 Found 244 hits with Last Name = 'mcgovern' and Initial = 'sl'
Found 244 hits with Last Name = 'mcgovern' and Initial = 'sl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2681
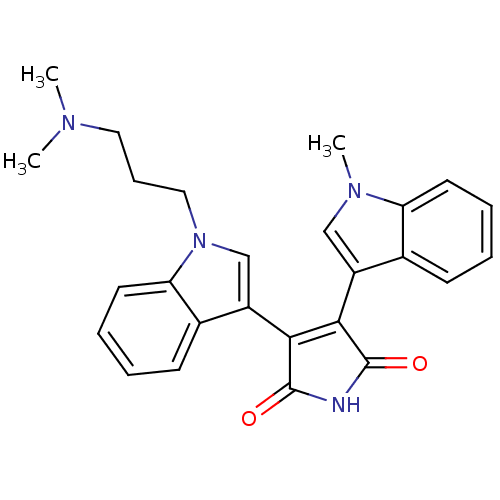
(3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H26N4O2/c1-28(2)13-8-14-30-16-20(18-10-5-7-12-22(18)30)24-23(25(31)27-26(24)32)19-15-29(3)21-11-6-4-9-17(19)21/h4-7,9-12,15-16H,8,13-14H2,1-3H3,(H,27,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3175
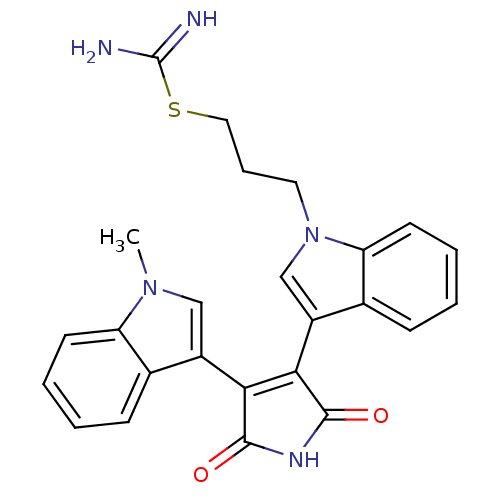
(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Therapeutic concentration on reverse transcriptase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM13336
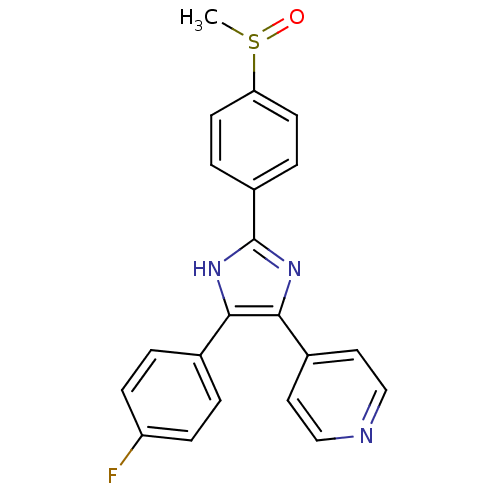
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Escherichia coli) | BDBM26139
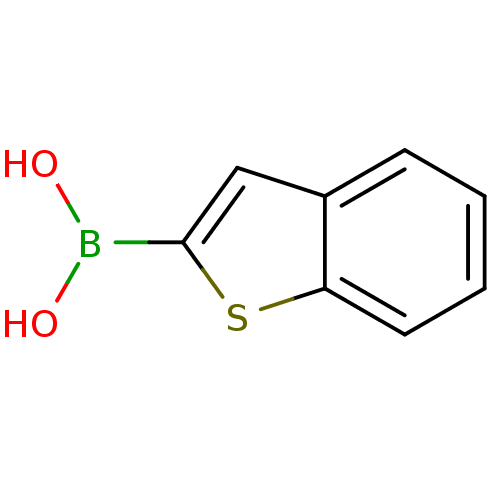
(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Escherichia coli) | BDBM26139

(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Amp C beta-Lactamase |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Escherichia coli) | BDBM26139

(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 50 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Escherichia coli) | BDBM26139

(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Escherichia coli) | BDBM26139

(1-benzothiophen-2-ylboranediol | 1-benzothiophen-2...)Show InChI InChI=1S/C8H7BO2S/c10-9(11)8-5-6-3-1-2-4-7(6)12-8/h1-5,10-11H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 500 mMKPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Gallus gallus (Chicken)) | BDBM50079267
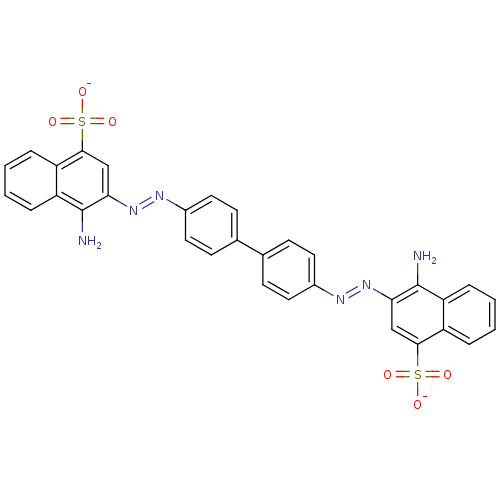
(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cloned Dihydrofolate reductase (cDHFR) |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111606
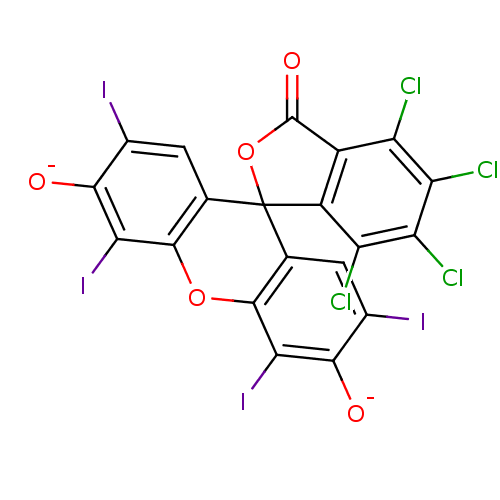
(4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-3-oxospi...)Show SMILES [O-]c1c(I)cc2c(Oc3c(I)c([O-])c(I)cc3C22OC(=O)c3c2c(Cl)c(Cl)c(Cl)c3Cl)c1I Show InChI InChI=1S/C20H4Cl4I4O5/c21-9-7-8(10(22)12(24)11(9)23)20(33-19(7)31)3-1-5(25)15(29)13(27)17(3)32-18-4(20)2-6(26)16(30)14(18)28/h1-2,29-30H/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111607

(3,4-Dichloro-N-[5-(5-chloro-benzothiazol-2-ylsulfa...)Show SMILES Clc1ccc2sc(Sc3nnc(NC(=O)c4ccc(Cl)c(Cl)c4)s3)nc2c1 Show InChI InChI=1S/C16H7Cl3N4OS3/c17-8-2-4-12-11(6-8)20-15(25-12)27-16-23-22-14(26-16)21-13(24)7-1-3-9(18)10(19)5-7/h1-6H,(H,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50369776
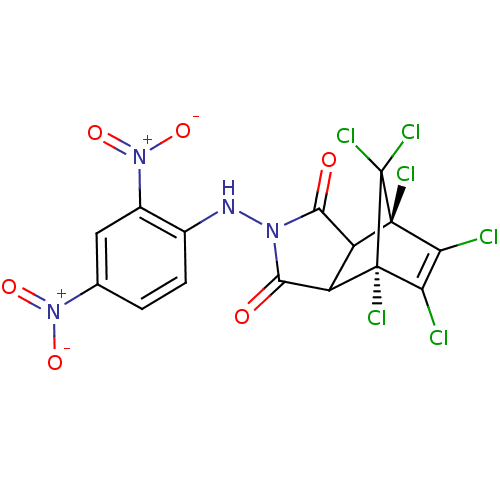
(CHEMBL298301)Show SMILES [O-][N+](=O)c1ccc(NN2C(=O)C3C(C2=O)[C@@]2(Cl)C(Cl)=C(Cl)[C@]3(Cl)C2(Cl)Cl)c(c1)[N+]([O-])=O |t:19,TLB:9:11:23:19.17,20:19:23:11.12,THB:13:12:23:19.17,18:17:23:11.12| Show InChI InChI=1S/C15H6Cl6N4O6/c16-9-10(17)14(19)8-7(13(9,18)15(14,20)21)11(26)23(12(8)27)22-5-2-1-4(24(28)29)3-6(5)25(30)31/h1-3,7-8,22H/t7?,8?,13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N2
(Homo sapiens (Human)) | BDBM14029

((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C related kinase 2 (PRK2) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50079267

(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14029

((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase ROCK2 (ROCKII) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50126829
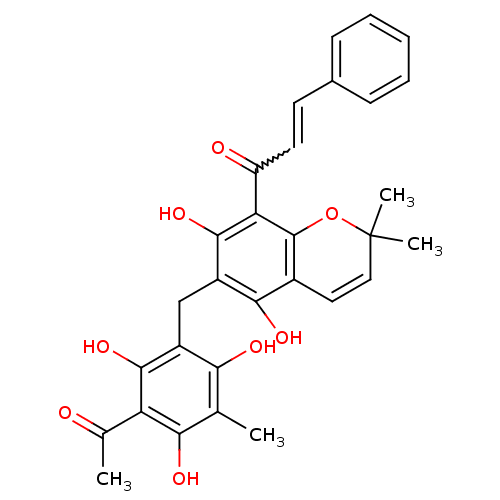
((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50108771

(2'-amino-3'-methoxyflavone | 2-(2-Amino-3-methoxy-...)Show InChI InChI=1S/C16H13NO3/c1-19-14-8-4-6-11(16(14)17)15-9-12(18)10-5-2-3-7-13(10)20-15/h2-9H,17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111589
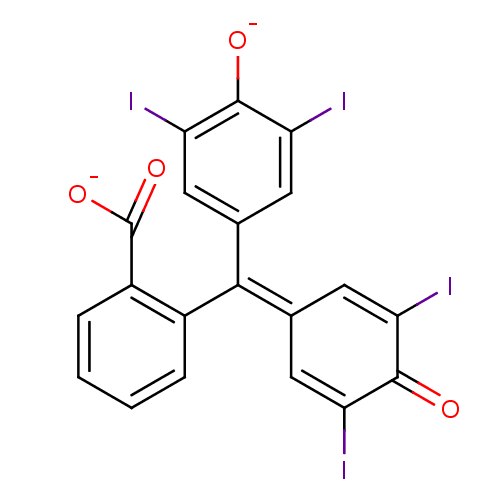
(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Amp C beta-Lactamase |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50336799
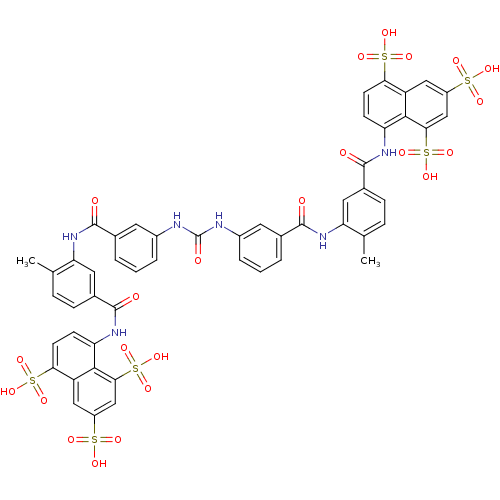
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA-DNA dependent protein kinase (DNA-PK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N1
(Homo sapiens (Human)) | BDBM14027
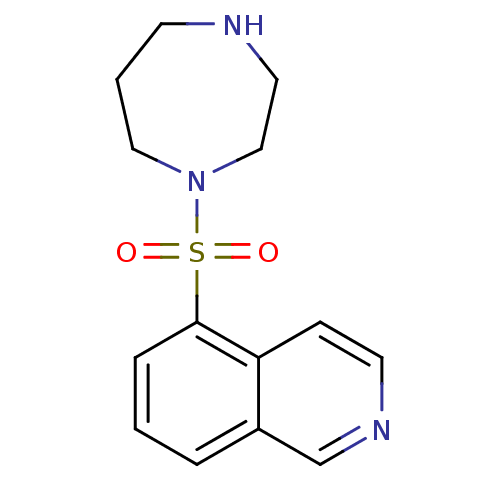
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C related kinase 1 (PRK1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of p38-regulated activated kinase (PRAK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Homo sapiens (Human)) | BDBM15234
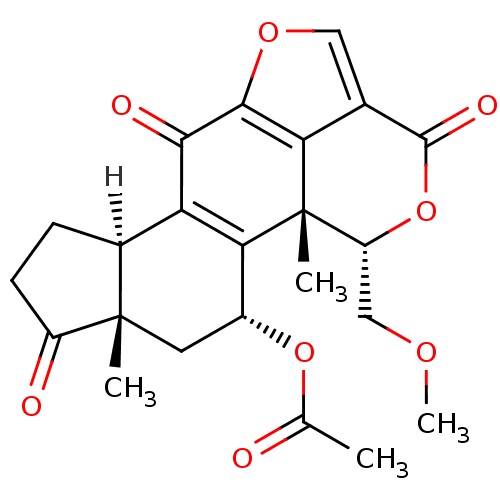
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase (mMLCK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14027

(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase ROCK2 (ROCKII) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13336

(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111605

(3-[2,2']Bithiophenyl-5-ylmethylene-1,3-dihydro-ind...)Show InChI InChI=1S/C17H11NOS2/c19-17-13(12-4-1-2-5-14(12)18-17)10-11-7-8-16(21-11)15-6-3-9-20-15/h1-10H,(H,18,19)/b13-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111590

(2-(4-Chloro-phenyl)-6-[4-(4-chloro-phenylsulfanyl)...)Show SMILES OC(=O)c1cc(nc(c1)-c1ccc(Sc2ccc(Cl)cc2)cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H15Cl2NO2S/c25-18-5-1-15(2-6-18)22-13-17(24(28)29)14-23(27-22)16-3-9-20(10-4-16)30-21-11-7-19(26)8-12-21/h1-14H,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50111590

(2-(4-Chloro-phenyl)-6-[4-(4-chloro-phenylsulfanyl)...)Show SMILES OC(=O)c1cc(nc(c1)-c1ccc(Sc2ccc(Cl)cc2)cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H15Cl2NO2S/c25-18-5-1-15(2-6-18)22-13-17(24(28)29)14-23(27-22)16-3-9-20(10-4-16)30-21-11-7-19(26)8-12-21/h1-14H,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against chymotrypsinogen |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111611
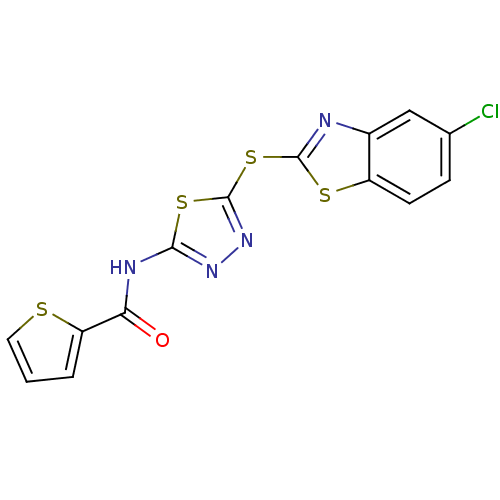
(CHEMBL49498 | Thiophene-2-carboxylic acid [5-(5-ch...)Show InChI InChI=1S/C14H7ClN4OS4/c15-7-3-4-9-8(6-7)16-13(22-9)24-14-19-18-12(23-14)17-11(20)10-2-1-5-21-10/h1-6H,(H,17,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50108771

(2'-amino-3'-methoxyflavone | 2-(2-Amino-3-methoxy-...)Show InChI InChI=1S/C16H13NO3/c1-19-14-8-4-6-11(16(14)17)15-9-12(18)10-5-2-3-7-13(10)20-15/h2-9H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen activated protein kinase kinase 1 (MKK1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50023867
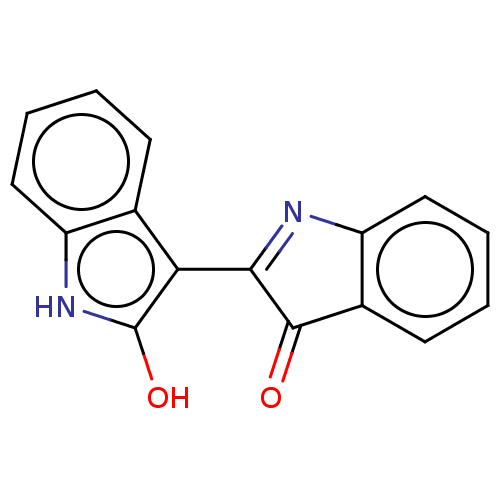
(Indirubin)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,18,20H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 (CDK2) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111585

(4-(4-Bromo-phenylazo)-phenol | 4-bromophenylazophe...)Show InChI InChI=1S/C12H9BrN2O/c13-9-1-3-10(4-2-9)14-15-11-5-7-12(16)8-6-11/h1-8,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2581
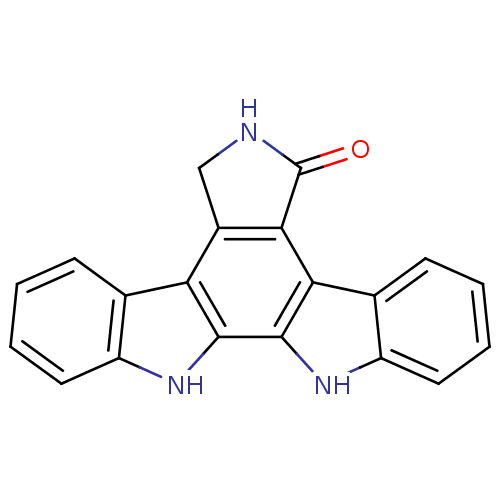
(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50111607

(3,4-Dichloro-N-[5-(5-chloro-benzothiazol-2-ylsulfa...)Show SMILES Clc1ccc2sc(Sc3nnc(NC(=O)c4ccc(Cl)c(Cl)c4)s3)nc2c1 Show InChI InChI=1S/C16H7Cl3N4OS3/c17-8-2-4-12-11(6-8)20-15(25-12)27-16-23-22-14(26-16)21-13(24)7-1-3-9(18)10(19)5-7/h1-6H,(H,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against chymotrypsinogen |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50369776

(CHEMBL298301)Show SMILES [O-][N+](=O)c1ccc(NN2C(=O)C3C(C2=O)[C@@]2(Cl)C(Cl)=C(Cl)[C@]3(Cl)C2(Cl)Cl)c(c1)[N+]([O-])=O |t:19,TLB:9:11:23:19.17,20:19:23:11.12,THB:13:12:23:19.17,18:17:23:11.12| Show InChI InChI=1S/C15H6Cl6N4O6/c16-9-10(17)14(19)8-7(13(9,18)15(14,20)21)11(26)23(12(8)27)22-5-2-1-4(24(28)29)3-6(5)25(30)31/h1-3,7-8,22H/t7?,8?,13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Amp C beta-Lactamase |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50111595
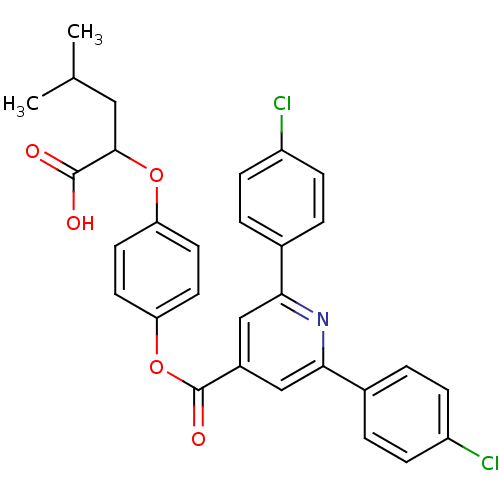
(2,6-Bis-(4-chloro-phenyl)-isonicotinic acid 4-(1-c...)Show SMILES CC(C)CC(Oc1ccc(OC(=O)c2cc(nc(c2)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2)cc1)C(O)=O Show InChI InChI=1S/C30H25Cl2NO5/c1-18(2)15-28(29(34)35)37-24-11-13-25(14-12-24)38-30(36)21-16-26(19-3-7-22(31)8-4-19)33-27(17-21)20-5-9-23(32)10-6-20/h3-14,16-18,28H,15H2,1-2H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against chymotrypsinogen |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111615
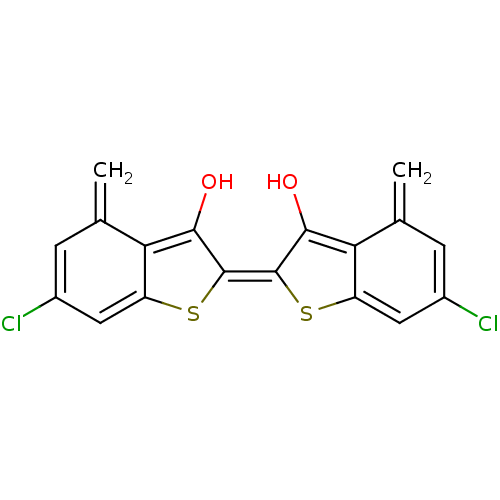
(6,6'-Dichloro-4,4'-dimethyl-[2,2']bi[benzo[b]thiop...)Show SMILES Oc1c2c(cc(Cl)cc2=C)s\c1=c1/sc2cc(Cl)cc(=C)c2c1O Show InChI InChI=1S/C18H10Cl2O2S2/c1-7-3-9(19)5-11-13(7)15(21)17(23-11)18-16(22)14-8(2)4-10(20)6-12(14)24-18/h3-6,21-22H,1-2H2/b18-17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111597
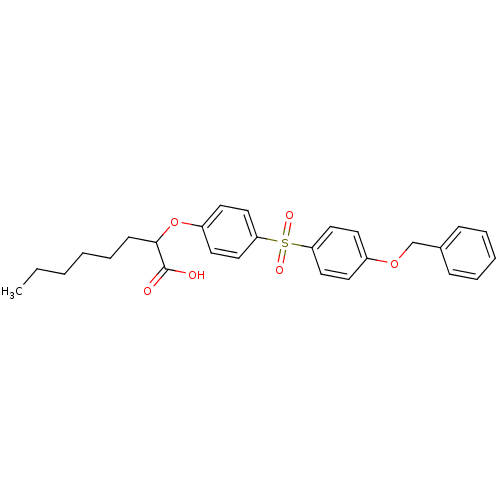
(2-[4-(4-Benzyloxy-benzenesulfonyl)-phenoxy]-octano...)Show SMILES CCCCCCC(Oc1ccc(cc1)S(=O)(=O)c1ccc(OCc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H30O6S/c1-2-3-4-8-11-26(27(28)29)33-23-14-18-25(19-15-23)34(30,31)24-16-12-22(13-17-24)32-20-21-9-6-5-7-10-21/h5-7,9-10,12-19,26H,2-4,8,11,20H2,1H3,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111591
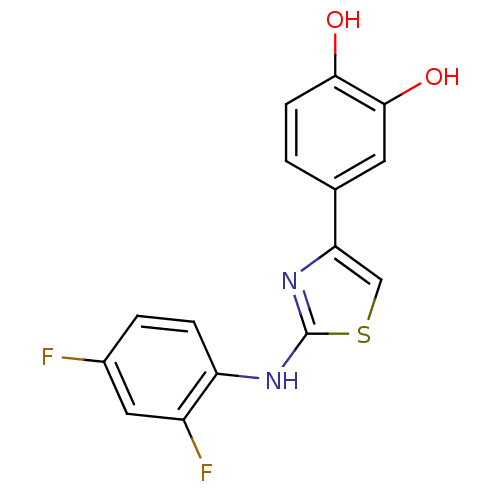
(4-[2-(2,4-Difluoro-phenylamino)-thiazol-4-yl]-benz...)Show InChI InChI=1S/C15H10F2N2O2S/c16-9-2-3-11(10(17)6-9)18-15-19-12(7-22-15)8-1-4-13(20)14(21)5-8/h1-7,20-21H,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 50 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against TEM-1 Beta-lactamase mutant G238A at 24 degree Centigrade |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50369776

(CHEMBL298301)Show SMILES [O-][N+](=O)c1ccc(NN2C(=O)C3C(C2=O)[C@@]2(Cl)C(Cl)=C(Cl)[C@]3(Cl)C2(Cl)Cl)c(c1)[N+]([O-])=O |t:19,TLB:9:11:23:19.17,20:19:23:11.12,THB:13:12:23:19.17,18:17:23:11.12| Show InChI InChI=1S/C15H6Cl6N4O6/c16-9-10(17)14(19)8-7(13(9,18)15(14,20)21)11(26)23(12(8)27)22-5-2-1-4(24(28)29)3-6(5)25(30)31/h1-3,7-8,22H/t7?,8?,13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Amp C beta-Lactamase |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against TEM-1 Beta-lactamase mutant M182T at 42 degree Centigrade |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Beta-lactamase from DMSO stock was determined |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against TEM-1 Beta-lactamase mutant M182T at 42 degree Centigrade |
J Med Chem 46: 4265-72 (2003)
Article DOI: 10.1021/jm030266r
BindingDB Entry DOI: 10.7270/Q29C6Z59 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta (PKCdelta) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50369776

(CHEMBL298301)Show SMILES [O-][N+](=O)c1ccc(NN2C(=O)C3C(C2=O)[C@@]2(Cl)C(Cl)=C(Cl)[C@]3(Cl)C2(Cl)Cl)c(c1)[N+]([O-])=O |t:19,TLB:9:11:23:19.17,20:19:23:11.12,THB:13:12:23:19.17,18:17:23:11.12| Show InChI InChI=1S/C15H6Cl6N4O6/c16-9-10(17)14(19)8-7(13(9,18)15(14,20)21)11(26)23(12(8)27)22-5-2-1-4(24(28)29)3-6(5)25(30)31/h1-3,7-8,22H/t7?,8?,13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against beta-lactamase in the presence of 50 mM KPi concentration of buffer |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data