

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50176259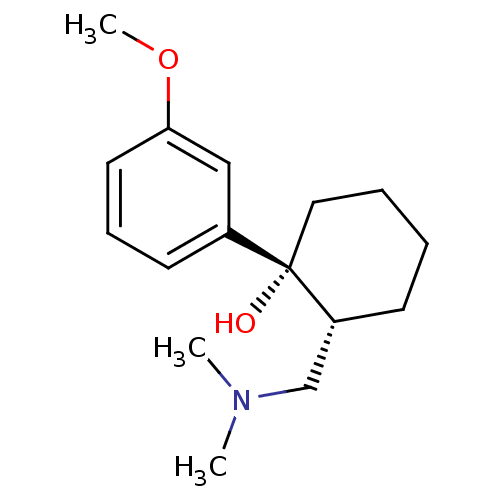 ((1R,2R)-2-[(dimethylamino)methyl]-1-(3-methoxyphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50353656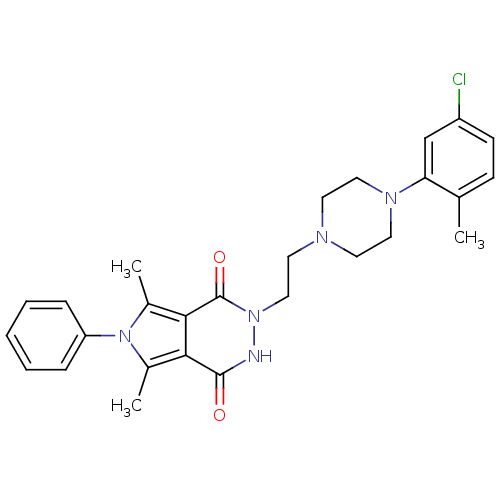 (CHEMBL1829950) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50353657 (CHEMBL1829947) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50326673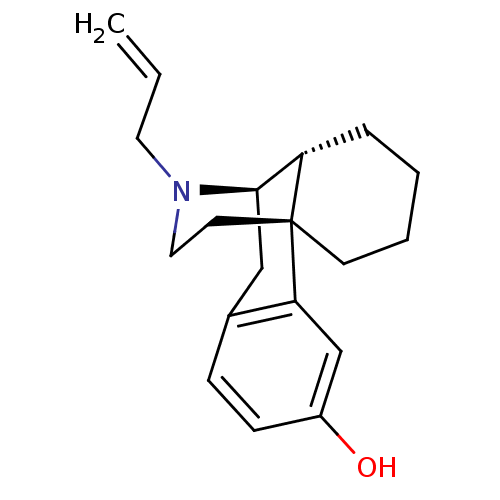 ((levallorphan)17-allyl-(1R,9R)-17-azatetracyclo[7....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.634 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 expressed in baculovirus infected Sf21 insect cells assessed as reduction in oxygen consumption using arachidonic acid as s... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50056998 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of sheep seminal vesicle COX-1 assessed as reduction in oxygen consumption using arachidonic acid as substrate incubated for 12 mins follo... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50074922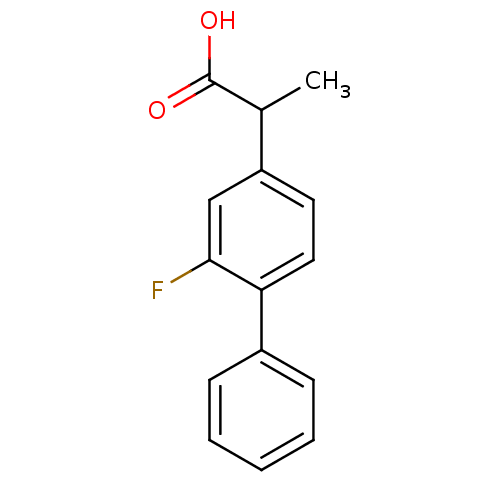 ((+-)-2-fluoro-alpha-methyl-4-biphenylacetic acid |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50074922 ((+-)-2-fluoro-alpha-methyl-4-biphenylacetic acid |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50353656 (CHEMBL1829950) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50353657 (CHEMBL1829947) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Displacement of [3H]-dihydromorphine from mu opioid receptor in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 46: 4992-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.006 BindingDB Entry DOI: 10.7270/Q27S7P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM85245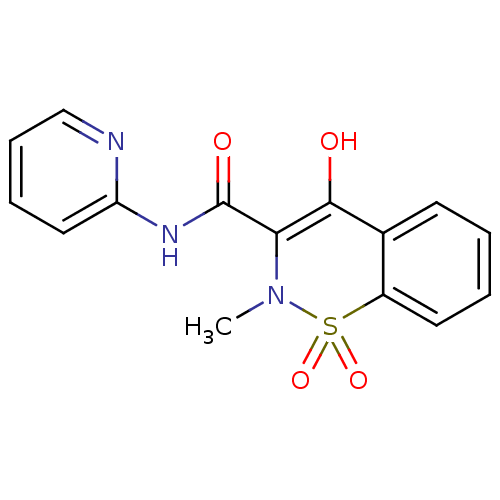 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM85245 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204369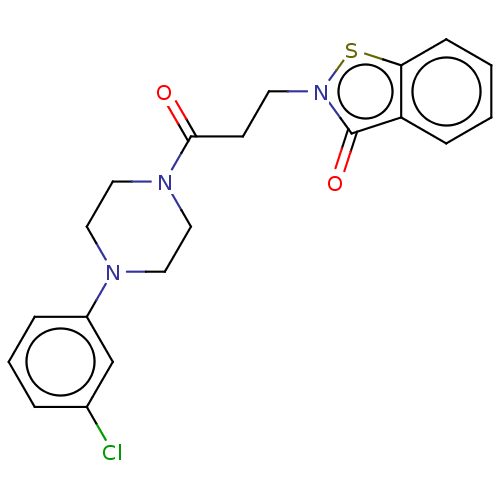 (CHEMBL3958352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204369 (CHEMBL3958352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204362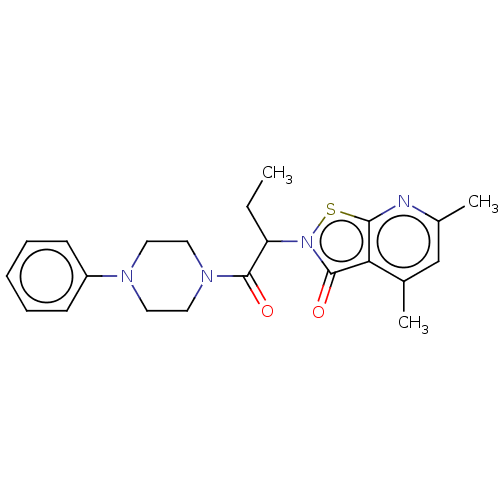 (CHEMBL3977032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204362 (CHEMBL3977032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204364 (CHEMBL3912571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204364 (CHEMBL3912571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204422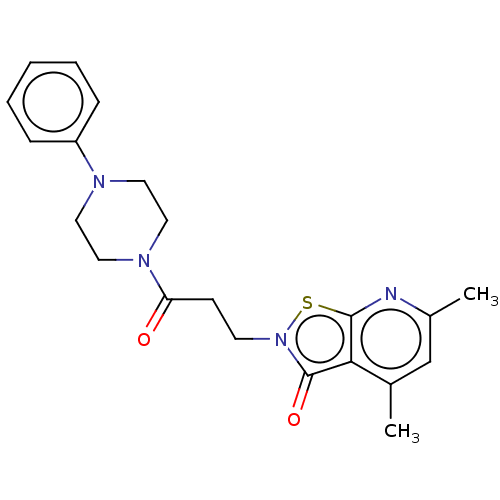 (CHEMBL3903347) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204422 (CHEMBL3903347) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204360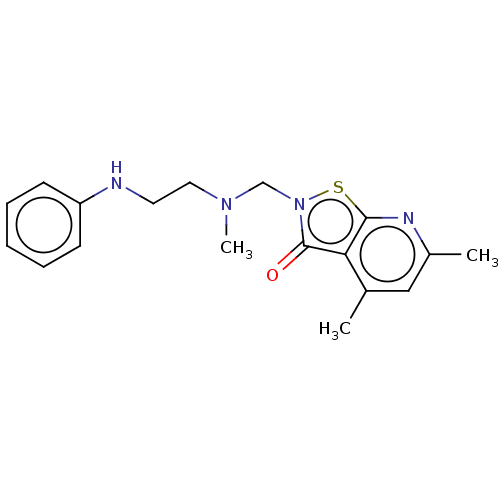 (CHEMBL3974473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204360 (CHEMBL3974473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204421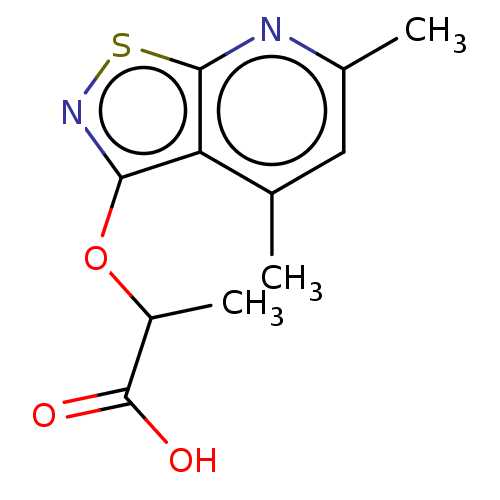 (CHEMBL3933250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204421 (CHEMBL3933250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204417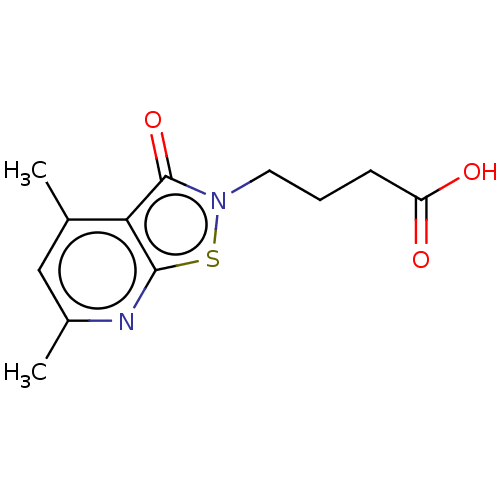 (CHEMBL3905241) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204417 (CHEMBL3905241) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204363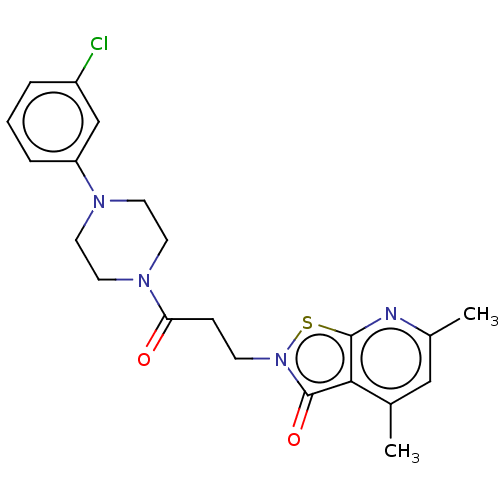 (CHEMBL3974298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204363 (CHEMBL3974298) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204366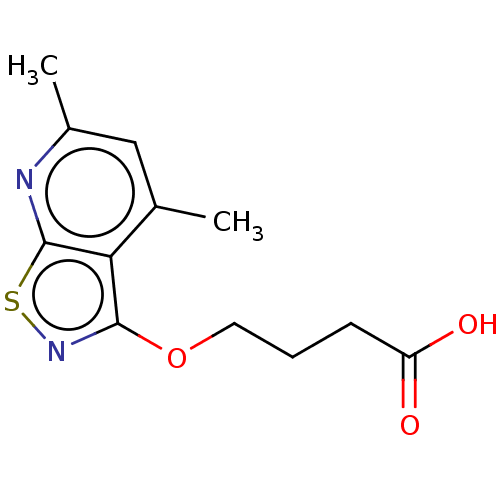 (CHEMBL3924311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204366 (CHEMBL3924311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204366 (CHEMBL3924311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM85245 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM85245 (CAS_36322-90-4 | NSC_4856 | Piroxicam) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204366 (CHEMBL3924311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204361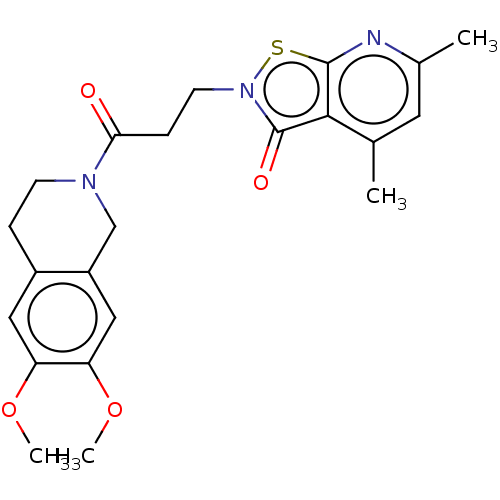 (CHEMBL3915322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204369 (CHEMBL3958352) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204417 (CHEMBL3905241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204417 (CHEMBL3905241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204361 (CHEMBL3915322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204421 (CHEMBL3933250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204421 (CHEMBL3933250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204369 (CHEMBL3958352) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204362 (CHEMBL3977032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204362 (CHEMBL3977032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204368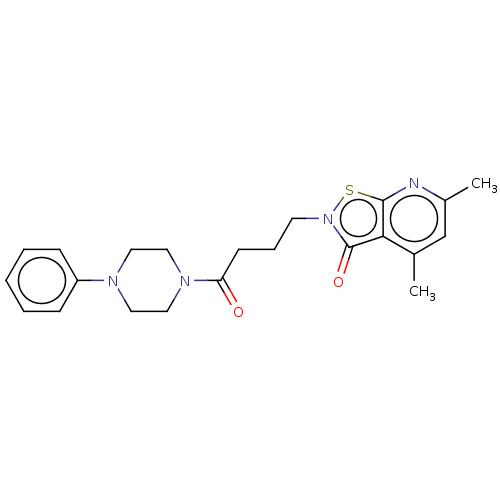 (CHEMBL3948850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50204368 (CHEMBL3948850) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-1 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204420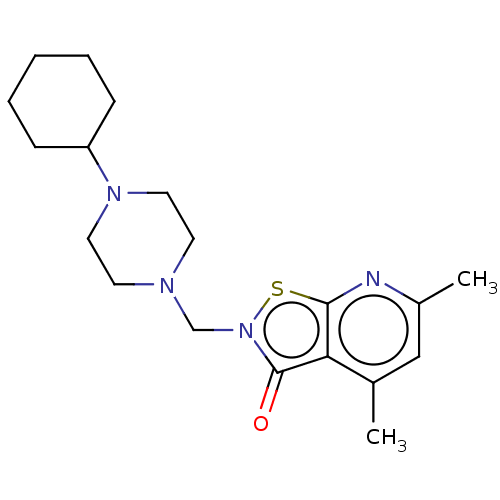 (CHEMBL3965233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204420 (CHEMBL3965233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204422 (CHEMBL3903347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50204422 (CHEMBL3903347) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw Medical University Curated by ChEMBL | Assay Description Inhibition of COX-2 (unknown origin) using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition in presence of TMPD b... | Bioorg Med Chem 25: 316-326 (2017) Article DOI: 10.1016/j.bmc.2016.10.036 BindingDB Entry DOI: 10.7270/Q21V5GZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |