 Found 497 hits with Last Name = 'mccann' and Initial = 'me'
Found 497 hits with Last Name = 'mccann' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293854
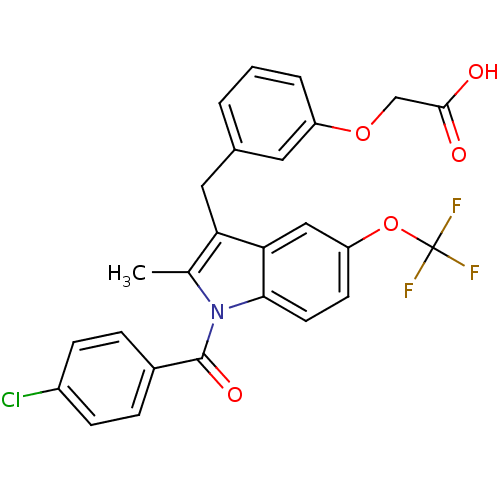
(2-(3-((1-(4-chlorobenzoyl)-2-methyl-5-(trifluorome...)Show SMILES Cc1c(Cc2cccc(OCC(O)=O)c2)c2cc(OC(F)(F)F)ccc2n1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H19ClF3NO5/c1-15-21(12-16-3-2-4-19(11-16)35-14-24(32)33)22-13-20(36-26(28,29)30)9-10-23(22)31(15)25(34)17-5-7-18(27)8-6-17/h2-11,13H,12,14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166300
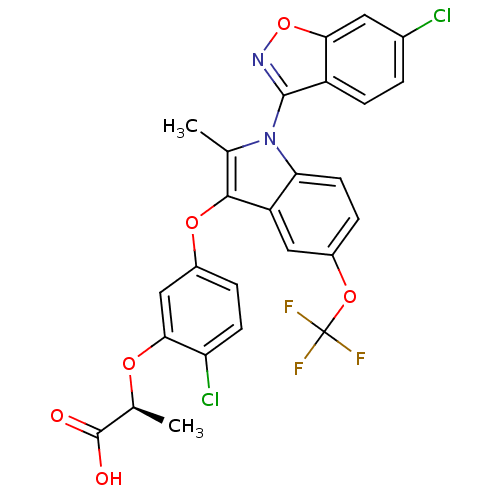
((S)-2-{2-Chloro-5-[1-(6-chloro-benzo[d]isoxazol-3-...)Show SMILES C[C@H](Oc1cc(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)ccc1Cl)C(O)=O |wU:1.0,(11.52,1.27,;11.04,-.19,;9.55,-.52,;8.51,.64,;7.01,.32,;5.98,1.43,;4.48,1.11,;4,-.36,;4.91,-1.61,;6.44,-1.61,;4,-2.84,;4.41,-4.33,;3.45,-5.51,;4.3,-6.81,;5.77,-6.42,;7.06,-7.25,;8.43,-6.56,;9.72,-7.37,;8.5,-5,;7.22,-4.17,;5.86,-4.89,;2.54,-2.38,;1.22,-3.14,;-.13,-2.38,;-.13,-.84,;-1.48,-.05,;-2.8,-.84,;-4.15,-1.63,;-3.59,.5,;-2.01,-2.17,;1.22,-.05,;2.54,-.82,;6.46,2.92,;7.95,3.24,;9,2.11,;10.5,2.41,;12.09,-1.33,;11.6,-2.79,;13.6,-1.01,)| Show InChI InChI=1S/C26H17Cl2F3N2O6/c1-12-23(37-15-4-7-19(28)22(11-15)36-13(2)25(34)35)18-10-16(38-26(29,30)31)5-8-20(18)33(12)24-17-6-3-14(27)9-21(17)39-32-24/h3-11,13H,1-2H3,(H,34,35)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157917

((2S)-2-(3-{[1-(4-METHOXYBENZOYL)-2-METHYL-5-(TRIFL...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50268271
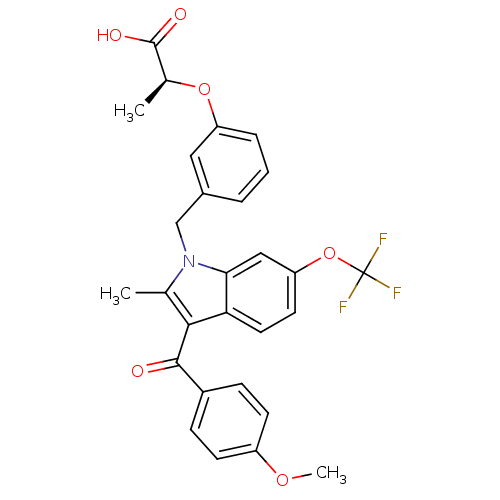
((S)-2-(3-((3-(4-methoxybenzoyl)-2-methyl-6-(triflu...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-25(26(33)19-7-9-20(36-3)10-8-19)23-12-11-22(38-28(29,30)31)14-24(23)32(16)15-18-5-4-6-21(13-18)37-17(2)27(34)35/h4-14,17H,15H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166292
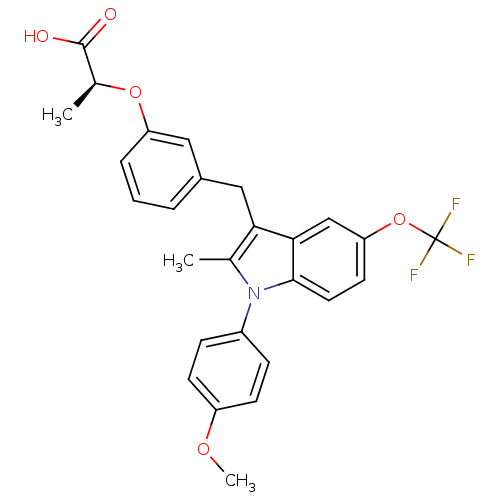
((S)-2-{3-[1-(4-Methoxy-phenyl)-2-methyl-5-trifluor...)Show SMILES COc1ccc(cc1)-n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 Show InChI InChI=1S/C27H24F3NO5/c1-16-23(14-18-5-4-6-21(13-18)35-17(2)26(32)33)24-15-22(36-27(28,29)30)11-12-25(24)31(16)19-7-9-20(34-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267990
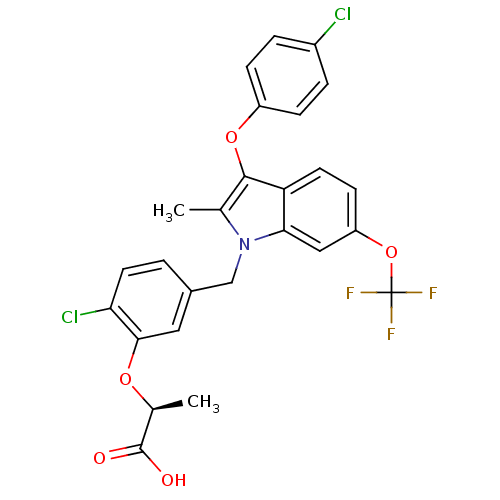
((S)-2-(2-chloro-5-((3-(4-chlorophenoxy)-2-methyl-6...)Show SMILES C[C@H](Oc1cc(Cn2c(C)c(Oc3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)ccc1Cl)C(O)=O |r| Show InChI InChI=1S/C26H20Cl2F3NO5/c1-14-24(36-18-6-4-17(27)5-7-18)20-9-8-19(37-26(29,30)31)12-22(20)32(14)13-16-3-10-21(28)23(11-16)35-15(2)25(33)34/h3-12,15H,13H2,1-2H3,(H,33,34)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244556
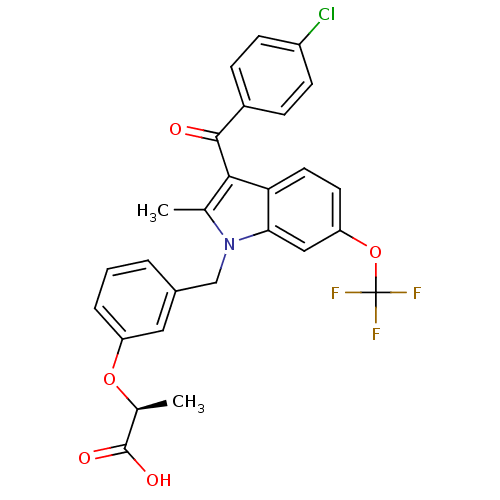
((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157917

((2S)-2-(3-{[1-(4-METHOXYBENZOYL)-2-METHYL-5-(TRIFL...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267989

((2S)-2-(4-chloro-3-((3-(6-methoxybenzo[d]isoxazol-...)Show SMILES COc1ccc2c(noc2c1)-c1c(C)n(Cc2cc(O[C@@H](C)C(O)=O)ccc2Cl)c2cc(OC(F)(F)F)ccc12 |r,wU:20.22,(26.73,-43.65,;26.71,-42.11,;25.37,-41.35,;25.35,-39.81,;24.02,-39.06,;22.71,-39.84,;21.24,-39.37,;20.34,-40.62,;21.26,-41.86,;22.72,-41.37,;24.05,-42.13,;20.75,-37.92,;21.65,-36.65,;23.19,-36.64,;20.73,-35.4,;21.2,-33.94,;22.71,-33.61,;23.74,-34.74,;25.24,-34.41,;26.28,-35.55,;27.78,-35.22,;28.25,-33.76,;28.82,-36.36,;30.32,-36.03,;28.35,-37.83,;25.71,-32.95,;24.66,-31.8,;23.16,-32.14,;22.12,-31.01,;19.26,-35.89,;17.92,-35.13,;16.6,-35.9,;15.26,-35.13,;15.26,-33.59,;15.25,-32.05,;16.8,-33.58,;13.72,-33.6,;16.59,-37.44,;17.92,-38.22,;19.27,-37.44,)| Show InChI InChI=1S/C28H22ClF3N2O6/c1-14-25(26-21-8-4-17(37-3)12-24(21)40-33-26)20-7-5-19(39-28(30,31)32)11-23(20)34(14)13-16-10-18(6-9-22(16)29)38-15(2)27(35)36/h4-12,15H,13H2,1-3H3,(H,35,36)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166295

((R)-2-{4-Chloro-3-[1-(6-methoxy-benzo[d]isoxazol-3...)Show SMILES COc1ccc2c(noc2c1)-n1c(C)c(Cc2cc(O[C@H](C)C(O)=O)ccc2Cl)c2cc(OC(F)(F)F)ccc12 |wD:20.22,(29.61,-7.83,;29.61,-6.29,;28.28,-5.52,;28.28,-3.99,;26.95,-3.22,;25.63,-3.99,;24.17,-3.51,;23.28,-4.76,;24.18,-5.99,;25.63,-5.52,;26.95,-6.28,;23.7,-2.06,;24.59,-.82,;26.14,-.82,;23.7,.43,;24.17,1.91,;25.67,2.23,;26.71,1.11,;28.2,1.44,;29.24,.28,;30.75,.6,;31.22,2.06,;31.78,-.54,;31.31,-2.01,;33.29,-.22,;28.68,2.9,;27.65,4.04,;26.15,3.72,;25.11,4.84,;22.23,-.03,;20.89,.74,;19.55,-.05,;18.2,.74,;16.86,-.05,;17.65,-1.38,;15.53,-.84,;16.08,1.3,;19.55,-1.58,;20.89,-2.36,;22.23,-1.58,)| Show InChI InChI=1S/C28H22ClF3N2O6/c1-14-21(11-16-10-18(5-8-23(16)29)38-15(2)27(35)36)22-12-19(39-28(30,31)32)6-9-24(22)34(14)26-20-7-4-17(37-3)13-25(20)40-33-26/h4-10,12-13,15H,11H2,1-3H3,(H,35,36)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293853
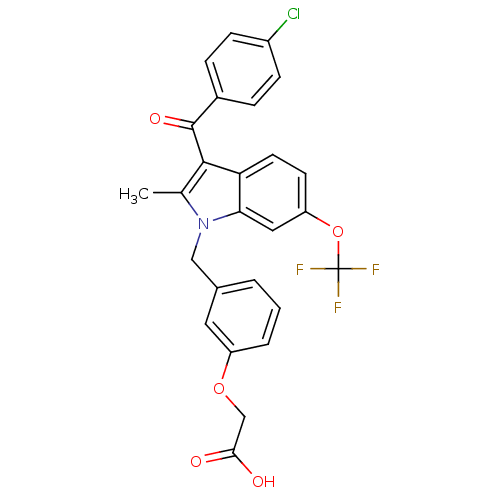
(2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(trifluorome...)Show SMILES Cc1c(C(=O)c2ccc(Cl)cc2)c2ccc(OC(F)(F)F)cc2n1Cc1cccc(OCC(O)=O)c1 Show InChI InChI=1S/C26H19ClF3NO5/c1-15-24(25(34)17-5-7-18(27)8-6-17)21-10-9-20(36-26(28,29)30)12-22(21)31(15)13-16-3-2-4-19(11-16)35-14-23(32)33/h2-12H,13-14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362957

(CHEMBL1946757)Show SMILES COc1ccc2c(noc2c1)-n1c2cc(ccc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O)C(F)(F)F |r,wU:27.30,(3.39,4.69,;2.06,5.47,;.72,4.7,;.72,3.15,;-.63,2.39,;-1.96,3.17,;-3.42,2.72,;-4.32,3.96,;-3.41,5.2,;-1.95,4.71,;-.61,5.48,;-4.18,1.38,;-5.65,.89,;-6.98,1.66,;-8.31,.89,;-8.31,-.66,;-6.98,-1.43,;-5.64,-.65,;-4.17,-1.12,;-4.14,-2.66,;-2.8,-3.41,;-2.78,-4.94,;-1.43,-5.69,;-.11,-4.9,;1.24,-5.64,;-.14,-3.35,;1.17,-2.55,;2.52,-3.3,;2.55,-4.84,;3.84,-2.5,;5.19,-3.24,;3.81,-.96,;-1.49,-2.61,;-3.27,.14,;-1.73,.15,;-9.64,1.66,;-9.64,3.2,;-10.97,.89,;-10.8,2.43,)| Show InChI InChI=1S/C26H19ClF3N3O6/c1-13(24(34)35)38-22-9-14(3-7-18(22)27)12-32-19-8-4-15(26(28,29)30)10-20(19)33(25(32)36)23-17-6-5-16(37-2)11-21(17)39-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50334486
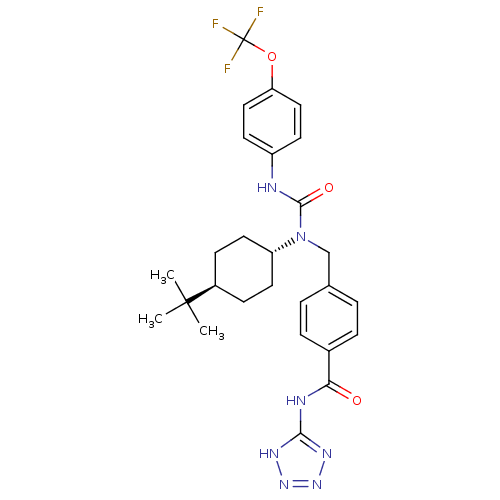
(CHEMBL1643951 | trans-4-((1-(4-tert-butylcyclohexy...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)Nc1nnn[nH]1)C(=O)Nc1ccc(OC(F)(F)F)cc1 |r,wU:7.10,wD:4.3,(26.38,-4.09,;27.72,-3.32,;29.05,-4.09,;27.7,-4.85,;27.72,-1.78,;29.05,-1.01,;29.05,.54,;27.72,1.29,;26.38,.53,;26.38,-1.01,;27.71,2.83,;26.38,3.6,;25.05,2.82,;25.05,1.27,;23.71,.5,;22.38,1.27,;22.38,2.82,;23.71,3.59,;21.05,.5,;21.04,-1.04,;19.71,1.27,;18.38,.5,;16.98,1.14,;15.95,-.01,;16.72,-1.34,;18.23,-1.02,;29.04,3.61,;29.04,5.15,;30.38,2.84,;31.71,3.62,;33.04,2.85,;34.37,3.62,;34.37,5.16,;35.7,5.94,;37.03,5.18,;38.36,5.96,;37.04,3.64,;38.36,4.41,;33.02,5.93,;31.7,5.15,)| Show InChI InChI=1S/C27H32F3N7O3/c1-26(2,3)19-8-12-21(13-9-19)37(25(39)31-20-10-14-22(15-11-20)40-27(28,29)30)16-17-4-6-18(7-5-17)23(38)32-24-33-35-36-34-24/h4-7,10-11,14-15,19,21H,8-9,12-13,16H2,1-3H3,(H,31,39)(H2,32,33,34,35,36,38)/t19-,21- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 21: 76-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.074
BindingDB Entry DOI: 10.7270/Q2R211PW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362956
(CHEMBL1946756)Show SMILES COc1ccc2c(noc2c1)-n1c2ccc(OC(F)(F)F)cc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O |r,wU:32.35,(27.18,-40.65,;25.85,-39.88,;24.51,-40.64,;24.51,-42.19,;23.16,-42.96,;21.83,-42.18,;20.37,-42.63,;19.47,-41.39,;20.38,-40.15,;21.85,-40.63,;23.18,-39.87,;19.61,-43.97,;18.15,-44.45,;16.81,-43.69,;15.48,-44.46,;15.48,-46,;14.15,-46.77,;12.81,-46,;11.48,-46.77,;12.81,-44.46,;11.47,-45.23,;16.82,-46.77,;18.16,-45.99,;19.63,-46.46,;19.65,-48,;21,-48.75,;21.01,-50.29,;22.36,-51.03,;23.68,-50.24,;25.03,-50.99,;23.65,-48.69,;24.97,-47.9,;26.32,-48.64,;26.35,-50.18,;27.63,-47.84,;28.98,-48.59,;27.6,-46.3,;22.3,-47.95,;20.53,-45.21,;22.07,-45.2,)| Show InChI InChI=1S/C26H19ClF3N3O7/c1-13(24(34)35)38-22-9-14(3-7-18(22)27)12-32-20-10-16(39-26(28,29)30)5-8-19(20)33(25(32)36)23-17-6-4-15(37-2)11-21(17)40-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293852
((2S)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CC[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C28H23ClF3NO5/c1-3-24(27(35)36)37-20-6-4-5-17(13-20)15-33-16(2)25(26(34)18-7-9-19(29)10-8-18)22-12-11-21(14-23(22)33)38-28(30,31)32/h4-14,24H,3,15H2,1-2H3,(H,35,36)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362955
(CHEMBL1946755)Show SMILES COc1ccc2c(noc2c1)-n1c2ccc(cc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O)C(F)(F)F |r,wU:27.30,(5.09,-40.95,;3.76,-40.17,;2.42,-40.94,;2.42,-42.49,;1.08,-43.25,;-.25,-42.47,;-1.71,-42.92,;-2.62,-41.68,;-1.7,-40.44,;-.24,-40.93,;1.09,-40.16,;-2.48,-44.26,;-3.94,-44.75,;-5.28,-43.98,;-6.61,-44.75,;-6.61,-46.3,;-5.27,-47.07,;-3.93,-46.29,;-2.46,-46.76,;-2.44,-48.3,;-1.09,-49.05,;-1.08,-50.58,;.27,-51.33,;1.59,-50.54,;2.94,-51.28,;1.56,-48.99,;2.88,-48.19,;4.23,-48.94,;4.26,-50.48,;5.54,-48.14,;6.89,-48.88,;5.51,-46.6,;.21,-48.25,;-1.56,-45.5,;-.02,-45.49,;-7.94,-47.07,;-9.28,-46.3,;-7.94,-48.61,;-9.29,-47.83,)| Show InChI InChI=1S/C26H19ClF3N3O6/c1-13(24(34)35)38-22-9-14(3-7-18(22)27)12-32-20-10-15(26(28,29)30)4-8-19(20)33(25(32)36)23-17-6-5-16(37-2)11-21(17)39-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362958

(CHEMBL1946758)Show SMILES COc1ccc2c(noc2c1)-n1c2cc(OC(F)(F)F)ccc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O |r,wU:32.35,(27.64,4.76,;26.31,5.54,;24.97,4.77,;24.97,3.22,;23.62,2.46,;22.29,3.24,;20.83,2.79,;19.93,4.03,;20.84,5.27,;22.31,4.78,;23.64,5.55,;20.07,1.45,;18.61,.96,;17.27,1.73,;15.94,.96,;14.61,1.73,;13.27,.96,;11.94,1.73,;13.27,-.58,;11.93,.19,;15.94,-.59,;17.27,-1.36,;18.61,-.58,;20.08,-1.05,;20.11,-2.59,;21.46,-3.34,;21.47,-4.87,;22.82,-5.62,;24.14,-4.83,;25.49,-5.57,;24.11,-3.28,;25.43,-2.48,;26.77,-3.23,;26.81,-4.77,;28.09,-2.43,;29.44,-3.17,;28.06,-.89,;22.76,-2.54,;20.98,.21,;22.52,.22,)| Show InChI InChI=1S/C26H19ClF3N3O7/c1-13(24(34)35)38-22-9-14(3-7-18(22)27)12-32-19-8-5-16(39-26(28,29)30)10-20(19)33(25(32)36)23-17-6-4-15(37-2)11-21(17)40-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293846
((+)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(trifluo...)Show SMILES CC[C@@](C)(Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-4-28(3,27(36)37)38-21-7-5-6-18(14-21)16-34-17(2)25(26(35)19-8-10-20(30)11-9-19)23-13-12-22(15-24(23)34)39-29(31,32)33/h5-15H,4,16H2,1-3H3,(H,36,37)/t28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293846

((+)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(trifluo...)Show SMILES CC[C@@](C)(Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-4-28(3,27(36)37)38-21-7-5-6-18(14-21)16-34-17(2)25(26(35)19-8-10-20(30)11-9-19)23-13-12-22(15-24(23)34)39-29(31,32)33/h5-15H,4,16H2,1-3H3,(H,36,37)/t28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166298

((2S)-2-(4-chloro-3-(1-(6-chlorobenzo[d]isoxazol-3-...)Show SMILES C[C@H](Oc1ccc(Cl)c(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r,wU:1.0,(28.69,2.15,;28.21,.68,;26.71,.36,;25.68,1.51,;26.15,2.97,;25.11,4.12,;23.61,3.79,;22.58,4.93,;23.14,2.33,;21.64,2.01,;21.16,.55,;22.08,-.71,;23.62,-.71,;21.17,-1.96,;21.64,-3.43,;20.75,-4.67,;21.65,-5.92,;23.11,-5.44,;24.44,-6.21,;25.77,-5.44,;27.1,-6.21,;25.76,-3.9,;24.44,-3.14,;23.11,-3.9,;19.69,-1.48,;18.35,-2.26,;17.02,-1.48,;17.02,.06,;15.69,.83,;15.69,2.37,;15.67,3.91,;17.23,2.38,;14.15,2.36,;18.35,.83,;19.69,.07,;24.17,1.18,;29.24,-.46,;30.75,-.14,;28.77,-1.93,)| Show InChI InChI=1S/C26H17Cl2F3N2O6/c1-12-23(37-22-11-15(4-7-19(22)28)36-13(2)25(34)35)18-10-16(38-26(29,30)31)5-8-20(18)33(12)24-17-6-3-14(27)9-21(17)39-32-24/h3-11,13H,1-2H3,(H,34,35)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166298

((2S)-2-(4-chloro-3-(1-(6-chlorobenzo[d]isoxazol-3-...)Show SMILES C[C@H](Oc1ccc(Cl)c(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r,wU:1.0,(28.69,2.15,;28.21,.68,;26.71,.36,;25.68,1.51,;26.15,2.97,;25.11,4.12,;23.61,3.79,;22.58,4.93,;23.14,2.33,;21.64,2.01,;21.16,.55,;22.08,-.71,;23.62,-.71,;21.17,-1.96,;21.64,-3.43,;20.75,-4.67,;21.65,-5.92,;23.11,-5.44,;24.44,-6.21,;25.77,-5.44,;27.1,-6.21,;25.76,-3.9,;24.44,-3.14,;23.11,-3.9,;19.69,-1.48,;18.35,-2.26,;17.02,-1.48,;17.02,.06,;15.69,.83,;15.69,2.37,;15.67,3.91,;17.23,2.38,;14.15,2.36,;18.35,.83,;19.69,.07,;24.17,1.18,;29.24,-.46,;30.75,-.14,;28.77,-1.93,)| Show InChI InChI=1S/C26H17Cl2F3N2O6/c1-12-23(37-22-11-15(4-7-19(22)28)36-13(2)25(34)35)18-10-16(38-26(29,30)31)5-8-20(18)33(12)24-17-6-3-14(27)9-21(17)39-32-24/h3-11,13H,1-2H3,(H,34,35)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362953
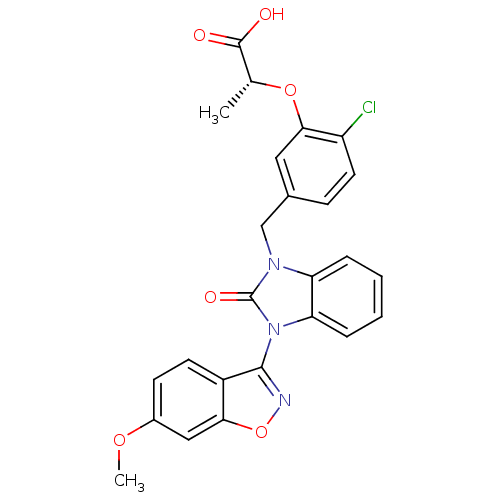
(CHEMBL1947143)Show SMILES COc1ccc2c(noc2c1)-n1c2ccccc2n(Cc2ccc(Cl)c(O[C@H](C)C(O)=O)c2)c1=O |r,wU:27.30,(27.1,-12.24,;25.78,-11.45,;24.43,-12.2,;24.41,-13.75,;23.06,-14.49,;21.74,-13.7,;20.27,-14.13,;19.39,-12.88,;20.31,-11.65,;21.77,-12.15,;23.11,-11.41,;19.49,-15.46,;18.02,-15.93,;16.7,-15.15,;15.36,-15.9,;15.34,-17.45,;16.66,-18.23,;18.01,-17.47,;19.48,-17.96,;19.48,-19.5,;20.82,-20.26,;20.82,-21.8,;22.15,-22.56,;23.49,-21.78,;24.82,-22.55,;23.47,-20.24,;24.8,-19.46,;26.14,-20.21,;26.15,-21.75,;27.47,-19.43,;28.81,-20.19,;27.46,-17.89,;22.14,-19.48,;20.39,-16.71,;21.93,-16.72,)| Show InChI InChI=1S/C25H20ClN3O6/c1-14(24(30)31)34-22-11-15(7-10-18(22)26)13-28-19-5-3-4-6-20(19)29(25(28)32)23-17-9-8-16(33-2)12-21(17)35-27-23/h3-12,14H,13H2,1-2H3,(H,30,31)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258479
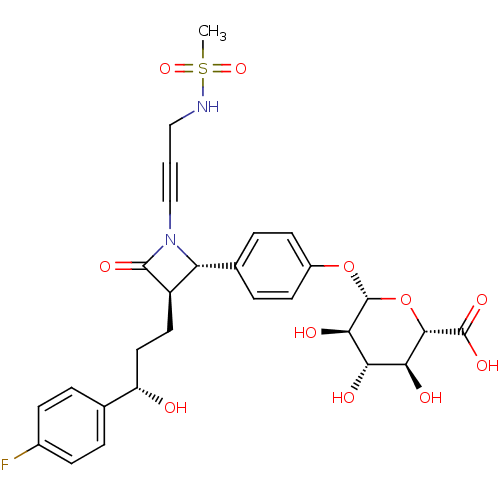
((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362967

(CHEMBL1947142)Show SMILES COc1ccc2c(noc2c1)-n1c2ccc(cc2n(Cc2cc(ccc2Cl)[C@@]2(C)OC(O)=NC2=O)c1=O)C(F)(F)F |r,wD:27.31,c:35,(5.18,-12.71,;3.86,-11.92,;2.52,-12.67,;2.49,-14.22,;1.14,-14.97,;-.18,-14.17,;-1.65,-14.61,;-2.53,-13.35,;-1.61,-12.12,;-.15,-12.63,;1.19,-11.88,;-2.43,-15.93,;-3.89,-16.4,;-5.22,-15.62,;-6.56,-16.38,;-6.58,-17.92,;-5.25,-18.71,;-3.9,-17.94,;-2.44,-18.43,;-2.43,-19.97,;-1.1,-20.73,;.22,-19.95,;1.56,-20.71,;1.57,-22.26,;.24,-23.03,;-1.1,-22.27,;-2.43,-23.04,;2.88,-19.93,;2.86,-21.47,;2.88,-18.39,;4.35,-17.92,;4.84,-16.46,;5.25,-19.18,;4.34,-20.42,;4.81,-21.88,;-1.53,-17.19,;.01,-17.2,;-7.92,-18.67,;-9.25,-17.89,;-7.94,-20.21,;-9.28,-19.42,)| Show InChI InChI=1S/C27H18ClF3N4O6/c1-26(23(36)32-24(37)40-26)14-3-7-18(28)13(9-14)12-34-20-10-15(27(29,30)31)4-8-19(20)35(25(34)38)22-17-6-5-16(39-2)11-21(17)41-33-22/h3-11H,12H2,1-2H3,(H,32,36,37)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Niemann-Pick C1-like 1 protein
(Canis familiaris) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293850
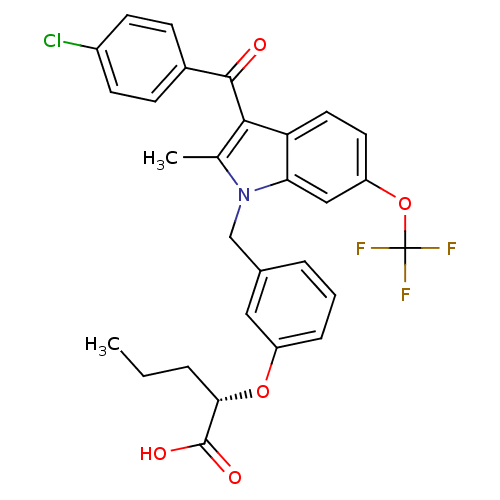
((2S)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CCC[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-3-5-25(28(36)37)38-21-7-4-6-18(14-21)16-34-17(2)26(27(35)19-8-10-20(30)11-9-19)23-13-12-22(15-24(23)34)39-29(31,32)33/h4,6-15,25H,3,5,16H2,1-2H3,(H,36,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293851
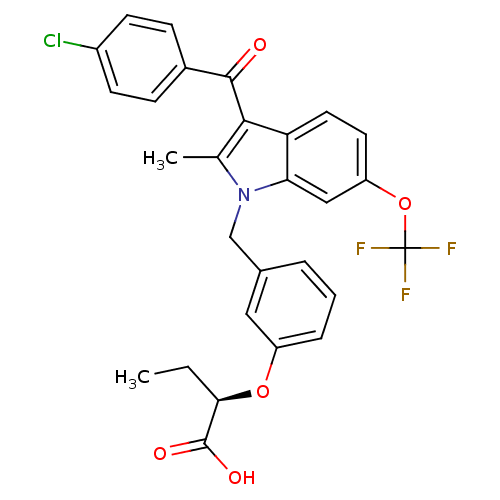
((2R)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CC[C@@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C28H23ClF3NO5/c1-3-24(27(35)36)37-20-6-4-5-17(13-20)15-33-16(2)25(26(34)18-7-9-19(29)10-8-18)22-12-11-21(14-23(22)33)38-28(30,31)32/h4-14,24H,3,15H2,1-2H3,(H,35,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267991
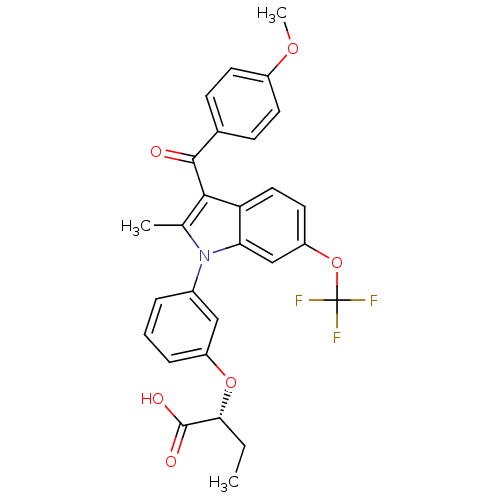
((R)-2-(3-(3-(4-methoxybenzoyl)-2-methyl-6-(trifluo...)Show SMILES CC[C@@H](Oc1cccc(c1)-n1c(C)c(C(=O)c2ccc(OC)cc2)c2ccc(OC(F)(F)F)cc12)C(O)=O |r| Show InChI InChI=1S/C28H24F3NO6/c1-4-24(27(34)35)37-20-7-5-6-18(14-20)32-16(2)25(26(33)17-8-10-19(36-3)11-9-17)22-13-12-21(15-23(22)32)38-28(29,30)31/h5-15,24H,4H2,1-3H3,(H,34,35)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362960

(CHEMBL1946953)Show SMILES COc1ccc2c(noc2c1)-n1c2ccc(cc2n(Cc2cc(O[C@H](C)C(O)=O)ccc2Cl)c1=O)C(F)(F)F |r,wU:24.27,(27.26,-10.89,;25.93,-10.12,;24.59,-10.88,;24.59,-12.44,;23.24,-13.2,;21.91,-12.42,;20.45,-12.87,;19.55,-11.63,;20.46,-10.39,;21.92,-10.87,;23.26,-10.11,;19.69,-14.21,;18.22,-14.69,;16.89,-13.93,;15.56,-14.7,;15.56,-16.24,;16.89,-17.02,;18.23,-16.24,;19.7,-16.7,;19.73,-18.24,;21.07,-18.99,;22.38,-18.2,;23.73,-18.94,;25.04,-18.14,;26.39,-18.88,;26.42,-20.42,;27.71,-18.09,;29.06,-18.83,;27.68,-16.55,;23.76,-20.48,;22.44,-21.28,;21.09,-20.53,;19.77,-21.32,;20.6,-15.45,;22.14,-15.44,;14.22,-17.01,;12.89,-16.24,;14.22,-18.55,;12.88,-17.77,)| Show InChI InChI=1S/C26H19ClF3N3O6/c1-13(24(34)35)38-17-5-7-19(27)14(9-17)12-32-21-10-15(26(28,29)30)3-8-20(21)33(25(32)36)23-18-6-4-16(37-2)11-22(18)39-31-23/h3-11,13H,12H2,1-2H3,(H,34,35)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362972

(CHEMBL1946571)Show SMILES C[C@@H](Oc1cc(Cn2c3cc(ccc3n(-c3noc4c(C)cccc34)c2=O)C(F)(F)F)ccc1Cl)C(O)=O |r,wU:1.0,(3.41,-20.44,;3.38,-18.9,;2.03,-18.16,;.71,-18.95,;-.63,-18.21,;-1.94,-19.01,;-3.29,-18.26,;-3.31,-16.72,;-4.78,-16.25,;-6.12,-17.03,;-7.46,-16.26,;-7.45,-14.72,;-6.12,-13.94,;-4.79,-14.71,;-3.33,-14.22,;-2.56,-12.89,;-3.46,-11.64,;-2.55,-10.4,;-1.09,-10.89,;.25,-10.13,;.25,-8.59,;1.58,-10.9,;1.57,-12.45,;.23,-13.21,;-1.1,-12.43,;-2.41,-15.47,;-.87,-15.46,;-8.79,-17.03,;-10.12,-16.26,;-8.79,-18.57,;-10.13,-17.79,;-1.92,-20.54,;-.58,-21.29,;.74,-20.5,;2.09,-21.25,;4.7,-18.1,;6.05,-18.84,;4.67,-16.56,)| Show InChI InChI=1S/C26H19ClF3N3O5/c1-13-4-3-5-17-22(13)38-31-23(17)33-19-9-7-16(26(28,29)30)11-20(19)32(25(33)36)12-15-6-8-18(27)21(10-15)37-14(2)24(34)35/h3-11,14H,12H2,1-2H3,(H,34,35)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362949
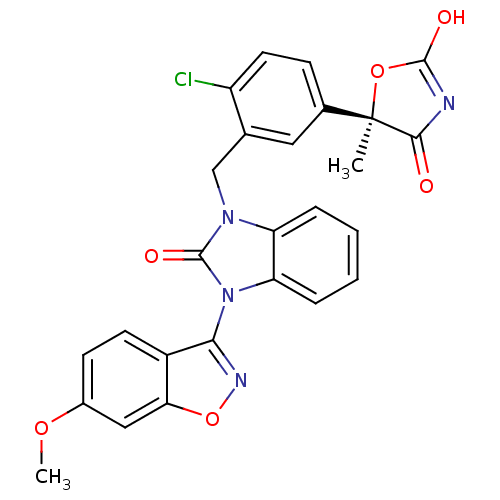
(CHEMBL1947147)Show SMILES COc1ccc2c(noc2c1)-n1c2ccccc2n(Cc2cc(ccc2Cl)[C@@]2(C)OC(O)=NC2=O)c1=O |r,wD:27.31,c:35,(27.37,-42.86,;26.05,-42.07,;24.71,-42.82,;24.68,-44.37,;23.33,-45.11,;22.01,-44.32,;20.54,-44.75,;19.66,-43.5,;20.58,-42.27,;22.04,-42.77,;23.38,-42.03,;19.76,-46.08,;18.3,-46.55,;16.97,-45.77,;15.63,-46.52,;15.61,-48.07,;16.94,-48.85,;18.29,-48.09,;19.75,-48.58,;19.76,-50.12,;21.09,-50.88,;22.41,-50.1,;23.75,-50.86,;23.76,-52.4,;22.43,-53.18,;21.09,-52.42,;19.76,-53.19,;25.07,-50.08,;25.05,-51.61,;25.07,-48.54,;26.54,-48.07,;27.03,-46.61,;27.44,-49.32,;26.53,-50.56,;27,-52.03,;20.66,-47.33,;22.2,-47.34,)| Show InChI InChI=1S/C26H19ClN4O6/c1-26(23(32)28-24(33)36-26)15-7-10-18(27)14(11-15)13-30-19-5-3-4-6-20(19)31(25(30)34)22-17-9-8-16(35-2)12-21(17)37-29-22/h3-12H,13H2,1-2H3,(H,28,32,33)/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293847
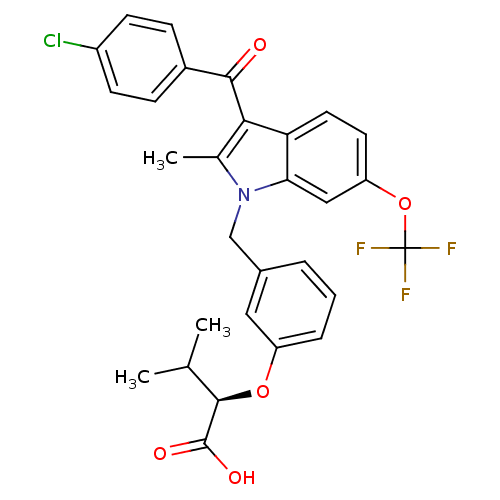
((2R)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CC(C)[C@@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-16(2)27(28(36)37)38-21-6-4-5-18(13-21)15-34-17(3)25(26(35)19-7-9-20(30)10-8-19)23-12-11-22(14-24(23)34)39-29(31,32)33/h4-14,16,27H,15H2,1-3H3,(H,36,37)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244809

((2R)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362973
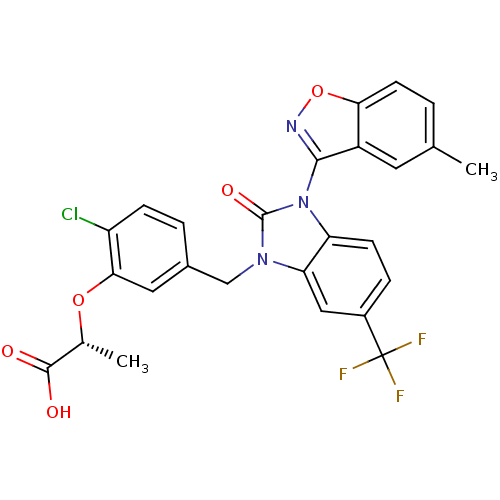
(CHEMBL1946572)Show SMILES C[C@@H](Oc1cc(Cn2c3cc(ccc3n(-c3noc4ccc(C)cc34)c2=O)C(F)(F)F)ccc1Cl)C(O)=O |r,wU:1.0,(27.94,-20.73,;27.91,-19.19,;26.56,-18.44,;25.24,-19.24,;23.9,-18.5,;22.59,-19.3,;21.24,-18.55,;21.22,-17.01,;19.75,-16.54,;18.41,-17.32,;17.08,-16.55,;17.08,-15,;18.41,-14.23,;19.74,-15,;21.2,-14.51,;21.97,-13.17,;21.07,-11.93,;21.98,-10.69,;23.44,-11.18,;24.78,-10.41,;26.11,-11.19,;26.1,-12.74,;27.43,-13.52,;24.76,-13.5,;23.43,-12.72,;22.12,-15.75,;23.66,-15.74,;15.74,-17.32,;14.41,-16.55,;15.74,-18.86,;14.4,-18.08,;22.61,-20.83,;23.95,-21.58,;25.27,-20.79,;26.62,-21.53,;29.23,-18.39,;30.58,-19.13,;29.2,-16.85,)| Show InChI InChI=1S/C26H19ClF3N3O5/c1-13-3-8-21-17(9-13)23(31-38-21)33-19-7-5-16(26(28,29)30)11-20(19)32(25(33)36)12-15-4-6-18(27)22(10-15)37-14(2)24(34)35/h3-11,14H,12H2,1-2H3,(H,34,35)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293849
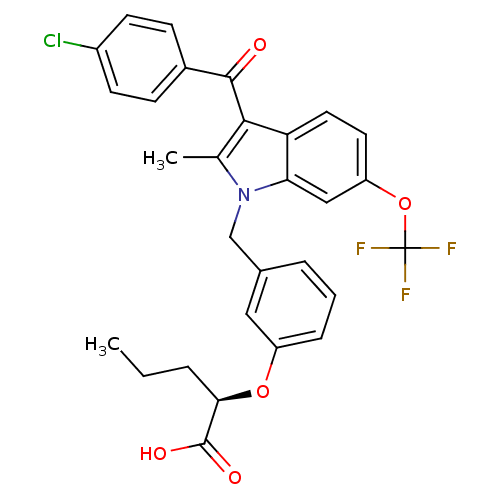
((2R)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CCC[C@@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-3-5-25(28(36)37)38-21-7-4-6-18(14-21)16-34-17(2)26(27(35)19-8-10-20(30)11-9-19)23-13-12-22(15-24(23)34)39-29(31,32)33/h4,6-15,25H,3,5,16H2,1-2H3,(H,36,37)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362948

(CHEMBL1947146)Show SMILES COc1ccc2c(noc2c1)-n1c2ccccc2n(Cc2cc(O[C@H](C)C(O)=O)ccc2Cl)c1=O |r,wU:24.27,(2.98,-42.51,;1.66,-41.72,;.32,-42.47,;.29,-44.02,;-1.06,-44.76,;-2.38,-43.97,;-3.85,-44.4,;-4.73,-43.15,;-3.81,-41.92,;-2.35,-42.42,;-1.01,-41.68,;-4.63,-45.73,;-6.1,-46.2,;-7.42,-45.42,;-8.76,-46.17,;-8.78,-47.72,;-7.46,-48.5,;-6.11,-47.74,;-4.64,-48.23,;-4.64,-49.77,;-3.3,-50.53,;-1.98,-49.75,;-.65,-50.51,;.68,-49.73,;2.02,-50.49,;2.03,-52.03,;3.35,-49.7,;4.69,-50.46,;3.34,-48.16,;-.63,-52.05,;-1.97,-52.83,;-3.3,-52.07,;-4.63,-52.84,;-3.73,-46.98,;-2.19,-46.99,)| Show InChI InChI=1S/C25H20ClN3O6/c1-14(24(30)31)34-17-8-10-19(26)15(11-17)13-28-20-5-3-4-6-21(20)29(25(28)32)23-18-9-7-16(33-2)12-22(18)35-27-23/h3-12,14H,13H2,1-2H3,(H,30,31)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50362974
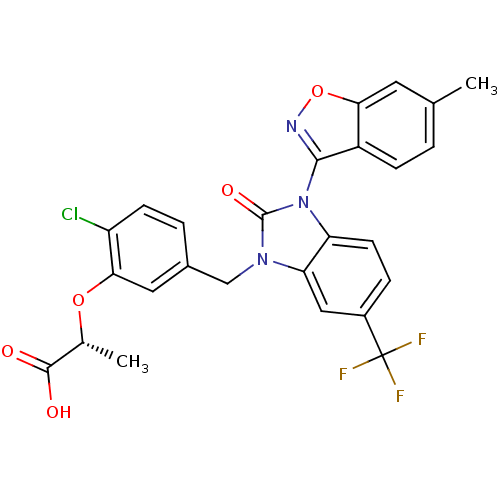
(CHEMBL1946753)Show SMILES C[C@@H](Oc1cc(Cn2c3cc(ccc3n(-c3noc4cc(C)ccc34)c2=O)C(F)(F)F)ccc1Cl)C(O)=O |r,wU:1.0,(3.59,-34.44,;3.56,-32.9,;2.21,-32.16,;.89,-32.95,;-.45,-32.21,;-1.76,-33.01,;-3.11,-32.26,;-3.13,-30.72,;-4.6,-30.25,;-5.94,-31.03,;-7.28,-30.26,;-7.27,-28.72,;-5.95,-27.94,;-4.61,-28.71,;-3.15,-28.22,;-2.38,-26.89,;-3.28,-25.64,;-2.37,-24.4,;-.91,-24.89,;.42,-24.13,;1.76,-24.9,;3.09,-24.13,;1.75,-26.45,;.41,-27.21,;-.92,-26.43,;-2.23,-29.47,;-.69,-29.46,;-8.61,-31.03,;-9.94,-30.26,;-8.61,-32.57,;-9.96,-31.79,;-1.74,-34.54,;-.4,-35.29,;.92,-34.5,;2.27,-35.25,;4.88,-32.1,;6.23,-32.84,;4.84,-30.56,)| Show InChI InChI=1S/C26H19ClF3N3O5/c1-13-3-6-17-21(9-13)38-31-23(17)33-19-8-5-16(26(28,29)30)11-20(19)32(25(33)36)12-15-4-7-18(27)22(10-15)37-14(2)24(34)35/h3-11,14H,12H2,1-2H3,(H,34,35)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma by scintillation proximity assay |
J Med Chem 54: 8541-54 (2011)
Article DOI: 10.1021/jm201061j
BindingDB Entry DOI: 10.7270/Q2542P11 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293844
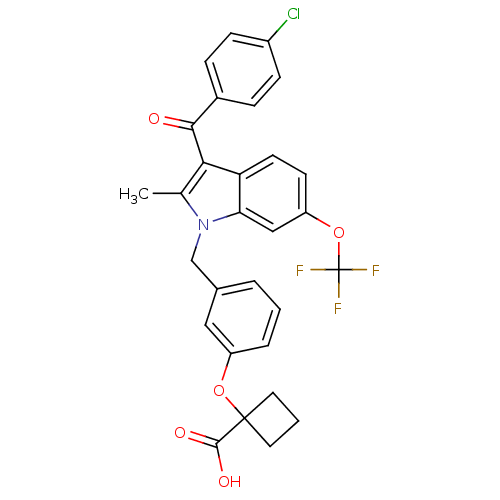
(1-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(trifluorome...)Show SMILES Cc1c(C(=O)c2ccc(Cl)cc2)c2ccc(OC(F)(F)F)cc2n1Cc1cccc(OC2(CCC2)C(O)=O)c1 Show InChI InChI=1S/C29H23ClF3NO5/c1-17-25(26(35)19-6-8-20(30)9-7-19)23-11-10-22(39-29(31,32)33)15-24(23)34(17)16-18-4-2-5-21(14-18)38-28(27(36)37)12-3-13-28/h2,4-11,14-15H,3,12-13,16H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Rattus norvegicus) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293848
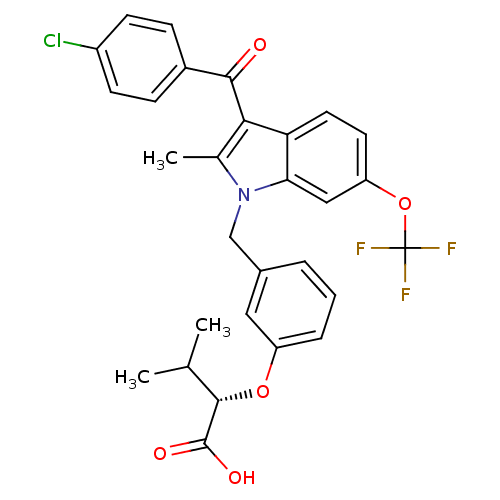
((2S)-2-{3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES CC(C)[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C29H25ClF3NO5/c1-16(2)27(28(36)37)38-21-6-4-5-18(13-21)15-34-17(3)25(26(35)19-7-9-20(30)10-8-19)23-12-11-22(14-24(23)34)39-29(31,32)33/h4-14,16,27H,15H2,1-3H3,(H,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 48: 141-51 (2005)
Article DOI: 10.1021/jm0493156
BindingDB Entry DOI: 10.7270/Q2HH6H8H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Niemann-Pick C1-like 1 protein
(Macaca mulatta) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
NPC1-like intracellular cholesterol transporter 1
(Homo sapiens (Human)) | BDBM50258479

((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorop...)Show SMILES CS(=O)(=O)NCC#CN1[C@@H]([C@@H](CC[C@H](O)c2ccc(F)cc2)C1=O)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1 |r| Show InChI InChI=1S/C28H31FN2O11S/c1-43(39,40)30-13-2-14-31-21(19(26(31)36)11-12-20(32)15-3-7-17(29)8-4-15)16-5-9-18(10-6-16)41-28-24(35)22(33)23(34)25(42-28)27(37)38/h3-10,19-25,28,30,32-35H,11-13H2,1H3,(H,37,38)/t19-,20+,21-,22+,23+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166302
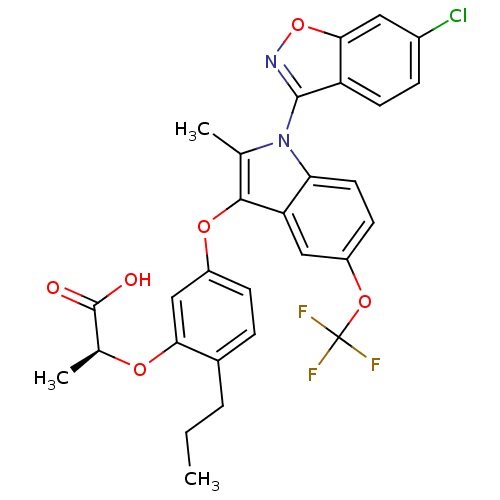
((S)-2-{5-[1-(6-Chloro-benzo[d]isoxazol-3-yl)-2-met...)Show SMILES CCCc1ccc(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)cc1O[C@@H](C)C(O)=O |wU:36.41,(13.07,5.11,;11.56,4.8,;11.09,3.32,;9.59,3.02,;8.54,4.16,;7.04,3.84,;6.56,2.36,;5.07,2.04,;4.59,.56,;5.49,-.7,;7.03,-.7,;4.59,-1.93,;5,-3.42,;4.03,-4.6,;4.88,-5.91,;6.35,-5.49,;7.65,-6.33,;9.02,-5.63,;10.31,-6.46,;9.09,-4.09,;7.81,-3.25,;6.45,-3.96,;3.12,-1.45,;1.8,-2.23,;.45,-1.45,;.45,.09,;-.9,.86,;-2.22,.09,;-3.57,-.71,;-3.01,1.42,;-1.43,-1.26,;1.8,.86,;3.12,.09,;7.6,1.23,;9.1,1.56,;10.14,.41,;11.63,.72,;12.11,2.19,;12.68,-.4,;12.19,-1.88,;14.19,-.09,)| Show InChI InChI=1S/C29H24ClF3N2O6/c1-4-5-17-6-8-19(14-24(17)38-16(3)28(36)37)39-26-15(2)35(27-21-10-7-18(30)12-25(21)41-34-27)23-11-9-20(13-22(23)26)40-29(31,32)33/h6-14,16H,4-5H2,1-3H3,(H,36,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50144009
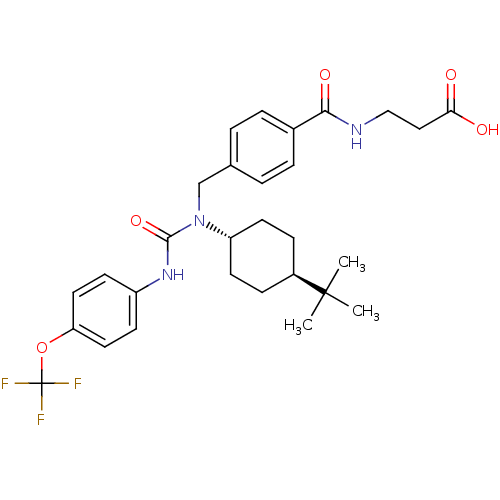
(3-{4-[1-(4-tert-Butyl-cyclohexyl)-3-(4-trifluorome...)Show SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)N(Cc1ccc(cc1)C(=O)NCCC(O)=O)C(=O)Nc1ccc(OC(F)(F)F)cc1 |wU:4.3,wD:7.10,(10.18,2.9,;9.39,1.56,;8.05,2.34,;8.62,.21,;10.74,.78,;12.08,1.53,;13.41,.77,;13.41,-.77,;12.07,-1.54,;10.74,-.77,;14.74,-1.54,;14.74,-3.07,;13.39,-3.86,;12.07,-3.1,;10.74,-3.86,;10.74,-5.4,;12.07,-6.17,;13.41,-5.4,;9.41,-6.19,;9.43,-7.72,;8.06,-5.42,;6.74,-6.2,;5.4,-5.44,;4.07,-6.23,;2.73,-5.47,;4.07,-7.77,;16.06,-.77,;16.06,.77,;17.39,-1.54,;18.71,-.77,;20.04,-1.53,;21.38,-.77,;21.36,.78,;22.71,1.56,;22.7,3.09,;21.17,3.11,;24.23,3.11,;22.71,4.62,;20.04,1.55,;18.71,.77,)| Show InChI InChI=1S/C29H36F3N3O5/c1-28(2,3)21-8-12-23(13-9-21)35(27(39)34-22-10-14-24(15-11-22)40-29(30,31)32)18-19-4-6-20(7-5-19)26(38)33-17-16-25(36)37/h4-7,10-11,14-15,21,23H,8-9,12-13,16-18H2,1-3H3,(H,33,38)(H,34,39)(H,36,37)/t21-,23- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 21: 76-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.074
BindingDB Entry DOI: 10.7270/Q2R211PW |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11160
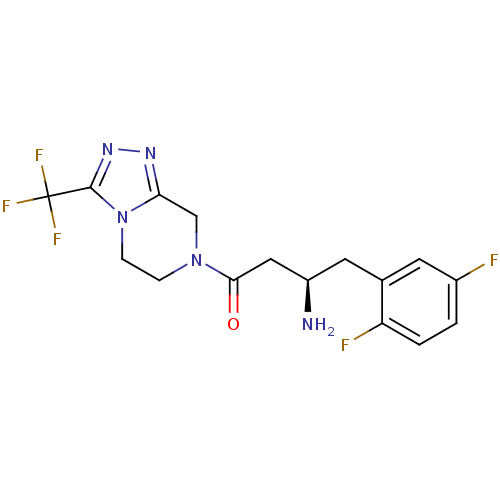
((3R)-3-amino-4-(2,5-difluorophenyl)-1-[3-(trifluor...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)ccc1F |r| Show InChI InChI=1S/C16H16F5N5O/c17-10-1-2-12(18)9(5-10)6-11(22)7-14(27)25-3-4-26-13(8-25)23-24-15(26)16(19,20)21/h1-2,5,11H,3-4,6-8,22H2/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Merck Research Laboratories
| Assay Description
The enzyme activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 360 nm and m... |
J Med Chem 48: 141-51 (2005)
Article DOI: 10.1021/jm0493156
BindingDB Entry DOI: 10.7270/Q2HH6H8H |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50334495

((R)-3-(4-((3-(3,5-dichlorophenyl)-5-(4-(trifluorom...)Show SMILES O[C@H](CNC(=O)c1ccc(Cn2nc(cc2-c2ccc(OC(F)(F)F)cc2)-c2cc(Cl)cc(Cl)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C27H20Cl2F3N3O5/c28-19-9-18(10-20(29)11-19)22-12-23(16-5-7-21(8-6-16)40-27(30,31)32)35(34-22)14-15-1-3-17(4-2-15)25(37)33-13-24(36)26(38)39/h1-12,24,36H,13-14H2,(H,33,37)(H,38,39)/t24-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]glucagon from human GCGR expressed in CHO cells |
Bioorg Med Chem Lett 21: 76-81 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.074
BindingDB Entry DOI: 10.7270/Q2R211PW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166296
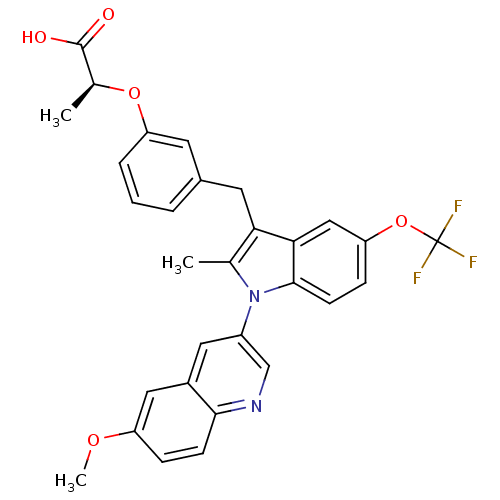
((S)-2-{3-[1-(6-Methoxy-quinolin-3-yl)-2-methyl-5-t...)Show SMILES COc1ccc2ncc(cc2c1)-n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 Show InChI InChI=1S/C30H25F3N2O5/c1-17-25(12-19-5-4-6-23(11-19)39-18(2)29(36)37)26-15-24(40-30(31,32)33)8-10-28(26)35(17)21-13-20-14-22(38-3)7-9-27(20)34-16-21/h4-11,13-16,18H,12H2,1-3H3,(H,36,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Niemann-Pick C1-like 1 protein
(Macaca mulatta) | BDBM50240720
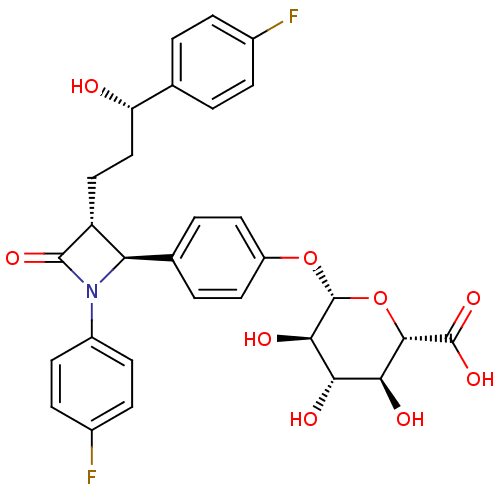
((2S,3S,4S,5R,6S)-6-(4-((2S,3R)-1-(4-fluorophenyl)-...)Show SMILES O[C@@H](CC[C@@H]1[C@H](N(C1=O)c1ccc(F)cc1)c1ccc(O[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)C(O)=O)cc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C30H29F2NO9/c31-17-5-1-15(2-6-17)22(34)14-13-21-23(33(28(21)38)19-9-7-18(32)8-10-19)16-3-11-20(12-4-16)41-30-26(37)24(35)25(36)27(42-30)29(39)40/h1-12,21-27,30,34-37H,13-14H2,(H,39,40)/t21-,22+,23-,24+,25+,26-,27+,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co
Curated by ChEMBL
| Assay Description
Displacement of [35S](2S,3S,4S,5R,6S)-6-(4-((2S,3R)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-1-(4-(3-(methylsulfonamido)prop-1-ynyl)phenyl)-4-oxoaz... |
Bioorg Med Chem Lett 19: 2965-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.031
BindingDB Entry DOI: 10.7270/Q2T153JQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166293

((S)-2-{3-[1-(6-Chloro-benzo[d]isoxazol-3-yl)-2-met...)Show SMILES C[C@H](Oc1cccc(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |wU:1.0,(13.85,3.3,;13.37,1.84,;11.87,1.53,;10.83,2.67,;11.32,4.14,;10.28,5.27,;8.78,4.95,;8.3,3.48,;6.81,3.15,;6.33,1.67,;7.23,.42,;8.77,.42,;6.33,-.81,;6.74,-2.3,;5.77,-3.48,;6.62,-4.79,;8.09,-4.38,;9.39,-5.21,;10.76,-4.51,;12.04,-5.34,;10.83,-2.97,;9.55,-2.14,;8.19,-2.84,;4.86,-.33,;3.54,-1.12,;2.19,-.33,;2.19,1.2,;.84,1.97,;-.48,1.2,;-1.27,2.53,;.31,-.14,;-1.83,.41,;3.54,1.97,;4.86,1.21,;9.34,2.35,;14.41,.7,;13.92,-.77,;15.92,1.02,)| Show InChI InChI=1S/C26H18ClF3N2O6/c1-13-23(36-17-5-3-4-16(11-17)35-14(2)25(33)34)20-12-18(37-26(28,29)30)7-9-21(20)32(13)24-19-8-6-15(27)10-22(19)38-31-24/h3-12,14H,1-2H3,(H,33,34)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data