 Found 596 hits with Last Name = 'maddox' and Initial = 'd'
Found 596 hits with Last Name = 'maddox' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236511
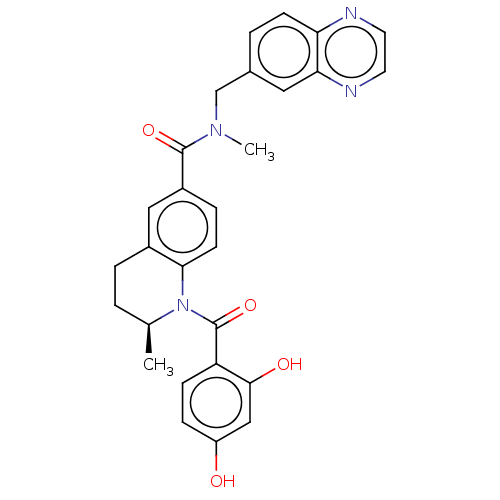
(CHEMBL3718319)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)C(=O)N(C)Cc1ccc2nccnc2c1 |r| Show InChI InChI=1S/C28H26N4O4/c1-17-3-5-19-14-20(6-10-25(19)32(17)28(36)22-8-7-21(33)15-26(22)34)27(35)31(2)16-18-4-9-23-24(13-18)30-12-11-29-23/h4,6-15,17,33-34H,3,5,16H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D2 (long) by [3H]-spiperone displacement. |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236516
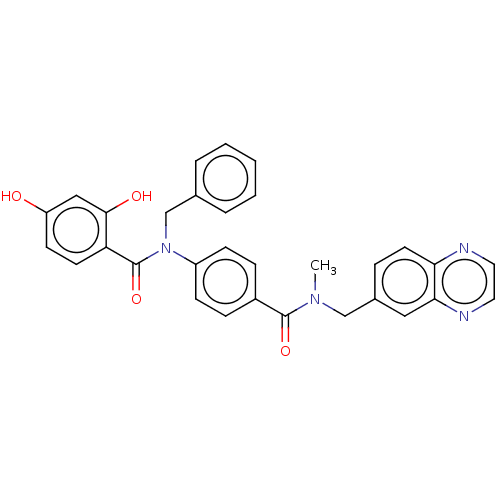
(CHEMBL3731789)Show SMILES CN(Cc1ccc2nccnc2c1)C(=O)c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C31H26N4O4/c1-34(19-22-7-14-27-28(17-22)33-16-15-32-27)30(38)23-8-10-24(11-9-23)35(20-21-5-3-2-4-6-21)31(39)26-13-12-25(36)18-29(26)37/h2-18,36-37H,19-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236530
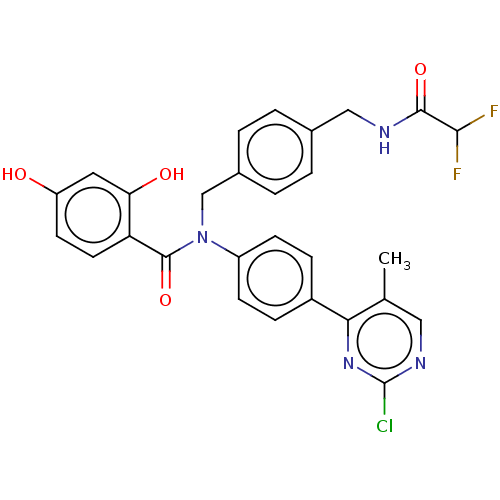
(CHEMBL3727577)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CNC(=O)C(F)F)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C28H23ClF2N4O4/c1-16-13-33-28(29)34-24(16)19-6-8-20(9-7-19)35(27(39)22-11-10-21(36)12-23(22)37)15-18-4-2-17(3-5-18)14-32-26(38)25(30)31/h2-13,25,36-37H,14-15H2,1H3,(H,32,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM265209
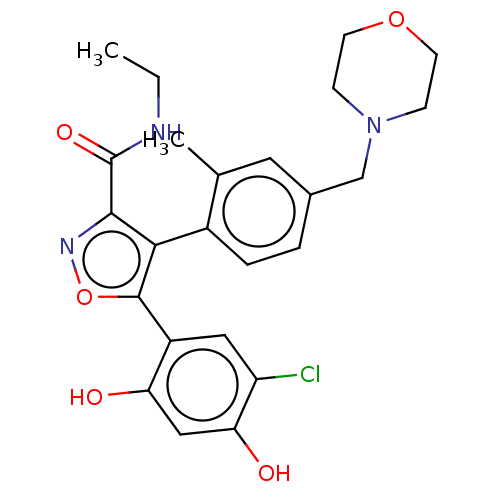
(US10413550, Example 41j | US11234987, Example 41j ...)Show SMILES CCNC(=O)c1noc(c1-c1ccc(CN2CCOCC2)cc1C)-c1cc(Cl)c(O)cc1O |(3.1,-6.74,;2.33,-5.41,;3.1,-4.07,;2.33,-2.74,;3.1,-1.41,;.79,-2.74,;-.12,-3.99,;-1.58,-3.51,;-1.58,-1.97,;-.12,-1.49,;.28,-.01,;-.81,1.08,;-.41,2.57,;1.08,2.97,;1.48,4.46,;2.96,4.85,;4.05,3.77,;5.54,4.16,;5.94,5.65,;4.85,6.74,;3.36,6.34,;2.17,1.88,;1.77,.39,;2.86,-.7,;-2.67,-.88,;-2.27,.61,;-3.36,1.7,;-2.96,3.18,;-4.85,1.3,;-5.94,2.39,;-5.25,-.19,;-4.16,-1.28,;-4.56,-2.77,)| Show InChI InChI=1S/C24H26ClN3O5/c1-3-26-24(31)22-21(23(33-27-22)17-11-18(25)20(30)12-19(17)29)16-5-4-15(10-14(16)2)13-28-6-8-32-9-7-28/h4-5,10-12,29-30H,3,6-9,13H2,1-2H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236519
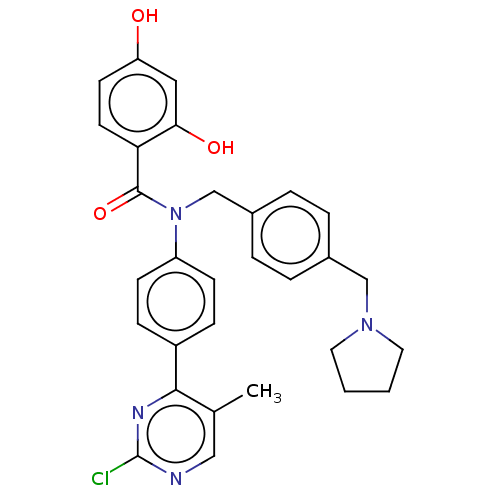
(CHEMBL3727843)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CN2CCCC2)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C30H29ClN4O3/c1-20-17-32-30(31)33-28(20)23-8-10-24(11-9-23)35(29(38)26-13-12-25(36)16-27(26)37)19-22-6-4-21(5-7-22)18-34-14-2-3-15-34/h4-13,16-17,36-37H,2-3,14-15,18-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236514
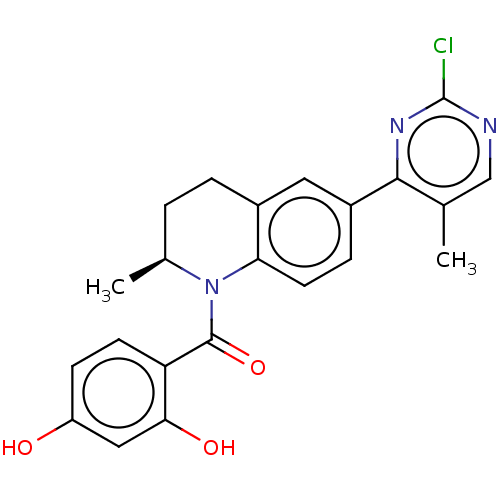
(CHEMBL4100504)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)ncc1C |r| Show InChI InChI=1S/C22H20ClN3O3/c1-12-11-24-22(23)25-20(12)15-5-8-18-14(9-15)4-3-13(2)26(18)21(29)17-7-6-16(27)10-19(17)28/h5-11,13,27-28H,3-4H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236518
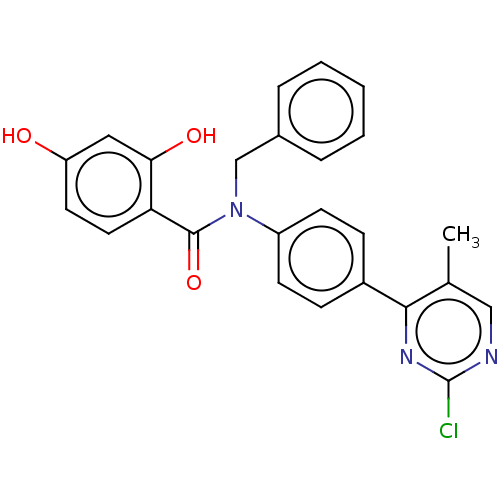
(CHEMBL3732469)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C25H20ClN3O3/c1-16-14-27-25(26)28-23(16)18-7-9-19(10-8-18)29(15-17-5-3-2-4-6-17)24(32)21-12-11-20(30)13-22(21)31/h2-14,30-31H,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236521
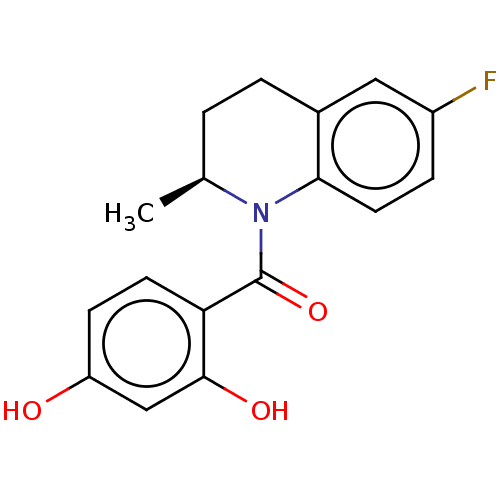
(CHEMBL3715843)Show SMILES C[C@H]1CCc2cc(F)ccc2N1C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236512
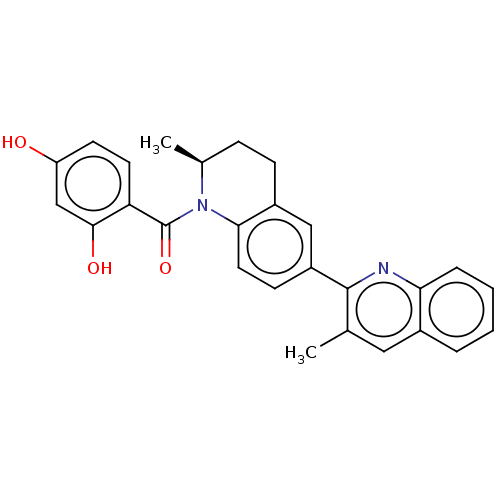
(CHEMBL3716663)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc2ccccc2cc1C |r| Show InChI InChI=1S/C27H24N2O3/c1-16-13-18-5-3-4-6-23(18)28-26(16)20-9-12-24-19(14-20)8-7-17(2)29(24)27(32)22-11-10-21(30)15-25(22)31/h3-6,9-15,17,30-31H,7-8H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM265209

(US10413550, Example 41j | US11234987, Example 41j ...)Show SMILES CCNC(=O)c1noc(c1-c1ccc(CN2CCOCC2)cc1C)-c1cc(Cl)c(O)cc1O |(3.1,-6.74,;2.33,-5.41,;3.1,-4.07,;2.33,-2.74,;3.1,-1.41,;.79,-2.74,;-.12,-3.99,;-1.58,-3.51,;-1.58,-1.97,;-.12,-1.49,;.28,-.01,;-.81,1.08,;-.41,2.57,;1.08,2.97,;1.48,4.46,;2.96,4.85,;4.05,3.77,;5.54,4.16,;5.94,5.65,;4.85,6.74,;3.36,6.34,;2.17,1.88,;1.77,.39,;2.86,-.7,;-2.67,-.88,;-2.27,.61,;-3.36,1.7,;-2.96,3.18,;-4.85,1.3,;-5.94,2.39,;-5.25,-.19,;-4.16,-1.28,;-4.56,-2.77,)| Show InChI InChI=1S/C24H26ClN3O5/c1-3-26-24(31)22-21(23(33-27-22)17-11-18(25)20(30)12-19(17)29)16-5-4-15(10-14(16)2)13-28-6-8-32-9-7-28/h4-5,10-12,29-30H,3,6-9,13H2,1-2H3,(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial
(Homo sapiens (Human)) | BDBM50236526
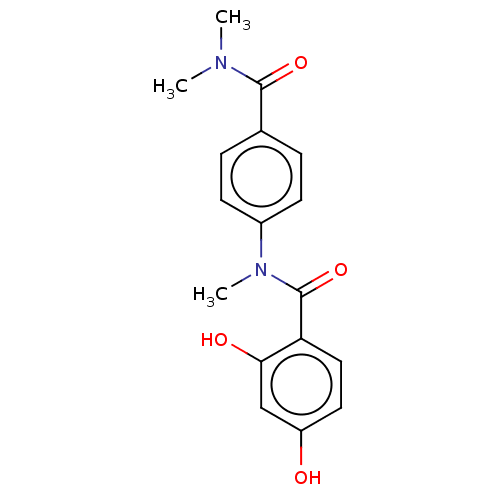
(CHEMBL4089806)Show InChI InChI=1S/C17H18N2O4/c1-18(2)16(22)11-4-6-12(7-5-11)19(3)17(23)14-9-8-13(20)10-15(14)21/h4-10,20-21H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK2 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236525

(CHEMBL4097485)Show SMILES CN(C(=O)c1ccc(O)cc1O)c1ccc(cc1)C(=O)N1CC(Oc2ccccc12)C(O)=O Show InChI InChI=1S/C24H20N2O7/c1-25(23(30)17-11-10-16(27)12-19(17)28)15-8-6-14(7-9-15)22(29)26-13-21(24(31)32)33-20-5-3-2-4-18(20)26/h2-12,21,27-28H,13H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236523
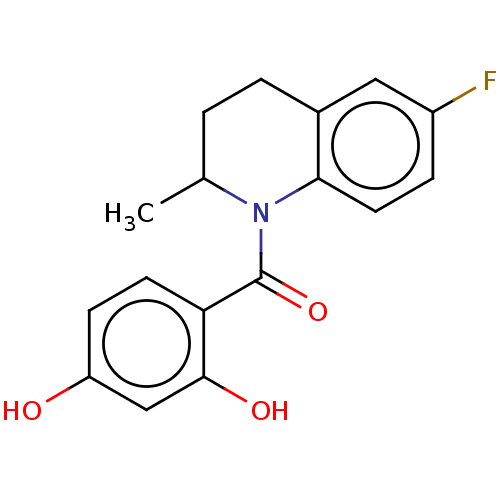
(CHEMBL3714988)Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236517
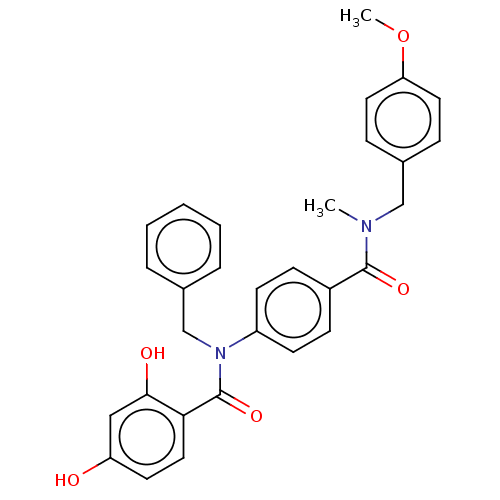
(CHEMBL3732579)Show SMILES COc1ccc(CN(C)C(=O)c2ccc(cc2)N(Cc2ccccc2)C(=O)c2ccc(O)cc2O)cc1 Show InChI InChI=1S/C30H28N2O5/c1-31(19-22-8-15-26(37-2)16-9-22)29(35)23-10-12-24(13-11-23)32(20-21-6-4-3-5-7-21)30(36)27-17-14-25(33)18-28(27)34/h3-18,33-34H,19-20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236525

(CHEMBL4097485)Show SMILES CN(C(=O)c1ccc(O)cc1O)c1ccc(cc1)C(=O)N1CC(Oc2ccccc12)C(O)=O Show InChI InChI=1S/C24H20N2O7/c1-25(23(30)17-11-10-16(27)12-19(17)28)15-8-6-14(7-9-15)22(29)26-13-21(24(31)32)33-20-5-3-2-4-18(20)26/h2-12,21,27-28H,13H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236515
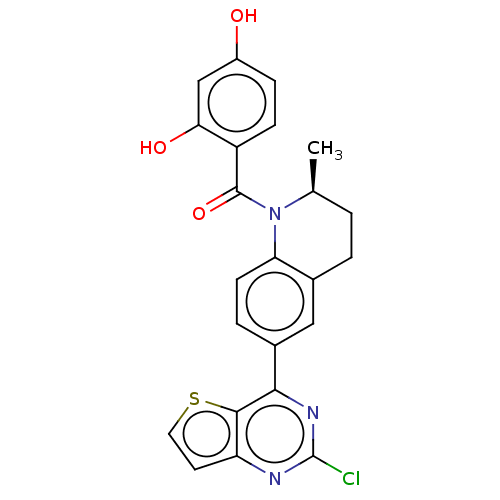
(CHEMBL3717621)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2ccsc12 |r| Show InChI InChI=1S/C23H18ClN3O3S/c1-12-2-3-13-10-14(20-21-17(8-9-31-21)25-23(24)26-20)4-7-18(13)27(12)22(30)16-6-5-15(28)11-19(16)29/h4-12,28-29H,2-3H2,1H3/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 3, mitochondrial
(Homo sapiens (Human)) | BDBM50236522
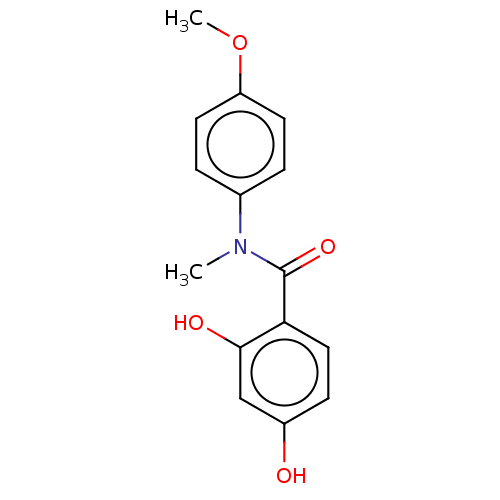
(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK3 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236513
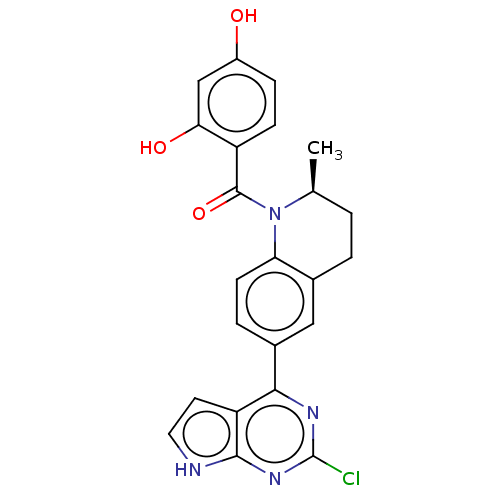
(CHEMBL3715902)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2[nH]ccc12 |r| Show InChI InChI=1S/C23H19ClN4O3/c1-12-2-3-13-10-14(20-17-8-9-25-21(17)27-23(24)26-20)4-7-18(13)28(12)22(31)16-6-5-15(29)11-19(16)30/h4-12,29-30H,2-3H2,1H3,(H,25,26,27)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236529
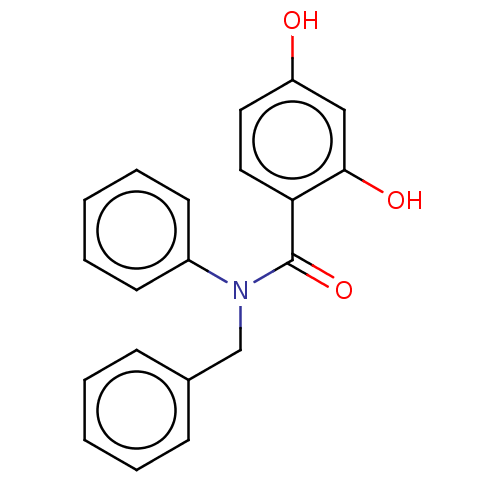
(CHEMBL3730154)Show InChI InChI=1S/C20H17NO3/c22-17-11-12-18(19(23)13-17)20(24)21(16-9-5-2-6-10-16)14-15-7-3-1-4-8-15/h1-13,22-23H,14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236516

(CHEMBL3731789)Show SMILES CN(Cc1ccc2nccnc2c1)C(=O)c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C31H26N4O4/c1-34(19-22-7-14-27-28(17-22)33-16-15-32-27)30(38)23-8-10-24(11-9-23)35(20-21-5-3-2-4-6-21)31(39)26-13-12-25(36)18-29(26)37/h2-18,36-37H,19-20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial
(Homo sapiens (Human)) | BDBM50236522

(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK2 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236524
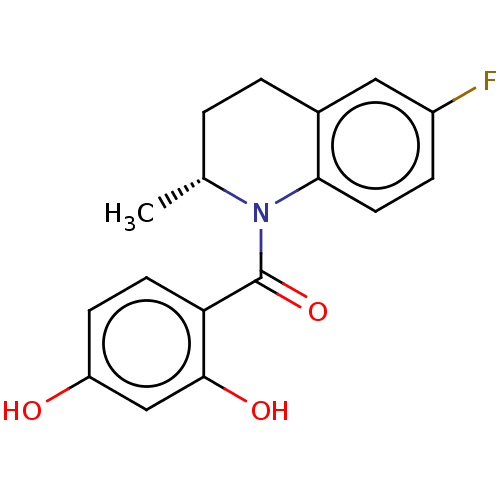
(CHEMBL3718004)Show SMILES C[C@@H]1CCc2cc(F)ccc2N1C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236526

(CHEMBL4089806)Show InChI InChI=1S/C17H18N2O4/c1-18(2)16(22)11-4-6-12(7-5-11)19(3)17(23)14-9-8-13(20)10-15(14)21/h4-10,20-21H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 4, mitochondrial
(Homo sapiens (Human)) | BDBM50236522

(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK4 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial
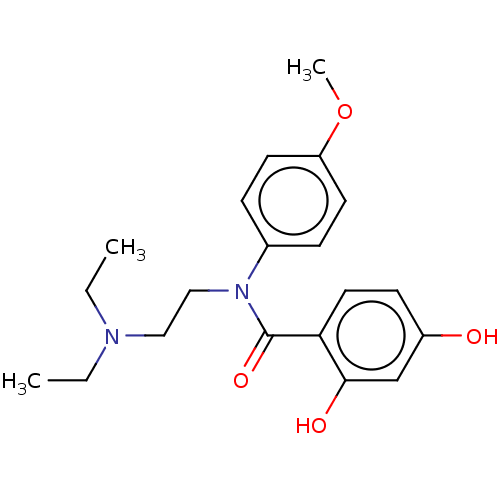
(Homo sapiens (Human)) | BDBM50236532
(CHEMBL4093486)Show InChI InChI=1S/C20H26N2O4/c1-4-21(5-2)12-13-22(15-6-9-17(26-3)10-7-15)20(25)18-11-8-16(23)14-19(18)24/h6-11,14,23-24H,4-5,12-13H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D2 (short) by [3H]-spiperone displacement. |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236522

(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236515

(CHEMBL3717621)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2ccsc12 |r| Show InChI InChI=1S/C23H18ClN3O3S/c1-12-2-3-13-10-14(20-21-17(8-9-31-21)25-23(24)26-20)4-7-18(13)27(12)22(30)16-6-5-15(28)11-19(16)29/h4-12,28-29H,2-3H2,1H3/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236522

(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236514

(CHEMBL4100504)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)ncc1C |r| Show InChI InChI=1S/C22H20ClN3O3/c1-12-11-24-22(23)25-20(12)15-5-8-18-14(9-15)4-3-13(2)26(18)21(29)17-7-6-16(27)10-19(17)28/h5-11,13,27-28H,3-4H2,1-2H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial
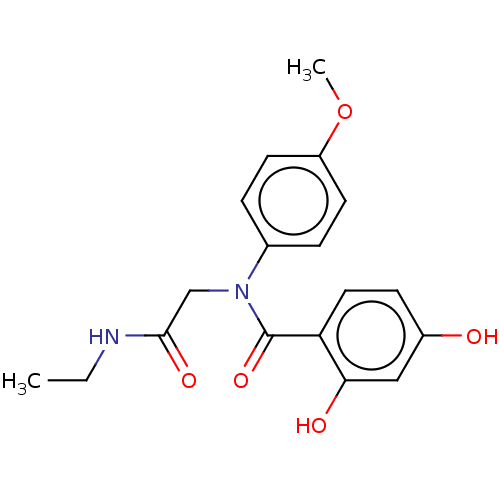
(Homo sapiens (Human)) | BDBM50236528
(CHEMBL4072775)Show InChI InChI=1S/C18H20N2O5/c1-3-19-17(23)11-20(12-4-7-14(25-2)8-5-12)18(24)15-9-6-13(21)10-16(15)22/h4-10,21-22H,3,11H2,1-2H3,(H,19,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK2 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236511

(CHEMBL3718319)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)C(=O)N(C)Cc1ccc2nccnc2c1 |r| Show InChI InChI=1S/C28H26N4O4/c1-17-3-5-19-14-20(6-10-25(19)32(17)28(36)22-8-7-21(33)15-26(22)34)27(35)31(2)16-18-4-9-23-24(13-18)30-12-11-29-23/h4,6-15,17,33-34H,3,5,16H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236519

(CHEMBL3727843)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CN2CCCC2)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C30H29ClN4O3/c1-20-17-32-30(31)33-28(20)23-8-10-24(11-9-23)35(29(38)26-13-12-25(36)16-27(26)37)19-22-6-4-21(5-7-22)18-34-14-2-3-15-34/h4-13,16-17,36-37H,2-3,14-15,18-19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236521

(CHEMBL3715843)Show SMILES C[C@H]1CCc2cc(F)ccc2N1C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236518

(CHEMBL3732469)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C25H20ClN3O3/c1-16-14-27-25(26)28-23(16)18-7-9-19(10-8-18)29(15-17-5-3-2-4-6-17)24(32)21-12-11-20(30)13-22(21)31/h2-14,30-31H,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Activity was evaluated in human mu opioid receptors transfected with CHO cells by [35S]GTP-gamma-S, assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236523

(CHEMBL3714988)Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236512

(CHEMBL3716663)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc2ccccc2cc1C |r| Show InChI InChI=1S/C27H24N2O3/c1-16-13-18-5-3-4-6-23(18)28-26(16)20-9-12-24-19(14-20)8-7-17(2)29(24)27(32)22-11-10-21(30)15-25(22)31/h3-6,9-15,17,30-31H,7-8H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236517

(CHEMBL3732579)Show SMILES COc1ccc(CN(C)C(=O)c2ccc(cc2)N(Cc2ccccc2)C(=O)c2ccc(O)cc2O)cc1 Show InChI InChI=1S/C30H28N2O5/c1-31(19-22-8-15-26(37-2)16-9-22)29(35)23-10-12-24(13-11-23)32(20-21-6-4-3-5-7-21)30(36)27-17-14-25(33)18-28(27)34/h3-18,33-34H,19-20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236513

(CHEMBL3715902)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2[nH]ccc12 |r| Show InChI InChI=1S/C23H19ClN4O3/c1-12-2-3-13-10-14(20-17-8-9-25-21(17)27-23(24)26-20)4-7-18(13)28(12)22(31)16-6-5-15(29)11-19(16)30/h4-12,29-30H,2-3H2,1H3,(H,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
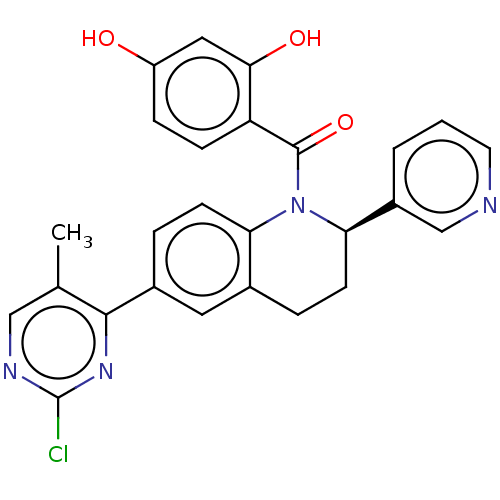
(Homo sapiens (Human)) | BDBM50236520
(CHEMBL3717438)Show SMILES Cc1cnc(Cl)nc1-c1ccc2N([C@H](CCc2c1)c1cccnc1)C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C26H21ClN4O3/c1-15-13-29-26(27)30-24(15)17-5-9-21-16(11-17)4-8-22(18-3-2-10-28-14-18)31(21)25(34)20-7-6-19(32)12-23(20)33/h2-3,5-7,9-14,22,32-33H,4,8H2,1H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236530

(CHEMBL3727577)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CNC(=O)C(F)F)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C28H23ClF2N4O4/c1-16-13-33-28(29)34-24(16)19-6-8-20(9-7-19)35(27(39)22-11-10-21(36)12-23(22)37)15-18-4-2-17(3-5-18)14-32-26(38)25(30)31/h2-13,25,36-37H,14-15H2,1H3,(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647518
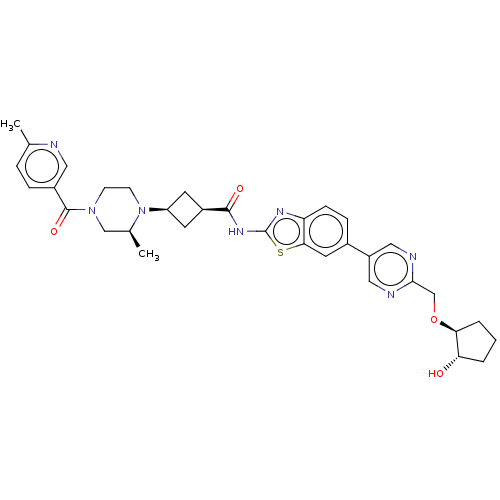
(US20240025893, Example b-04-44)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccc(C)nc1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-14.57,3.75,;-15.34,5.08,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647516
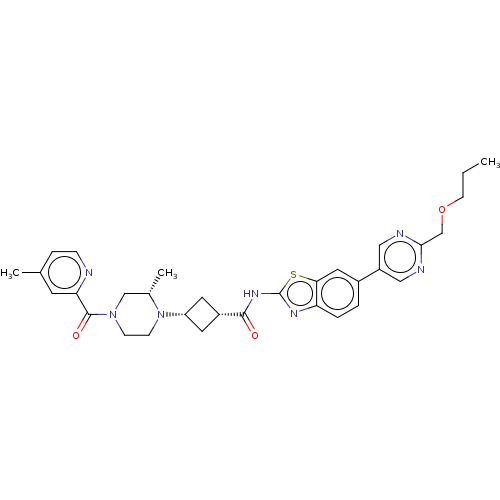
(US20240025893, Example b-04-42)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3cc(C)ccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.85,;14.15,-3.08,;12.82,-3.85,;11.49,-3.08,;10.15,-3.85,;8.82,-3.08,;7.49,-3.85,;6.15,-3.08,;6.15,-1.54,;7.49,-.77,;8.82,-1.54,;4.82,-.77,;4.82,.77,;3.48,1.54,;2.15,.77,;.69,1.25,;-.22,,;-1.76,,;-2.53,-1.33,;-1.76,-2.67,;-4.07,-1.33,;-5.16,-.24,;-6.25,-1.33,;-5.16,-2.42,;-7.79,-1.33,;-8.56,,;-10.11,,;-10.87,-1.33,;-10.11,-2.67,;-8.56,-2.67,;-7.79,-4,;-12.41,-1.33,;-13.18,-2.67,;-13.18,,;-12.41,1.33,;-13.18,2.67,;-12.41,4,;-14.72,2.67,;-15.49,1.33,;-14.72,,;.69,-1.25,;2.15,-.77,;3.48,-1.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647522
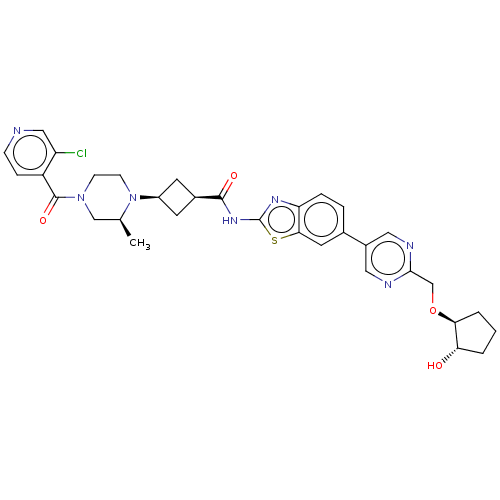
(US20240025893, Example b-04-48)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccncc1Cl |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.25,;-8.41,-.92,;-9.96,-.92,;-10.72,.41,;-9.96,1.75,;-8.41,1.75,;-7.64,.41,;-6.1,.41,;-5.01,1.5,;-3.92,.41,;-5.01,-.68,;-2.38,.41,;-1.61,-.92,;-1.61,1.75,;-.07,1.75,;.83,2.99,;2.3,2.52,;3.63,3.29,;4.97,2.52,;4.97,.98,;3.63,.21,;2.3,.98,;.83,.5,;6.3,.21,;6.3,-1.33,;7.63,-2.1,;8.97,-1.33,;10.3,-2.1,;11.63,-1.33,;12.97,-2.1,;14.43,-1.63,;15.34,-2.87,;14.43,-4.12,;12.97,-3.64,;11.63,-4.41,;8.97,.21,;7.63,.98,;-12.26,.41,;-13.03,-.92,;-13.03,1.75,;-12.26,3.08,;-13.03,4.41,;-14.57,4.41,;-15.34,3.08,;-14.57,1.75,;-15.34,.41,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647521

(US20240025893, Example b-04-47)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cc(C)ccn1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-12.26,5.08,;-14.57,3.75,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647520
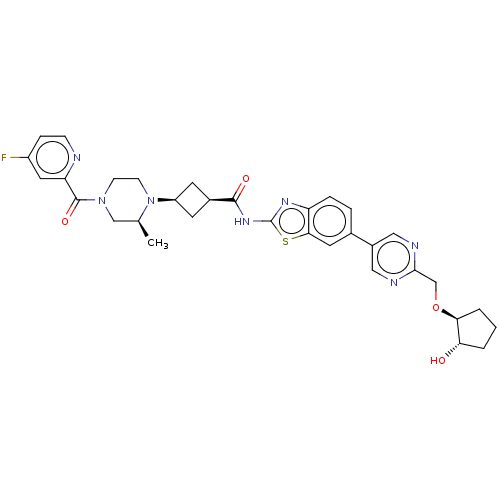
(US20240025893, Example b-04-46)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1cc(F)ccn1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.64,-2.92,;-8.41,-1.59,;-9.96,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-12.26,-.25,;-13.03,-1.59,;-13.03,1.08,;-12.26,2.41,;-13.03,3.75,;-12.26,5.08,;-14.57,3.75,;-15.34,2.41,;-14.57,1.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647517
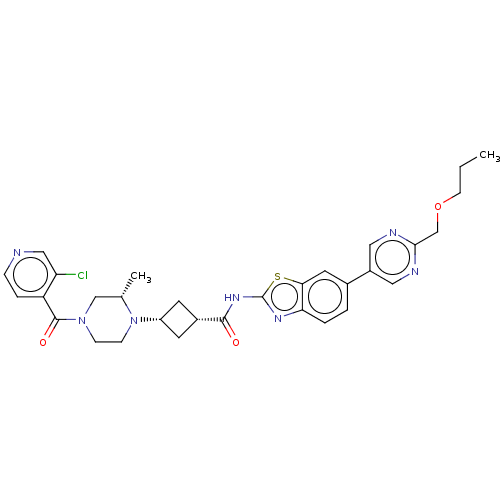
(US20240025893, Example b-04-43)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3ccncc3Cl)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.18,;14.15,-2.41,;12.82,-3.18,;11.49,-2.41,;10.15,-3.18,;8.82,-2.41,;7.49,-3.18,;6.15,-2.41,;6.15,-.87,;7.49,-.1,;8.82,-.87,;4.82,-.1,;4.82,1.44,;3.48,2.21,;2.15,1.44,;.69,1.91,;-.22,.67,;-1.76,.67,;-2.53,-.67,;-1.76,-2,;-4.07,-.67,;-5.16,.42,;-6.25,-.67,;-5.16,-1.76,;-7.79,-.67,;-8.56,.67,;-10.11,.67,;-10.87,-.67,;-10.11,-2,;-8.56,-2,;-7.79,-3.33,;-12.41,-.67,;-13.18,-2,;-13.18,.67,;-12.41,2,;-13.18,3.33,;-14.72,3.33,;-15.49,2,;-14.72,.67,;-15.49,-.67,;.69,-.58,;2.15,-.1,;3.48,-.87,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647513
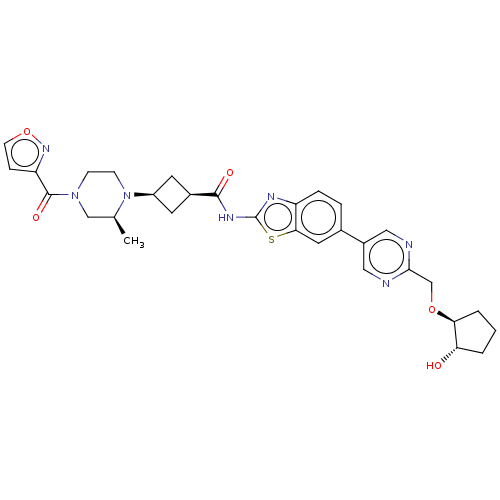
(US20240025893, Example b-04-39)Show SMILES C[C@H]1CN(CCN1[C@H]1C[C@H](C1)C(=O)Nc1nc2ccc(cc2s1)-c1cnc(CO[C@H]2CCC[C@@H]2O)nc1)C(=O)c1ccon1 |r,wU:29.32,7.7,9.12,1.0,wD:33.38,(-7.79,-2.11,;-8.56,-.77,;-10.1,-.77,;-10.87,.56,;-10.1,1.9,;-8.56,1.9,;-7.79,.56,;-6.25,.56,;-5.16,1.65,;-4.07,.56,;-5.16,-.53,;-2.53,.56,;-1.76,-.77,;-1.76,1.9,;-.22,1.9,;.69,3.14,;2.15,2.67,;3.49,3.44,;4.82,2.67,;4.82,1.13,;3.49,.36,;2.15,1.13,;.69,.65,;6.15,.36,;6.15,-1.18,;7.49,-1.95,;8.82,-1.18,;10.15,-1.95,;11.49,-1.18,;12.82,-1.95,;14.29,-1.48,;15.19,-2.72,;14.29,-3.97,;12.82,-3.49,;11.49,-4.26,;8.82,.36,;7.49,1.13,;-12.41,.56,;-13.18,-.77,;-13.18,1.9,;-12.7,3.36,;-13.95,4.26,;-15.19,3.36,;-14.72,1.9,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647519

(US20240025893, Example b-04-45)Show SMILES COc1ccc(cn1)C(=O)N1CCN([C@H]2C[C@H](C2)C(=O)Nc2nc3ccc(cc3s2)-c2cnc(CO[C@H]3CCC[C@@H]3O)nc2)[C@@H](C)C1 |r,wU:36.39,14.14,16.19,44.50,wD:40.45,(-14.35,6.26,;-15.34,5.08,;-14.57,3.75,;-13.03,3.75,;-12.26,2.41,;-13.03,1.08,;-14.57,1.08,;-15.34,2.41,;-12.26,-.25,;-13.03,-1.59,;-10.72,-.25,;-9.96,1.08,;-8.41,1.08,;-7.64,-.25,;-6.1,-.25,;-5.01,.83,;-3.92,-.25,;-5.01,-1.34,;-2.38,-.25,;-1.61,-1.59,;-1.61,1.08,;-.07,1.08,;.83,2.33,;2.3,1.85,;3.63,2.62,;4.97,1.85,;4.97,.31,;3.63,-.46,;2.3,.31,;.83,-.17,;6.3,-.46,;6.3,-2,;7.63,-2.77,;8.97,-2,;10.3,-2.77,;11.63,-2,;12.97,-2.77,;14.43,-2.29,;15.34,-3.54,;14.43,-4.79,;12.97,-4.31,;11.63,-5.08,;8.97,-.46,;7.63,.31,;-8.41,-1.59,;-7.64,-2.92,;-9.96,-1.59,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647515

(US20240025893, Example b-04-41)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3cc(F)ccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.85,;14.15,-3.08,;12.82,-3.85,;11.49,-3.08,;10.15,-3.85,;8.82,-3.08,;7.49,-3.85,;6.15,-3.08,;6.15,-1.54,;7.49,-.77,;8.82,-1.54,;4.82,-.77,;4.82,.77,;3.48,1.54,;2.15,.77,;.69,1.25,;-.22,,;-1.76,,;-2.53,-1.33,;-1.76,-2.67,;-4.07,-1.33,;-5.16,-.24,;-6.25,-1.33,;-5.16,-2.42,;-7.79,-1.33,;-8.56,,;-10.11,,;-10.87,-1.33,;-10.11,-2.67,;-8.56,-2.67,;-7.79,-4,;-12.41,-1.33,;-13.18,-2.67,;-13.18,,;-12.41,1.33,;-13.18,2.67,;-12.41,4,;-14.72,2.67,;-15.49,1.33,;-14.72,,;.69,-1.25,;2.15,-.77,;3.48,-1.54,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM647498
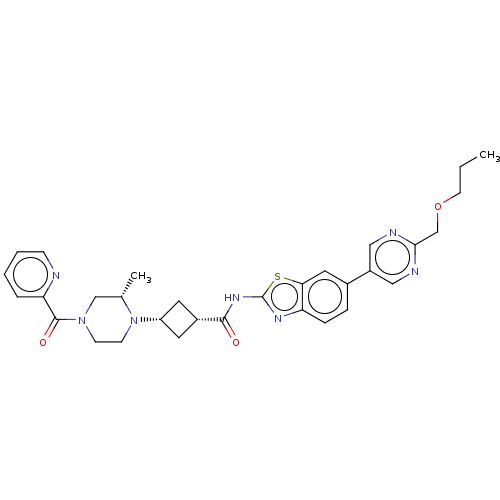
(US20240025893, Example b-04-24)Show SMILES CCCOCc1ncc(cn1)-c1ccc2nc(NC(=O)[C@@H]3C[C@@H](C3)N3CCN(C[C@@H]3C)C(=O)c3ccccn3)sc2c1 |r,wU:22.25,20.20,29.32,(15.49,-3.18,;14.15,-2.41,;12.82,-3.18,;11.49,-2.41,;10.15,-3.18,;8.82,-2.41,;7.49,-3.18,;6.15,-2.41,;6.15,-.87,;7.49,-.1,;8.82,-.87,;4.82,-.1,;4.82,1.44,;3.48,2.21,;2.15,1.44,;.69,1.91,;-.22,.67,;-1.76,.67,;-2.53,-.67,;-1.76,-2,;-4.07,-.67,;-5.16,.42,;-6.25,-.67,;-5.16,-1.76,;-7.79,-.67,;-8.56,.67,;-10.11,.67,;-10.87,-.67,;-10.11,-2,;-8.56,-2,;-7.79,-3.33,;-12.41,-.67,;-13.18,-2,;-13.18,.67,;-12.41,2,;-13.18,3.33,;-14.72,3.33,;-15.49,2,;-14.72,.67,;.69,-.58,;2.15,-.1,;3.48,-.87,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data