

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759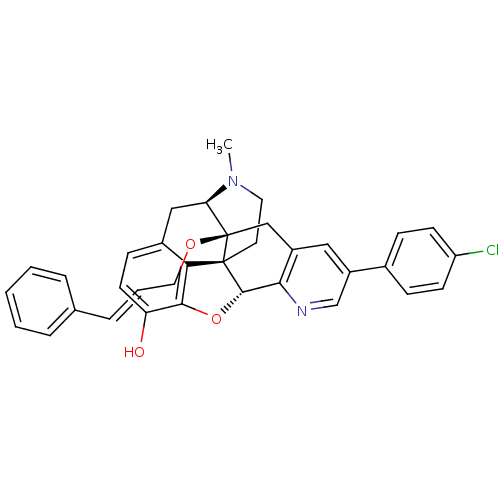 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342116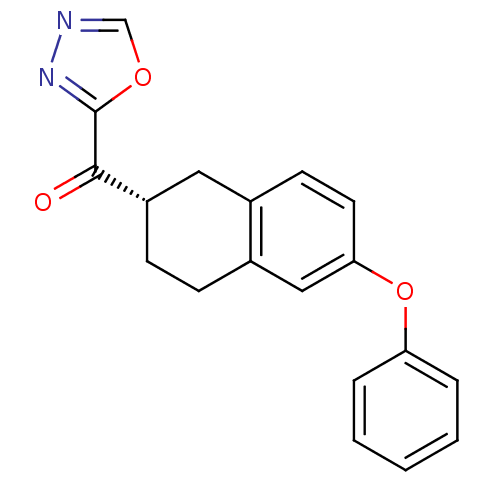 ((S)-(1,3,4-oxadiazol-2-yl)(6-phenoxy-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398750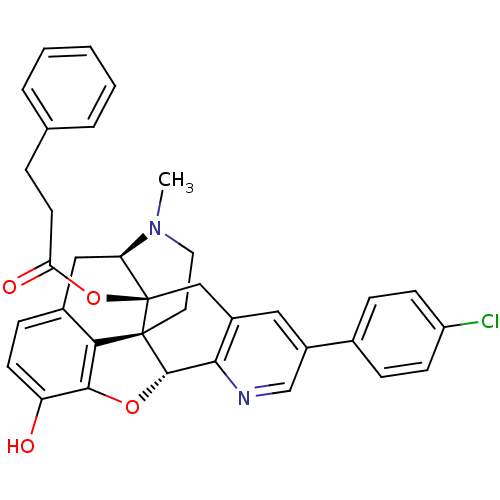 (CHEMBL2179662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398751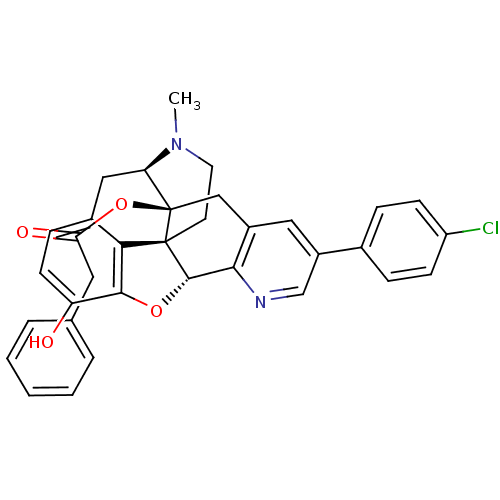 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342118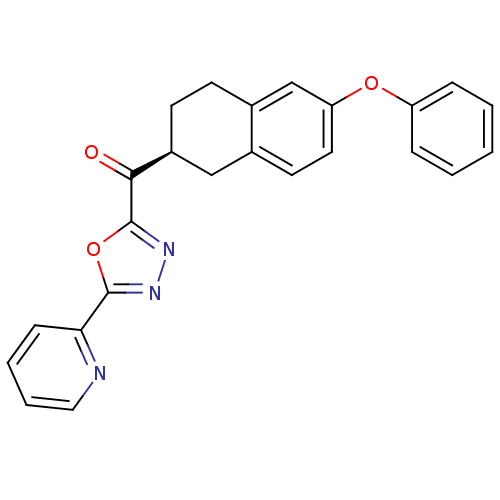 ((S)-(6-phenoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398753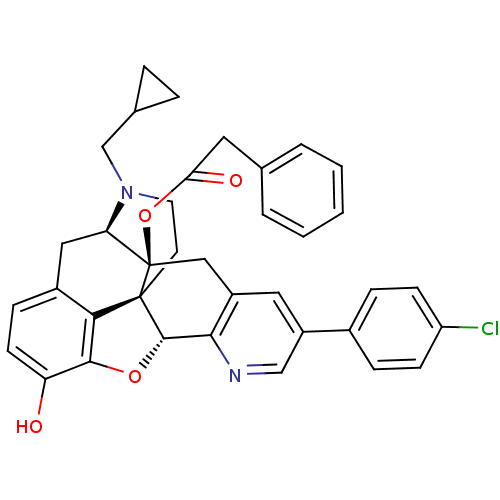 (CHEMBL2179658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342064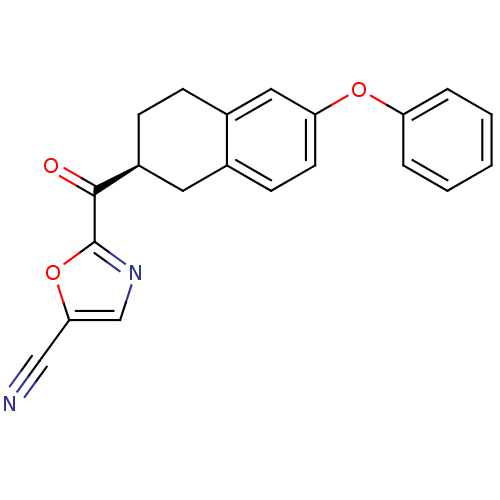 ((S)-2-(6-phenoxy-1,2,3,4-tetrahydronaphthalene-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342123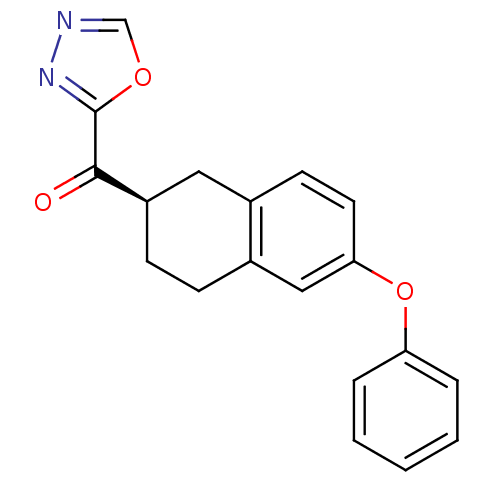 ((R)-(1,3,4-oxadiazol-2-yl)(6-phenoxy-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342104 ((S)-(5-(benzyloxy)-2,3-dihydro-1H-inden-2-yl)(5-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342117 ((S)-methyl 5-(6-phenoxy-1,2,3,4-tetrahydronaphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398755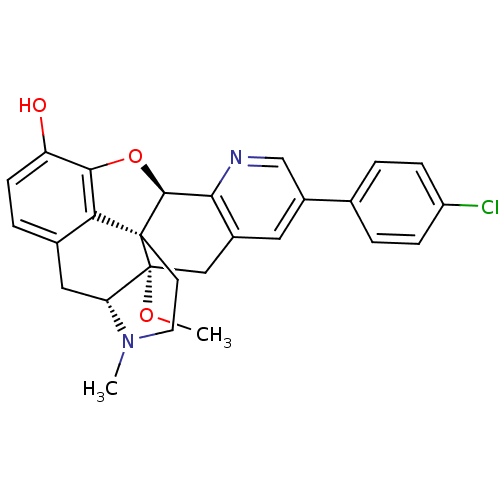 (CHEMBL2179653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342124 ((R)-methyl 5-(6-phenoxy-1,2,3,4-tetrahydronaphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398756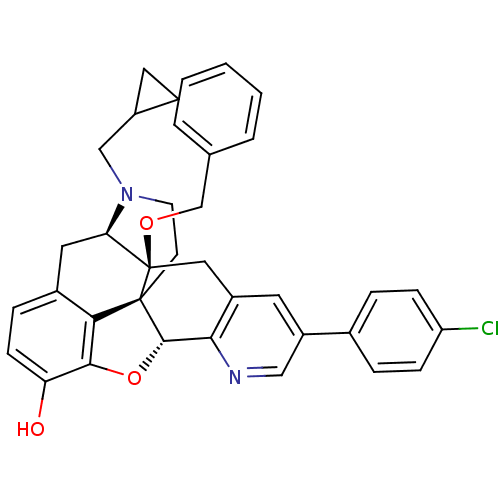 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342096 ((S)-(5-phenyl-2,3-dihydro-1H-inden-2-yl)(5-(pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398765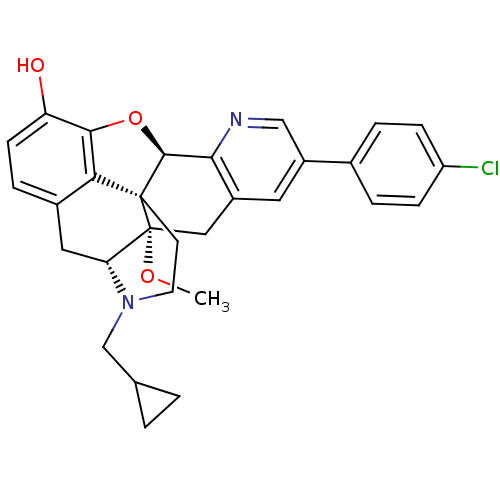 (CHEMBL2179649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342100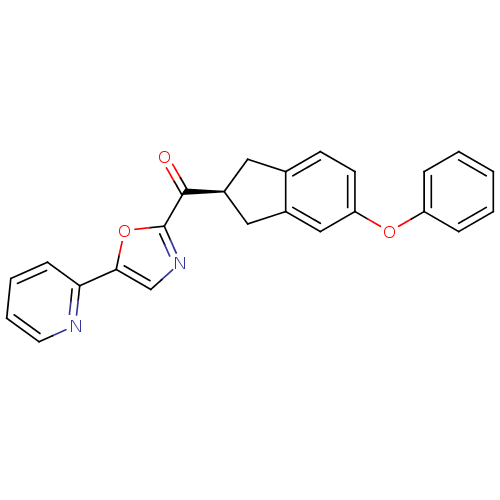 ((S)-(5-phenoxy-2,3-dihydro-1H-inden-2-yl)(5-(pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342062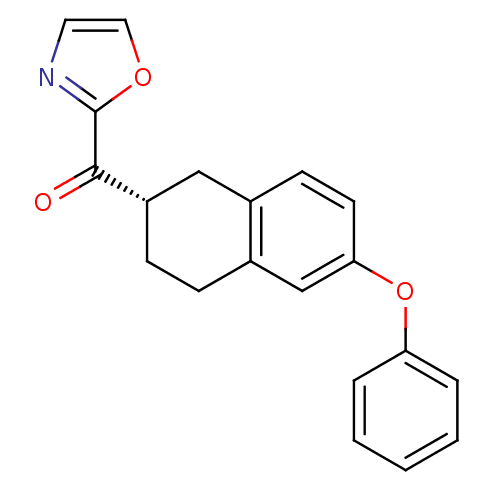 ((S)-oxazol-2-yl(6-phenoxy-1,2,3,4-tetrahydronaphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342066 ((S)-methyl 6-(2-(6-phenoxy-1,2,3,4-tetrahydronapht...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50080467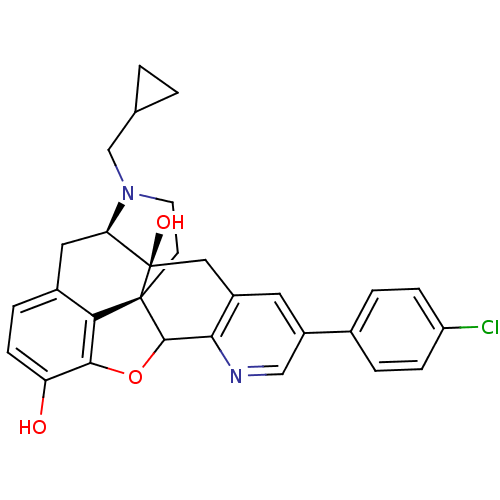 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398752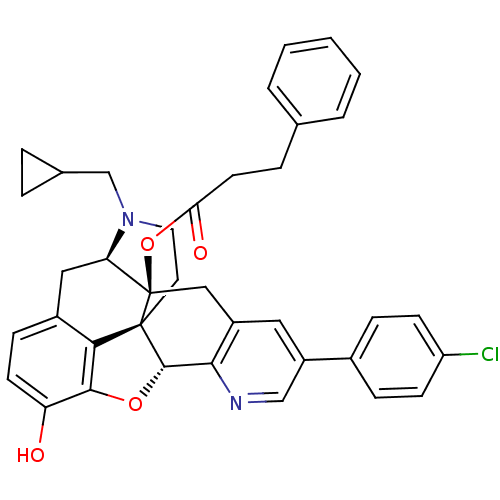 (CHEMBL2179659) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398762 (CHEMBL2179663) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342069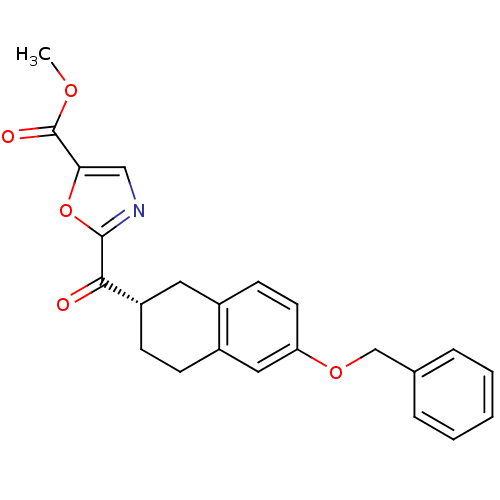 ((S)-methyl 2-(6-(benzyloxy)-1,2,3,4-tetrahydronaph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342070 ((S)-2-(6-(benzyloxy)-1,2,3,4-tetrahydronaphthalene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342071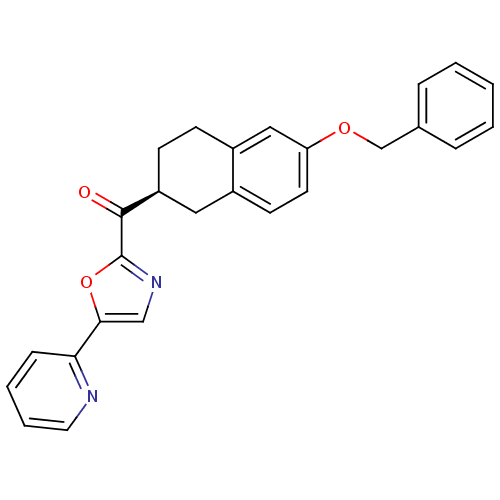 ((S)-(6-(benzyloxy)-1,2,3,4-tetrahydronaphthalen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342058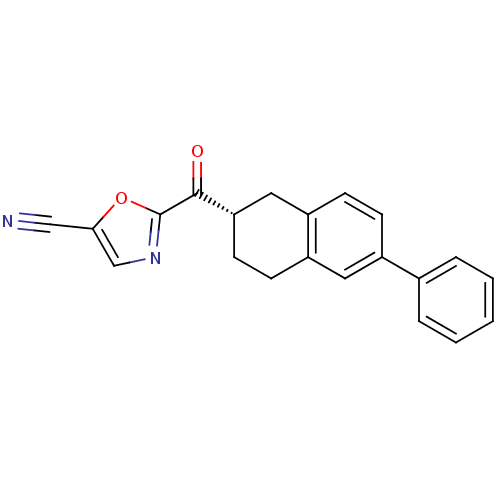 ((S)-2-(6-phenyl-1,2,3,4-tetrahydronaphthalene-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398764 (CHEMBL2179651) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398761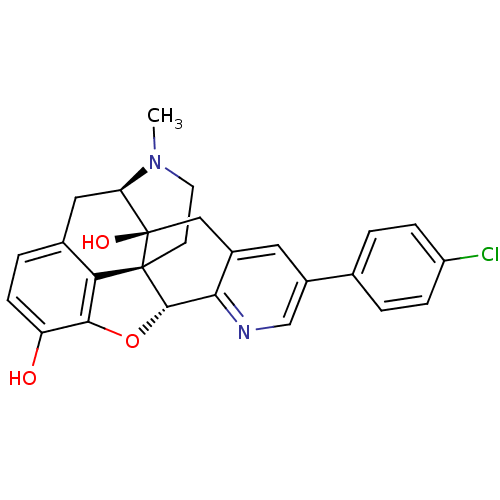 (CHEMBL611438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor by radioligand binding assay | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398752 (CHEMBL2179659) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342093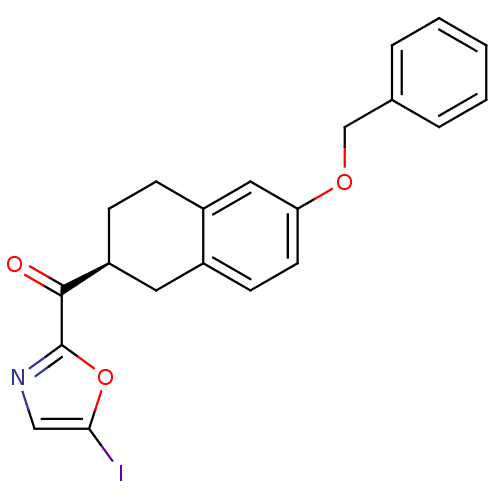 ((S)-(6-(benzyloxy)-1,2,3,4-tetrahydronaphthalen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50342065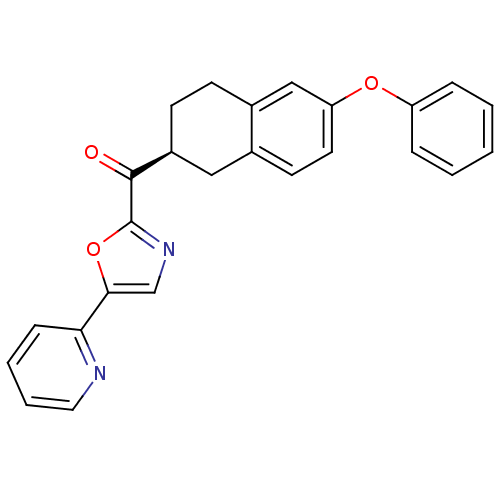 ((S)-(6-phenoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of rat recombinant FAAH expressed in Escherichia coli assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50342065 ((S)-(6-phenoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of full-length human FAAH expressed in COS-7 cells assessed as [14C]-labeled oleamide to oleic acid conversion | J Med Chem 54: 2805-22 (2011) Article DOI: 10.1021/jm101597x BindingDB Entry DOI: 10.7270/Q2ZK5H08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398756 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398765 (CHEMBL2179649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |