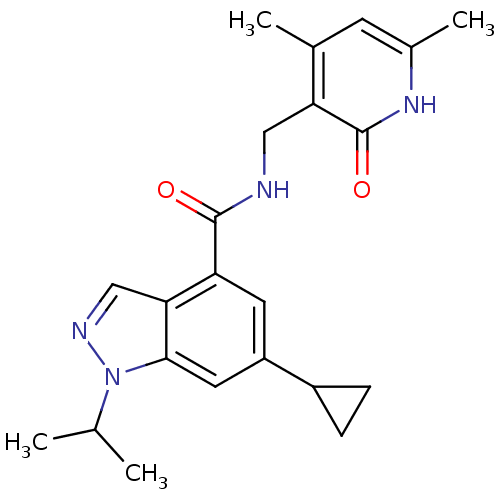 (CHEMBL2204999) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400783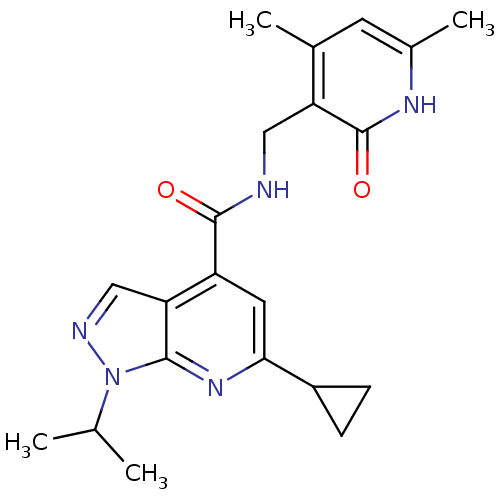 (CHEMBL1608462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of DNMT1 using polydi-dc as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT5 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT5 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT5 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETMAR (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETMAR (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETMAR (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H1 (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H1 (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H1 (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H2 (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SUV39H2 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SUV39H2 (Homo sapiens (Human)) | BDBM50400781 (CHEMBL2204997) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of SUV39H2 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase 2A (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of MLL1 using core histone as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT4 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50400779 (CHEMBL2204998) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50400778 (CHEMBL2204995) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PRMT1 using histone H4 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins | ACS Med Chem Lett 3: 1091-1096 (2012) Article DOI: 10.1021/ml3003346 BindingDB Entry DOI: 10.7270/Q2NK3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |