

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358044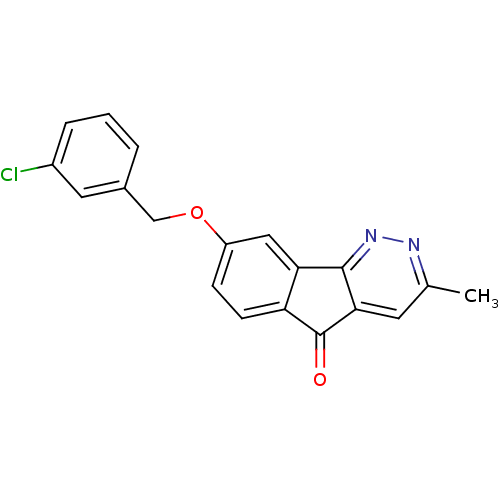 (CHEMBL1917940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358044 (CHEMBL1917940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM21975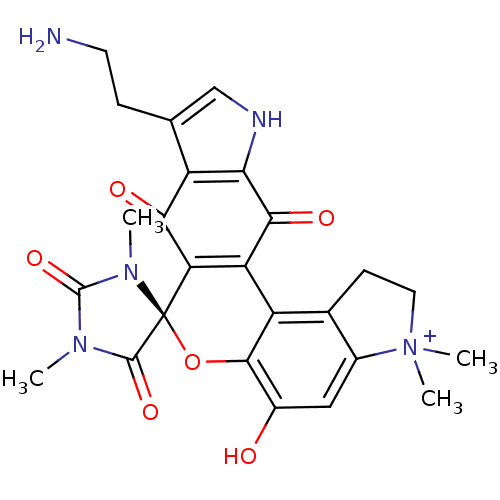 ((4S)-16'-(2-aminoethyl)-9'-hydroxy-1,3,6',6'-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50121688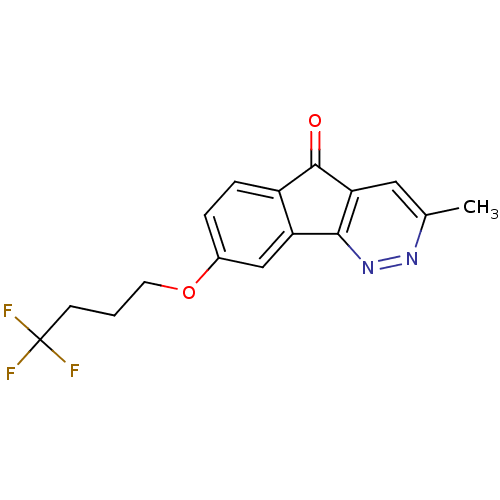 (3-Methyl-8-(4,4,4-trifluoro-butoxy)-indeno[1,2-c]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50358045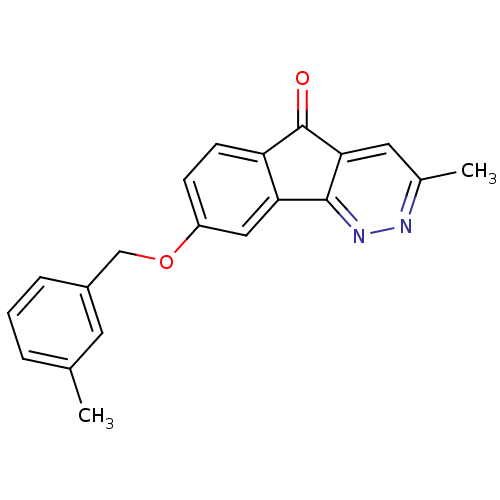 (CHEMBL1914652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis | Eur J Med Chem 46: 6104-11 (2011) Article DOI: 10.1016/j.ejmech.2011.09.042 BindingDB Entry DOI: 10.7270/Q2BC3ZXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428658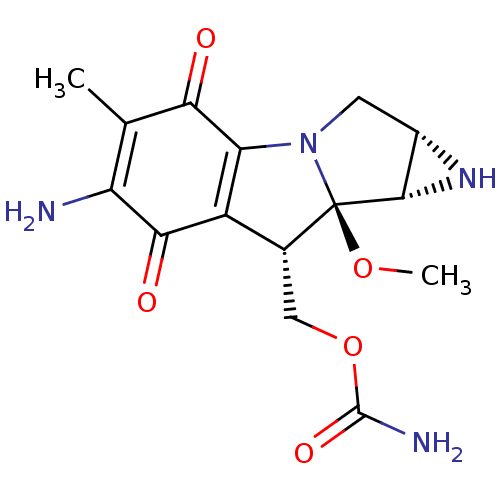 (MITOMYCIN | Mitomycin C | Mitosol | Mitozytrex | M...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Uncompetitive inhibition of hexahistidyl-tagged human IDO1 | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM100152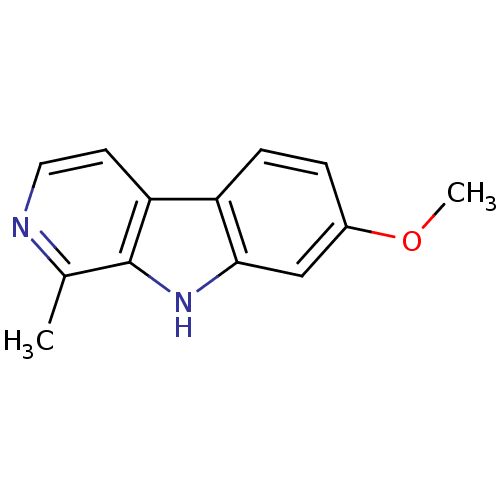 (7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of human DYRK1A kinase expressed in Escherichia coli cells | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428674 (CHEMBL576950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428673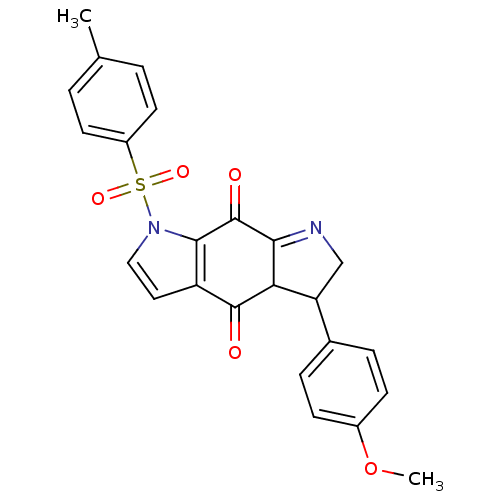 (CHEMBL574910) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428672 (CHEMBL572914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428671 (CHEMBL574690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268684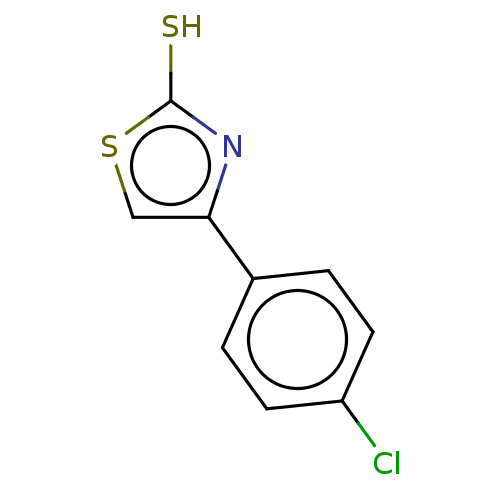 (CHEMBL1501761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428670 (CHEMBL576757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268684 (CHEMBL1501761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268688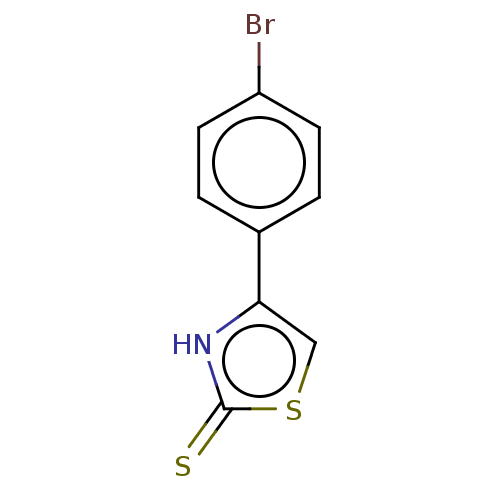 (CHEMBL4070094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268688 (CHEMBL4070094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428669 (CHEMBL575549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428668 (CHEMBL575780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428667 (CHEMBL575331) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428666 (CHEMBL574691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268675 (CHEMBL1558960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50268675 (CHEMBL1558960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428665 (CHEMBL573179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428664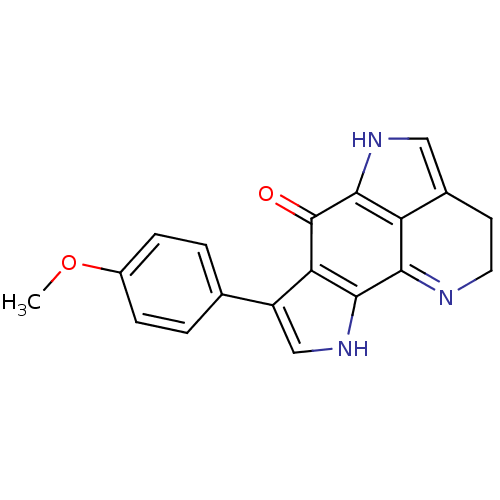 (CHEMBL576344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428663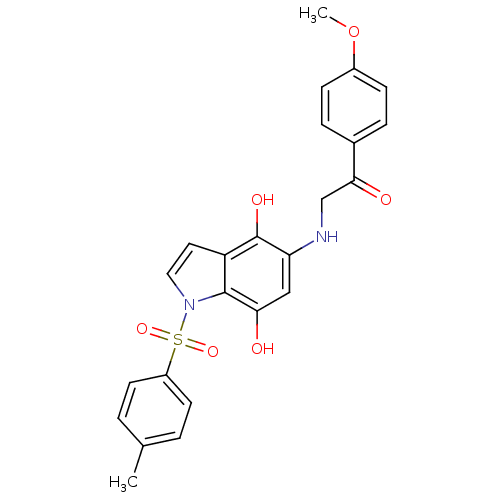 (CHEMBL574896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395379 (CHEMBL2164712 | US9168247, CV12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50333423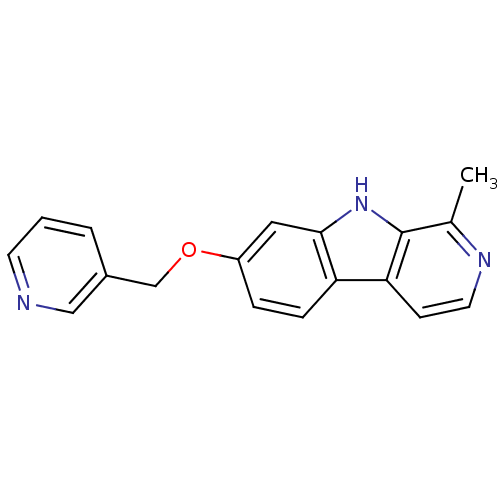 (7-(Pyridin-3-ylmethoxy)-1-methyl-beta-carboline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50333423 (7-(Pyridin-3-ylmethoxy)-1-methyl-beta-carboline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395379 (CHEMBL2164712 | US9168247, CV12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428662 (CHEMBL573014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428661 (CHEMBL575568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428660 (CHEMBL2332688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50333416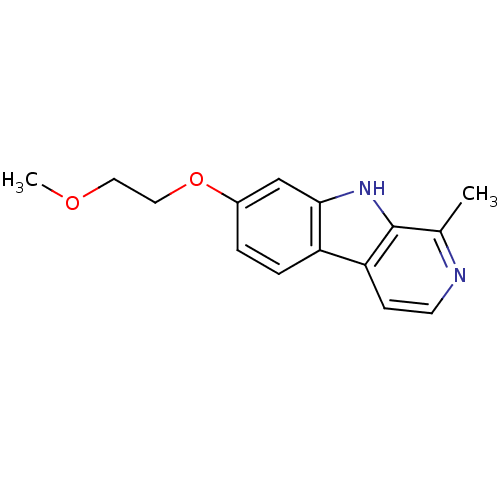 (7-(2-Methoxyethoxy)-1-methyl-beta-carboline | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50333416 (7-(2-Methoxyethoxy)-1-methyl-beta-carboline | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395380 (CHEMBL2164709 | US9168247, JR84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395380 (CHEMBL2164709 | US9168247, JR84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395381 (CHEMBL2164716 | US9168247, JR95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395381 (CHEMBL2164716 | US9168247, JR95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428659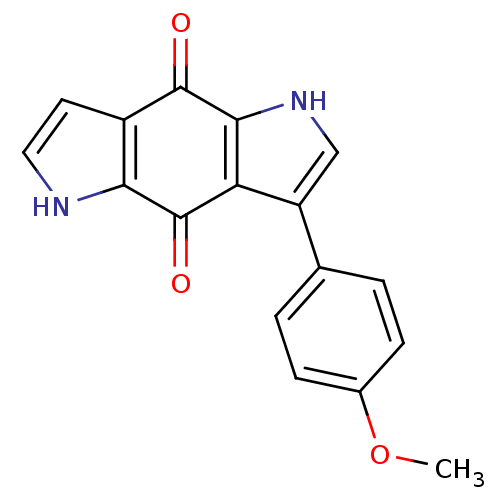 (CHEMBL583951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910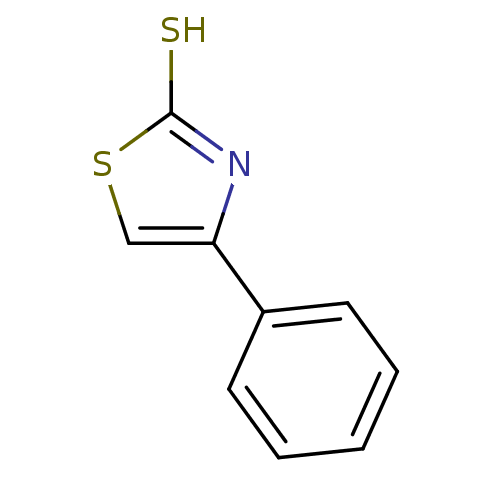 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50428657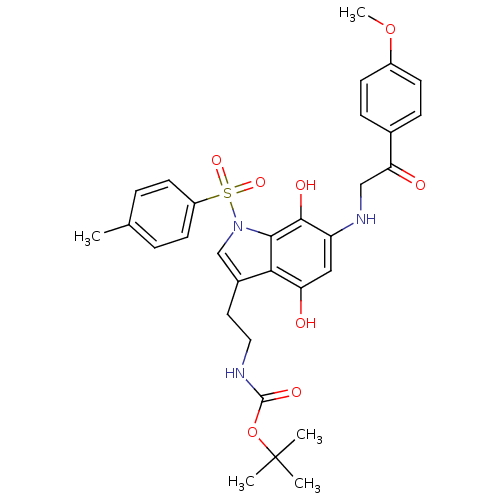 (CHEMBL573203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395378 (CHEMBL2164696 | US9168247, CV24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
FACULTES UNIVERSITAIRES NOTRE DAME DE LA PAIX; UNIVERSITE LIBRE BRUXELLES US Patent | Assay Description A radiometric protein kinase assay (33PanQinase Activity Assay) was used for measuring the kinase activity of DYRK1A protein kinase. All kinase assay... | US Patent US9168247 (2015) BindingDB Entry DOI: 10.7270/Q2X63KQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50395378 (CHEMBL2164696 | US9168247, CV24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP) Curated by ChEMBL | Assay Description Inhibition of DYRK1A kinase using RBER-CHKtide substrate by radiometric protein kinase assay | J Med Chem 55: 6489-501 (2012) Article DOI: 10.1021/jm300542e BindingDB Entry DOI: 10.7270/Q2GM88FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.39E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-Trp as substrate assessed as kynurenine level after 30 mins | Bioorg Med Chem Lett 23: 47-54 (2012) Article DOI: 10.1016/j.bmcl.2012.11.036 BindingDB Entry DOI: 10.7270/Q2JM2C03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||